Abstract
Purpose
Our purpose was to report outcomes of elderly patients who underwent definitive treatment involving radiation therapy for esophageal cancer at our institution.
Methods and Materials
We performed a retrospective review of patients aged ≥75 years with esophageal cancer treated with definitive radiation therapy (≥45 Gy) at our institution from 1997 to 2019. Acute and late Radiation Therapy Oncology Group grade 3+ toxicities were recorded. Survival was estimated using the Kaplan-Meier method.
Results
Of the 89 patients included, median age was 80 and 78% were male. Median adjusted Charlson Comorbidity Index and Karnofsky Performance Status were 5 (3-12) and 80 (50-100), respectively. The majority of cancers were adenocarcinoma (58%), distal (67%), and stage III (62%). Fifty-eight percent underwent definitive chemoradiotherapy, and one-third underwent preoperative intent chemoradiotherapy. Median prescribed dose was 50 Gy (45-66 Gy), and intensity modulated radiation therapy was used in 76%. Eighty-five percent completed the radiation therapy course. Among these, 20% had radiation therapy breaks. For those receiving concurrent chemotherapy, 37% had a dose reduction and 39.5% had a break/cycle reduction. Acute grade 3+ toxicity was 22%, with 2% grade 5 toxicity. Twenty-one of the 29 patients (72%) treated with preoperative intent underwent surgery. There were no deaths 90 days postoperatively. For patients who underwent surgery, 1- and 2-year overall survival were 95% and 84%. For those who did not undergo surgery, 1- and 2-year overall survival were 70% and 52%.
Conclusions
There is a role for aggressive radiation therapy in well-selected elderly patients with esophageal cancer. However, optimization of supportive care, chemotherapy regimens, radiation therapy dose/fractionation, and surgical indications are needed to reduce toxicity.
Introduction
For patients with locally advanced esophageal cancer, preoperative chemoradiotherapy improves survival compared with surgery alone.1 For patients who cannot undergo surgery, definitive chemoradiotherapy can achieve long-term disease control.2 However, elderly patients are underrepresented in randomized trials,3 raising the question of the generalizability of these results for the elderly. Although Radiation Therapy Oncology Group (RTOG) 85-01 included 26% of patients aged ≥70 years, outcomes for this group were not specifically evaluated. In the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS trial), only patients aged 18 to 75 were eligible, and the median age was 60. Yet, one-third of patients diagnosed with esophageal cancer are >75 years of age (Surveillance, Epidemiology, and End Results 21, 2012-2016).
Because many patients with esophageal cancer are elderly with comorbidities and poor performance status, a substantial proportion are not considered for chemoradiation or surgery, given concerns for toxicity. In RTOG 85-01, the rate of acute life-threatening/fatal toxicities associated with chemoradiotherapy was 10%. Esophagectomy is associated with perioperative mortality rates as high as 20%.4, 5, 6 Accordingly, elderly patients with esophageal cancer are less likely to be referred to a cancer specialist and, if referred, are less likely to receive surgery or chemotherapy, regardless of tumor stage or comorbidities.6, 7, 8
To reduce missed opportunities for curative treatment based on age alone, further evaluation of efficacy and tolerability of multimodality therapy in elderly patients is critical. Although some series have demonstrated favorable outcomes in well-selected elderly patients, they often focused on patients ≥65 years of age.9, 10, 11 However, older patients tend to fare worse with multimodality treatment.6,12,13 In a National Cancer Database analysis, octogenarians had worse survival with multimodality treatment versus nonoctogenarians, with no survival benefit compared with surgery alone.6 In addition, age >80 was predictive of worse survival after esophagectomy (with 30-day perioperative mortality >20%), independent of comorbidities.5,12 Thus, we sought to evaluate our institutional outcomes for elderly patients (age ≥75 years) with esophageal cancer who underwent treatment with definitive radiation therapy.
Methods and Materials
Study cohort
In this institutional review board–approved study, we performed a retrospective review of patients aged ≥75 years with biopsy-proven esophageal cancer treated with definitive radiation therapy from 1997 to 2019. All cancers were prescribed a dose ≥45 Gy using 3-dimensional (3D)–conformal or intensity modulated radiation therapy (IMRT). Stage IVB oligometastatic and postoperative radiation therapy patients were permitted, as the study intent was to evaluate toxicity/tolerability of definitive radiation therapy doses. Treatment intent (definitive, preoperative, or postoperative) was determined based on clinical notes. Decision to pursue surgery was based on patient preference and medical/surgical evaluation. Date of diagnosis was defined as the date of biopsy-proven diagnosis. Staging was reported as American Joint Committee on Cancer, eighth edition. Charlson Comorbidity Index (CCI) was used to quantify patients’ comorbidities14 and was adjusted to exclude the esophageal cancer diagnosis (“adjusted CCI”) to account for patients with a second solid malignancy.
Treatment
Generally, patients were simulated supine and immobilized with a Vac-Lok bag/wing board for midthoracic to distal esophageal tumors or long mask for upper esophageal tumors. Four-dimensional computed tomography (CT) and respiratory gating were performed for midthoracic/distal cancers; otherwise, planning was based on 3DCT with intravenous contrast. Positron emission tomography (PET)/CT was either done at the time of simulation or staging. PET was imported and fused to the simulation CT. Gross tumor volume was delineated based on PET and CT. For >0.5 cm of diaphragmatic/tumor motion throughout the respiratory cycle, an internal target volume was generated based on the four-dimensional CT. Clinical target volume was defined as a margin 3 to 4 cm craniocaudally and 1 to 2 cm radially (but <5 mm extension into lung parenchyma). Clinical target volume included elective nodal regions as recommended in the IMRT consensus guideline.15 Planned target volume was defined as a 5-mm margin. A simultaneous or sequential boost was frequently incorporated and prescribed to the gross tumor volume with 1- to 2-cm isotropic margin. Typical constraints included combined lung V20 < 20%, V30 < 20%, and mean <18 Gy; heart V30 < 50% and mean <20 Gy; and spinal cord maximum ≤45 Gy. Dosimetric planning was performed with an Eclipse (Varian) treatment planning system. Patients were assessed by their radiation oncologist every 5 fractions.
Outcomes
Radiation breaks were defined as documented breaks or calculated elapsed days >2 days from expected completion date without documentation of a break. RTOG grade 3+ toxicities (G3+) were noted from treatment start to 90 days posttreatment (acute) and from 90 days to last follow-up (late), and included toxicity thought to be secondary to chemotherapy, radiation, or surgery. Overall survival (OS) and progression-free survival (PFS) were calculated from treatment start. Emergency department (ED) visits and intensive care unit admissions during or within 30 days of treatment were documented. Presence of gastrotomy or jejunostomy tubes were categorized by placement before, during, or after radiation; those placed as adjunct to surgery were excluded. Follow-up visits typically occurred at 4 to 6 weeks post radiation therapy and at 6-month intervals with CT and/or PET in the first 5 years.
Statistical analysis
Baseline characteristics were summarized as frequencies for categorical variables and median values for continuous variables. Chi-square and Fisher's exact test (when observed cell counts were <5) were used for group comparisons. OS and PFS were estimated using the Kaplan-Meier method. Difference in survival between groups was determined with the log-rank test.
Tests were 2-sided, and P < .05 was considered statistically significant. Statistical analyses were performed with SAS University Edition version 3.8 (SAS Institute Inc, Cary, NC). Survival graphs were generated with Prism version 8 (GraphPad Software, San Diego, CA).
Results
Patient characteristics
Eighty-nine patients were eligible, and their characteristics are summarized in Table 1. Median age at treatment was 80 (interquartile range 76-84, with 7 patients ≥90, age deidentified per Health Insurance Portability and Accountability Act regulations), and 78% were male. Median adjusted CCI and Karnofsky Performance Status were 5 (3-12) and 80 (50-100), respectively. The majority of tumors were adenocarcinoma (58%), distal (67%), and stage III (62%). Seven (7.9%) patients had oligometastatic IVB disease (distant metastases, M1).
Table 1.
Patient characteristics
| Characteristics | All patients (n = 89) | Age ≥80 y (n = 49) | Underwent surgery∗ (n = 21) |
|---|---|---|---|
| Age, median (IQR) | 80 (76-84) | 84 (82-86) | 77 (76-78) |
| Male, n (%) | 69 (78) | 38 (78) | 16 (76) |
| Adjusted CCI, median (range) | 5 (3-12) | 6 (4-12) | 4 (3-6) |
| KPS, median (range) | 80 (50-100) | 85 (50-100) | 90 (70-100) |
| Histology, n (%) | |||
| Adenocarcinoma | 52 (58) | 27 (55) | 14 (67) |
| Squamous cell | 35 (40) | 20 (41) | 7 (33) |
| Poorly differentiated | 2 (2) | 2 (4) | 0 (0) |
| Location of primary, n (%) | |||
| Cervical | 8 (9) | 3 (6) | 0 (0) |
| Upper-middle thoracic | 21 (23.6) | 13 (27) | 2 (9.5) |
| Distal/GEJ | 60 (67.4) | 33 (67) | 19 (90.5) |
| Stage, n (%) | |||
| I | 4 (4.5) | 2 (4.1) | 0 (0) |
| II | 16 (18) | 10 (20.4) | 2 (9.5) |
| III | 55 (61.8) | 29 (59.2) | 17 (81) |
| IV† | 14 (15.7) | 8 (16.3) | 2 (9.5)‡ |
| Year of treatment, n (%) | |||
| 1997-2002 | 15 (16.9) | 5 (10.2) | 3 (14.3) |
| 2003-2008 | 19 (21.3) | 13 (26.5) | 2 (9.5) |
| 2009-2014 | 25 (28.1) | 12 (24.5) | 7 (33.3) |
| 2015-2019 | 30 (33.7) | 19 (38.8) | 9 (42.9) |
| Follow-up (median, IQR) | 16 mo (6-29) | 16 mo (6-24) | 24 mo (14-44) |
Abbreviations: CCI = Charlson Comorbidity Index; GEJ = gastroesophageal junction; IQR = interquartile range; KPS = Karnofsky Performance Status.
Underwent preoperative chemoradiation and surgery. Excludes those who underwent upfront surgery and postop radiation.
Includes 7 patients with distantly oligometastatic disease (stage IVB).
All stage IVA (locoregionally advanced due to tumor and/or nodal disease, depends on histology).
Treatment regimens
Eighty-one patients (91%) received concurrent chemotherapy, most commonly carboplatin-paclitaxel (46%) or a platinum-fluoropyrimidine (30%). Median prescribed radiation therapy dose was 50 Gy (45-66 Gy), with IMRT used in 76%. There were 2 definitive chemoradiation cases with prescribed dose of 45 Gy (both squamous cell carcinomas); all other cases were prescribed to at least 50 Gy.
Twenty-nine patients (33%) underwent preoperative intent chemoradiotherapy (Table 2). The majority (72%) treated with preoperative intent underwent surgery. Eight patients did not undergo surgery because of performance decline after chemoradiotherapy (n = 3), disease progression (n = 2), or patient preference (n = 3). Surgery was performed a median 60 days (range, 27-127 days) after completing radiation therapy. Two patients demonstrated complete pathologic response (both adenocarcinomas), and 15 were down-staged.
Table 2.
Treatment regimens
| All patients (n = 89) | Age ≥80 y (n = 49) | Underwent surgery∗ (n = 21) | |
|---|---|---|---|
| Intent, n (%) | |||
| CRT without preoperative intent | 52 (58) | 33 (67) | 0 (0) |
| CRT with preoperative intent | 29 (33) | 11 (22) | 21 (100) |
| Definitive radiation alone | 5 (6) | 3 (6) | 0 (0) |
| Postoperative radiation | 3 (3) | 2 (4) | 0 (0) |
| Chemotherapy, n (%) | |||
| Carboplatin-paclitaxel | 41 (46) | 22 (45) | 15 (71) |
| Platinum-fluoropyrimidine | 27 (30) | 12 (24) | 6 (29) |
| Fluoropyrimidine alone | 14 (16) | 11 (22) | 0 (0) |
| Other | 2 (2) | 1 (2) | 0 (0) |
| None | 5 (6) | 3 (6) | 0 (0) |
| Radiation | |||
| Dose, median (range) | 50 Gy (45-66 Gy) | 50 Gy (50-60 Gy) | 50 Gy (45-55.8 Gy) |
| IMRT modality | 68 (76%) | 39 (80%) | 17 (81%) |
Abbreviations: CRT = chemoradiation; IMRT = intensity modulated radiation therapy.
Underwent preoperative chemoradiation and surgery. Excludes those who underwent upfront surgery and postop radiation.
Three patients with presumed early stage cancers were surgically upstaged and thus received postoperative chemoradiotherapy. In these patients, radiation therapy was delivered a median of 168 days (range, 109-160 days) after surgery to a median dose of 50 Gy (range, 45-50 Gy) and was given with concurrent chemotherapy.
Toxicities
Thirteen patients (15%) did not complete the planned radiation therapy course (Table 3); they stopped at a median dose of 28 Gy (range, 3.6-53.6 Gy) owing to clinical decompensation or treatment intolerance. Two patients deteriorated after unrelated hospitalizations (inguinal hernia incarceration and hemorrhage of a psoas metastasis). One patient had fatal hemoptysis in the setting of thrombocytopenia.
Table 3.
Treatment outcomes
| All patients (n = 89) | Age 75-79 y (n = 40) | Age ≥80 y∗ (n = 49) | Underwent surgery† (n = 21) | |||
|---|---|---|---|---|---|---|
| Any RT or chemotherapy break/dose reduction/discontinuation, n (%) | 50 (58) | 16 (40) | 34 (74) | P = .01 | 8 (38) | P = .02 |
| Radiation | ||||||
| Did not complete, n (%) | 13 (15) | 4 (10) | 9 (18) | P = .27 | 0 (0) | P = .02 |
| RT break, n (%) | 18 (20) | 7 (18) | 11 (22) | P = .56 | 1 (5) | P = .03 |
| RT break duration (median, range) | 3 days (1-10) | 4 days (3-10) | 3 days (1-9) | 3 days (N/A) | ||
| Chemotherapy | ||||||
| Dose-reduced, n (%)‡ | 30 (37) | 5 (13) | 25 (57) | P < .001 | 3 (15) | P = .02 |
| Chemo break, n (%) | 10 (11.5) | 4 (10) | 6 (13) | P = .75 | 3 (14) | P = .70 |
| Reduced cycles/discontinuation, n (%) | 24 (28) | 8 (20) | 16 (34) | P = .14 | 3 (14) | P = .11 |
| Underwent surgery, n (%) | 21 of 89 (24) | 16 of 40 (40) | 5 of 49 (10) | 21 of 21 (100) | ||
| Hospitalizations | ||||||
| ED visit, n (%) | 26 (29) | 11 (28) | 15 (31) | P = .75 | 1 (5) | P = .003 |
| ICU admission, n (%) | 5§ (5.6‖) | 4§ (10) | 1 (2) | P = .17 | 3§ (14) | P = .08 |
| Acute toxicities, n (%) | ||||||
| G3 | 18 (20) | 6 (15) | 8 (16) | P = .31 | 3# (14) | P = .19 |
| G4/G5 | 0 (0)/2 (2) | 0 (0)/1 (2.5) | 0 (0)/1 (2) | P = .50 | 0 (0)/0 (0) | P = .58 |
| Late toxicities,∗∗ n (%) | ||||||
| G3 | 8 (9) | 6 (15) | 0 (0) | P = .01 | 2 (9) | P = .33 |
| G4/5 | 2 (2)/0 (0) | 2 (5)/0 (0) | 0 (0)/0 (0) | P = .19 | 1 (5)/0 (0) | P = .39 |
| 30-day mortality | 4 (4) | 1 (4) | 3 (6) | P = .30 | 0 (0) | P = .33 |
| 90-day mortality | 13 (15) | 5 (15) | 8 (16) | P = .61 | 0 (0) | P = .02 |
| G/J tube, n (%) | 23 (26) | 8 (20) | 15 (31) | P = .19 | 4 (19) | P = .23 |
| Placed before RT | 12 (52) | 3 (37.5) | 9 (60) | 2 (50) | ||
Abbreviations: ED = emergency department; G3 = grade 3; G/J = gastrotomy or jejunostomy; ICU = intensive care unit; RT = radiation therapy.
P values (X2 or Fisher’s exact) reported compared with age 75 to 79; P value is bolded if significant (P < .05).
Underwent preoperative chemoradiation and surgery. Excludes those who underwent upfront surgery and postop radiation. P values calculated with Fisher’s exact (given observed cell counts <5), compared with those who did not undergo surgery. P value is bolded if significant (P <.05).
Excludes cases where dose was unknown (n = 3)
Includes n = 3 nonfatal postop complications in surgical group, which required ICU stay.
Other cases: Fatal hemoptysis and complications from esophago-pleural fistula, which both occurred during RT course.
All acute toxicities were dysphagia-related. Two were present preoperatively (and thus likely due to chemoradiotherapy), whereas the third was present postoperatively (and thus likely due to surgery).
All late toxicities were dysphagia-related.
Of those who did complete radiation therapy, 18 (20%) required radiation therapy breaks (median, 3 days; range, 1-10 days). Reasons for breaks could be determined for 61% of patients and were mostly due to pharyngitis/mucositis/dehydration. Three breaks were due to unrelated events (hospitalizations for incarcerated hernia, ureteral stent placement, and gastrointestinal bleed secondary to supratherapeutic international normalised ratio). For those receiving concurrent chemotherapy, 30 (37%) were dose reduced and 34 (39.5%) required a chemotherapy cycle reduction or break primarily owing to G1 to G2 cytopenia (n = 10) and less commonly owing to mucositis/dehydration, diarrhea, or hand-foot syndrome (from capecitabine). The older age group demonstrated a higher proportion of chemotherapy dose reductions/adjustments (Table 3).
Twenty-six patients (29%) had an ED visit during or within 30 days of completing radiation therapy. The majority of visits (41%) were for G3 dysphagia, and 19% were for sepsis (primarily from aspiration pneumonia). Two patients had intensive care unit admissions for fatal complications (esophago-pleural fistula diagnosed during radiation therapy course and fatal hemoptysis in the setting of thrombocytopenia). Feeding tubes were placed in 26% of patients; of these, 52% were placed before radiation therapy, primarily for prophylactic intent. In 2 cases, the feeding tube was permanent (both placed before radiation therapy).
The rates of acute G3, G4, and G5 toxicity were 20%, 0%, and 2%. The rates of late G3, G4, and G5 toxicity were 9% (all for dysphagia/stricture requiring dilations), 2% (n = 2, nonfatal esophageal perforation/fistula), and 0%. Acute and late G3+ toxicities differed by chemotherapy regimens: platinum-fluoropyrimidine demonstrated higher G3+ toxicities versus carboplatin-paclitaxel (acute, 31% vs 17%, P = .004; late, 25% vs 5.5%, P = .004), whereas fluoropyrimidine alone demonstrated the lowest toxicities (acute, 7.1%; late, 0%). Acute G3+ toxicity was also statistically significantly different among adjusted CCI stratifications: CCI 1 to 4 (30%), 9 to 12 (22%), then 5 to 8 (13%) (P = .012). Treatment breaks did not significantly differ among the subgroups, but chemotherapy dose reductions/reduced cycles were significantly different (15%, 30%, and 66%, for CCI 1-4, 5-8, and 9-12, respectively, P = .04).
Of the 21 patients who underwent surgery, 14% (n = 3) had nonfatal acute surgical complications (acute respiratory distress syndrome, anastomotic leak, and ventilation-dependence requiring tracheostomy). There were no cases of 30- or 90-day mortality, and ED visits were significantly less compared with those who did not undergo surgery (P = .003; Table 3).
Survival outcomes
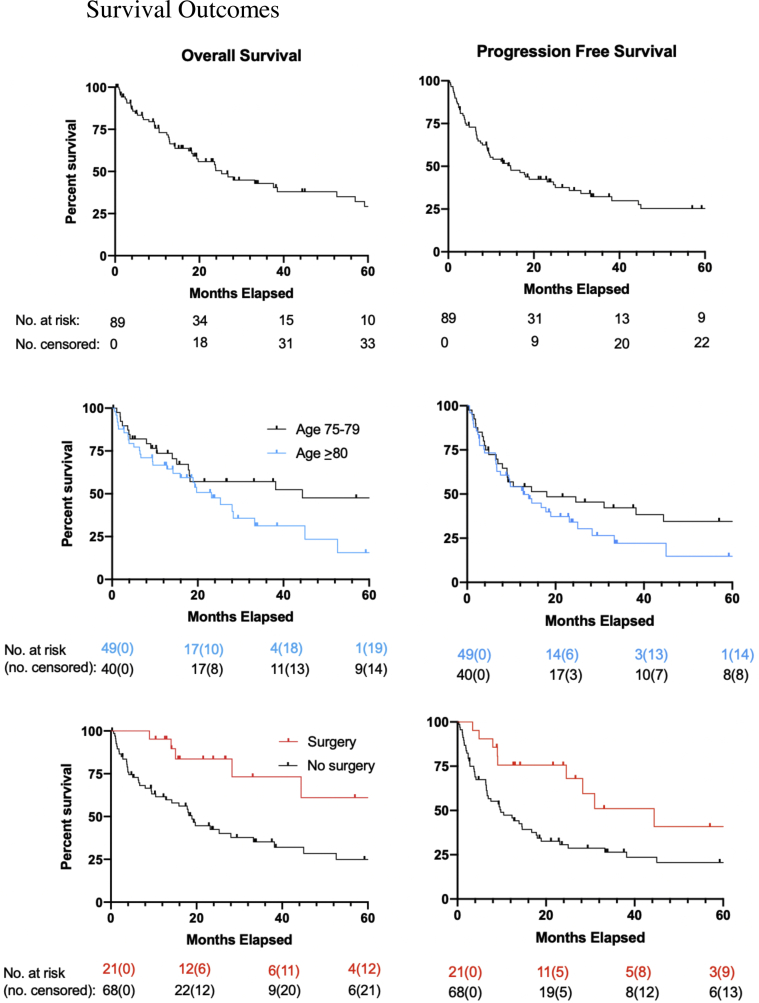
For the entire cohort, median follow-up was 16 months (interquartile range, 6-29 months). Median OS and PFS were 28 months (95% confidence interval [CI], 18-45 months) and 15 months (CI, 9.1-25 months), respectively (Fig 1). One- and 2-year OS were 70% and 52%, respectively. Outcomes were similar with the 3 postoperative radiation therapy patients and 7 oligometastatic patients excluded (73% and 54%, respectively). Of the 34 patients (38%) who developed progression, 16 (47%) had distant progression alone, 3 (9%) had synchronous distant and locoregional progression, and 15 (44%) had locoregional progression alone. Patients with IVB disease demonstrated worse median OS (3.1 months; 95% CI, 0.9-33 months vs 28 months; CI, 18-53 months, P = .0003) and PFS (2.4 months; 95% CI, 0.5-33 months vs 15 months; CI, 9-25 months, P = .005).
Figure 1.
Overall survival (left panel) and progression-free survival (right panel). Overall study population is shown in the top panel, stratification by age group is shown in the middle panel, and surgical management is shown in the bottom panel.
Those who underwent a radiation break (n = 18) appeared to have worse OS compared with those who did not, although the difference was not statistically significant (median, 8 months; 95% CI, 1.88 months [nonreportable upper limit] vs 28 months; CI, 19-62 months, P = .067). PFS was not statistically significant between the 2 groups (radiation therapy break median 6.5 months; 95% CI, 1.9 months [nonreportable upper limit] vs 17 months; CI, 9.4-25 months, P = .45). There was no difference in median OS or PFS for those who had a chemotherapy dose-reduction/break/discontinuation (OS: 33 months; 95% CI, 18-53 months vs 38 months; CI, 9.0-85 months, P = .58; median PFS: 25 months; 95% CI, 9.6-45 months vs 9 months; CI, 4.9-25 months, P = .11).
Given prior studies demonstrating worse outcomes in octogenarians, and given the median age of our cohort was 80, we compared outcomes between patients aged 75 to 79 and ≥80 years. Median survival between the groups was numerically but not statistically significantly different (OS: 44 months; 95% CI, 15-122 months vs 23 months; CI, 12-33 months, P = .17; PFS: 18 months; 95% CI, 8.0-63 months vs 13 months; CI, 6.7-23 months, P = .31).
Patients who underwent preoperative chemoradiotherapy and surgery demonstrated significantly better median OS (86 months; 95% CI, 28-133 months vs 19 months; CI, 10-33 months, P = .009) and PFS (44 months; 95% CI, 25-133 months vs 9.8 months; CI, 6.5-17 months, P = .024). One- and 2-year OS were 95% and 84%. Of the 7 patients (33%) who progressed after surgery, 4 (57%) had distant progression alone, 1 (14%) had synchronous distant and locoregional progression, and 2 (29%) had locoregional progression alone; all of these patients had residual pathologic disease (ypT2-4 ypN0-3).
Discussion
Since the publication of RTOG 85-01 and CROSS,1,2 standard of care for resectable locally advanced esophageal cancer has comprised preoperative chemoradiotherapy followed by surgery. However, the efficacy and tolerability of aggressive multimodality therapy in elderly patients are not clear. We report our experience with definitive radiation therapy for elderly patients, over half of whom were ≥80 years of age, and found survival comparable to historical outcomes.
A National Cancer Database analysis of over 4000 patients with locally advanced esophageal cancer treated with definitive chemoradiation showed 3-year OS of 24%; median age was 67 years (range, 22-90 years).16 Several randomized trials have demonstrated 2-year OS ~35% to 40% and 5-year OS ~20% for all-comers treated with definitive chemoradiotherapy,2,17, 18, 19 typically in cohorts with median age in the mid-60s. Although cross-retrospective comparisons have significant limitations, our older age cohort demonstrated 3-year OS of 44%, suggesting reasonable outcomes. The CROSS trial reported 3-year OS ~60% in patients who underwent trimodality treatment, with median OS ~50 months; median age of the CROSS cohort was 60 years. The surgical patients from our study were aged 75 to 85 and demonstrated 3-year OS of 75%, again within limitations of a cross-study comparison and short follow-up. Overall, our results demonstrate favorable survival outcomes in carefully selected elderly patients who undergo multimodality treatment, similar to other retrospective studies that have focused on elderly cohorts, typically aged 65 to 70 years and up,9, 10, 11,13 including a recent systematic review of 19 retrospective studies.10 In this review, despite favorable survival outcomes, a high rate of toxicities was noted with definitive bi/trimodality treatment, similar to our study. G3+ toxicities were 22% to 36% in the chemoradiotherapy group and 27% to 69% in the trimodality group. Our reported surgical complications were low, possibly owing to careful patient selection and optimal surgical techniques anticipated from a specialized high-volume center.
A recent Japanese retrospective study evaluated outcomes in elderly patients with a higher age threshold of ≥7520 and similarly found good oncologic outcomes, with 2-year OS of 53%; notably, patients older than 80 years demonstrated decreased tolerance for concurrent chemotherapy, were less likely to receive chemotherapy, and still demonstrated higher G3+ toxicities compared with the younger elderly patients. Other retrospective studies have similarly suggested decreased tolerance/survival benefit of chemotherapy in patients over 80,21 unlike younger elderly cohorts.22 In our study, we similarly saw a higher prevalence of chemotherapy dose reductions in this older age group, although there did not appear to be increased toxicity, possibly owing to planned rather than reactionary dose reductions. Notably, patients with metastatic disease demonstrated poor survival despite definitive treatment. Although conclusions are limited by the small sample size of this group (n = 7) and long study period (2000-2018), with changes in practice patterns and treatment techniques, these results highlight the importance of patient selection.
The majority of patients in our study received concurrent chemotherapy. Only 4 received definitive radiation therapy alone, either due to patient refusal or a medical contraindication, such as myelodysplasia. Almost one-quarter of patients underwent surgery, with good surgical outcomes; there was no 90-day postoperative mortality, and there were only 3 instances of nonfatal acute surgical complications. Notably, our overall and surgical study populations represent a favorable group of elderly patients, with minimal comorbidities. Although rates of life-threatening and fatal toxicities were low, a significant proportion of patients had grade 3 toxicity, radiation therapy/chemotherapy breaks, and ED visits. The majority of acute G3+ toxicities were attributed to chemotherapy-induced cytopenia and dysphagia (often difficult to ascertain from tumor- vs treatment-related); these were also primary reasons for treatment breaks. We were unable to find a correlation between age and toxicity apart from significantly lower late G3 toxicity among those ≥80 years of age. We suspect this may be due to increased dose reductions in this group versus limitations in follow-up documentation. These data emphasize a critical role of rigorous supportive care; for example, prophylactic intravenous hydration, nutrition consults, and more frequent on-treatment visits toward the end of treatment could reduce the number of ED visits and treatment breaks in this vulnerable population. Acute nonfatal toxicities were highest among those with low CCI, which may in part be due to more intense chemotherapy/fewer dose reductions; notably, there are limitations with CCI, as it includes a select group of comorbidities and does not necessarily correlate with frailty. As may be expected, our data showed higher nonfatal acute toxicity among platinum-fluoropyrimidine versus carboplatin-paclitaxel regimens, and lowest toxicity among fluoropyrimidine alone. Carboplatin-paclitaxel is considered more tolerable than platinum-fluoropyrimidine based on comparison of the CROSS trial with historical controls (ie, RTOG 8501 and Intergroup [INT] 0123) and retrospective studies.1,23, 24, 25 The ongoing PReoperative Chemoradiation for Resectable Esophageal and Junctional Cancer (PROTECT-1402) phase II trial will compare preoperative folinic acid, 5-FU, and oxaliplatin to carboplatin-paclitaxel.26 Single-agent chemotherapy typically is considered noncurative27; notably, 20% of our study population received fluoropyrimidine alone.
Further optimization of therapy can also reduce toxicity. The European CROSS trial used a preoperative radiation dose to 41.4 Gy in 23 fractions. Our institutional practice has been to use a preoperative dose of ≥45 Gy. Thus, we defined definitive dose as ≥45 Gy. In the nonsurgical setting, most practitioners consider ≥50 Gy as definitive per the negative dose-escalation study INT0123.28 In our study, there were 2 cases of definitive chemoradiotherapy with 45 Gy (both were squamous cell carcinomas, which is thought to be more radiosensitive with increased pathologic complete response, as reflected in the CROSS trial). All other cases were prescribed to ≥50 Gy. Although there is no convincing prospective data for dose-escalation >50 Gy, there were limitations/criticisms of INT0123. Thus, some practitioners still escalate to 60 to 70 Gy,29 particularly for adenocarcinoma, which is thought to be more radioresistant.1
Optimal radiation technique has also not been well evaluated, as there are no prospective trials comparing IMRT versus 3D conformal radiation therapy in esophageal cancer.30 The majority of our cohort were treated with IMRT after its introduction at our institution in the late 1990s. Most seminal trials, including CROSS, used 3D conformal radiotherapy. Given the conformality of IMRT, it theoretically could reduce toxicity to allow for dose escalation. With advances in the planning and delivery of conformal radiation, there has also been growing interest in hypofractionation (>2 Gy per daily fraction with lower cumulative dose).31 This approach may be beneficial for elderly patients given reduced treatment duration with potentially improved tolerability.
Study limitations are primarily due to the retrospective nature and associated inherent biases. It was single institutional, which may limit generalizability, and the study duration spanned over 2 decades, during which time treatment techniques have evolved/improved. There was inconsistent documentation regarding posttreatment PET/CTs and endoscopies, which limited our ability to report clinical response. Toxicity assessment may be incomplete, as we relied on radiation oncology on-treatment notes (which contain weekly toxicity assessments) for grading of acute toxicity during radiation therapy, whereas toxicity after radiation therapy was determined indirectly based on follow-up notes. Attributing toxicity to different treatment modalities retrospectively was challenging, and hence not attempted to avoid bias. Furthermore, the effect of treatment on patient-reported toxicity and quality of life are critical areas for research in future prospective studies, given known discrepancies between patient- and physician-reported toxicities.32 Retrospective assessment of chemotherapy doses and duration of feeding tubes may also be incomplete.
Conclusions
Definitive treatment for esophageal cancer should not be withheld for carefully selected elderly patients. However, given the high rate of G3+ toxicities and treatment breaks or dose-reductions, patient selection and proactive supportive care are critical. We were not able to find clear correlatives of who may be at highest risk for toxicity, and prospective studies are needed to better assess outcomes in elderly patients receiving definitive therapy and to optimize chemoradiotherapy regimens and surgical indications/techniques in this unique population.
Footnotes
Sources of support: No funding to report.
Disclosures: D.C. reports grants from Varian Medical Systems, and E.P. reports an honorarium from Accuray. Both are outside the scope of the submitted work.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Shapiro J., van Lanschot J.J.B., Hulshof M.C.C.M. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 2.Cooper J.S., Guo M.D., Herskovic A. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 3.Scher K.S., Hurria A. Under-representation of older adults in cancer registration trials: Known problem, little progress. J Clin Oncol. 2012;30:2036–2038. doi: 10.1200/JCO.2012.41.6727. [DOI] [PubMed] [Google Scholar]
- 4.Finlayson E.V., Birkmeyer J.D. Operative mortality with elective surgery in older adults. Eff Clin Pract. 2001;4:172–177. [PubMed] [Google Scholar]
- 5.Steyerberg E.W., Neville B.A., Koppert L.B. Surgical mortality in patients with esophageal cancer: Development and validation of a simple risk score. J Clin Oncol. 2006;24:4277–4284. doi: 10.1200/JCO.2005.05.0658. [DOI] [PubMed] [Google Scholar]
- 6.Bakhos C.T., Salami A.C., Kaiser L.R., Petrov R.V., Abbas A.E. Outcomes of octogenarians with esophageal cancer: An analysis of the National Cancer Database. Dis Esophagus. 2019;32:1–8. doi: 10.1093/dote/doy128. [DOI] [PubMed] [Google Scholar]
- 7.Steyerberg E.W., Neville B., Weeks J.C., Earle C.C. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: A population-based analysis of elderly patients. J Clin Oncol. 2007;25:2389–2396. doi: 10.1200/JCO.2006.09.7931. [DOI] [PubMed] [Google Scholar]
- 8.Cronin-Fenton D.P., Sharp L., Carsin A.-E., Comber H. Patterns of care and effects on mortality for cancers of the oesophagus and gastric cardia: A population-based study. Eur J Cancer. 2007;43:565–575. doi: 10.1016/j.ejca.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Semrau R., Herzog S.L., Vallbohmer D., Kocher M., Holscher A., Muller R.-P. Radiotherapy in elderly patients with inoperable esophageal cancer. Is there a benefit? Strahlenther Onkol. 2012;188:226–232. doi: 10.1007/s00066-011-0039-2. [DOI] [PubMed] [Google Scholar]
- 10.Skorus U.A., Kenig J. Outcome of esophageal cancer in the elderly - systematic review of the literature. Wideochir Inne Tech Maloinwazyjne. 2017;12:341–349. doi: 10.5114/wiitm.2017.72318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Won E., Ilson D.H. Management of localized esophageal cancer in the older patient. Oncologist. 2014;19:367–374. doi: 10.1634/theoncologist.2013-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moskovitz A.H., Rizk N.P., Venkatraman E. Mortality increases for octogenarians undergoing esophagogastrectomy for esophageal cancer. Ann Thorac Surg. 2006;82:2031–2036. doi: 10.1016/j.athoracsur.2006.06.053. discussion 2036. [DOI] [PubMed] [Google Scholar]
- 13.Walter F., Bockle D., Schmidt-Hegemann N.-S. Clinical outcome of elderly patients (≥ 70 years) with esophageal cancer undergoing definitive or neoadjuvant radio(chemo)therapy: A retrospective single center analysis. Radiat Oncol. 2018;13:93. doi: 10.1186/s13014-018-1044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin S.R., Wong Y.-N., Uzzo R.G., Beck J.R., Egleson B.L. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser score work. Med Care. 2015;53:e65–e72. doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu A.J., Bosch W.R., Chang D.T. Expert consensus contouring guidelines for intensity modulated radiation therapy in esophageal and gastroesophageal junction cancer. Int J Radiat Oncol Biol Phys. 2015;92:911–920. doi: 10.1016/j.ijrobp.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao M.S., Wong A.T., Schwartz D., Weiner J.P., Schreiber D. Definitive or preoperative chemoradiation therapy for esophageal cancer: Patterns of care and survival outcomes. Ann Thorac Surg. 2016;101:2148–2154. doi: 10.1016/j.athoracsur.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 17.Stahl M., Stuschke M., Lehmann N. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 18.Bedenne L., Michel P., Bouche O. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 19.Stahl M., Budach W. Definitive chemoradiotherapy. J Thorac Dis. 2017;9:S792–S798. doi: 10.21037/jtd.2017.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki G., Yamazaki H., Aibe N. Definitive radiotherapy for older patients aged ≥75 years with localized esophageal cancer. In Vivo. 2019;33:925–932. doi: 10.21873/invivo.11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y.C., Chen L.-L., Xu H.-B. Aging-related prognosis analysis of definitive radiotherapy for very elderly esophageal cancer. Cancer Med. 2018;7:1837–1844. doi: 10.1002/cam4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P., Xi M., Zhao L. Is there a benefit in receiving concurrent chemoradiotherapy for elderly patients with inoperable thoracic esophageal squamous cell carcinoma? PLoS One. 2014;9 doi: 10.1371/journal.pone.0105270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu X.M., Biagi J.J., Hopman W.M., Mahmud A. Shifting practice in definitive chemoradiation for localized esophageal cancer. Curr Oncol. 2017;24:e379–e387. doi: 10.3747/co.24.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honing J., Smit J.K., Muijs C.T. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol. 2014;25:638–643. doi: 10.1093/annonc/mdt589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blom R.L., Sosef M.N., Nap M. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Dis Esophagus. 2014;27:380–387. doi: 10.1111/dote.12110. [DOI] [PubMed] [Google Scholar]
- 26.Messager M., Mirabel X., Tresch E. Preoperative chemoradiation with paclitaxel-carboplatin or with fluorouracil-oxaliplatin-folinic acid (FOLFOX) for resectable esophageal and junctional cancer: The PROTECT-1402, randomized phase 2 trial. BMC Cancer. 2016;16:318. doi: 10.1186/s12885-016-2335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilson D.H. Esophageal cancer chemotherapy: Recent advances. Gastrointest Cancer Res. 2008;2:85–92. [PMC free article] [PubMed] [Google Scholar]
- 28.Minsky B.D., Pajak T.F., Ginsberg R.J. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Zhu H.-P., Wang T. What is the optimal radiation dose for non-operable esophageal cancer? Dissecting the evidence in a meta-analysis. Oncotarget. 2017;8:89095–89107. doi: 10.18632/oncotarget.18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu D., Li G., Li H., Jia F. Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer: A systematic review and meta-analysis. Med (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyu J., Liu T., Li T. Comparison of efficacy, safety, and costs between neoadjuvant hypofractionated radiotherapy and conventionally fractionated radiotherapy for esophageal carcinoma. Cancer Med. 2019;8:3710–3718. doi: 10.1002/cam4.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao C., Polomano R., Bruner D.W. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs. 2013;36:E1–E16. doi: 10.1097/NCC.0b013e318269040f. [DOI] [PubMed] [Google Scholar]