Abstract
Purpose
The Gamma Knife (GK) Icon allows for the delivery of stereotactic radiosurgery using a thermoplastic mask in combination with intrafraction motion monitoring using high definition motion management. The system pauses treatment if the magnitude of motion in all directions exceeds 1 to 1.5 mm, causing a break in treatment and prolongation of the session. We reviewed the records of patients treated in a frameless manner on our GK Icon system to determine predictors for treatment interruption.
Methods and Materials
We reviewed the records of patients treated between May 2019 and May 2020 on the GK Icon using a frameless technique for brain metastases, gliomas, schwannomas, and meningiomas. We recorded treatment time as noted in the plan document, actual treatment delivery time, and any pauses in treatment. We tabulated baseline characteristics including age, gender, diagnosis, performance status, and shifts at time of treatment. We used a receiver operating curve analysis to determine a timepoint corresponding with treatment interruption. We then conducted a logistic regression analysis to generate odds ratios for likelihood of treatment.
Results
We identified 150 patients meeting inclusion criteria. The majority (82%) were patients with brain metastases. The median age was 63 and the median dose was 27 Gy (16-30 Gy) in 3 fractions (1-5 fractions). The median treatment time was 23 minutes (4-108 minutes). Sixty-nine patients (46%) had at least 1 pause in treatment (range, 1-7). Receiver operating curve analysis revealed treatment time >19 minutes and rotation >0.47 degrees to be associated with interruption. Multivariable logistic regression revealed rotation >0.47 degrees and treatment time >19 minutes as predictive of interruption.
Conclusions
For patients with rotations exceeding 0.47 degrees or an extended treatment time, physicians should expect treatment interruptions, consider fractionation to lessen table time, or use a frame-based approach.
Introduction
The Gamma Knife (GK; Elekta, Stockholm, Sweden) has been a tool in the radiosurgeon’s armamentarium since the late 1980s in the United States.1 It offers noninvasive delivery of ablative doses of radiation for a variety of benign and malignant conditions. Traditionally, the GK has immobilized patients using a frame, which requires light sedation and local anesthesia for placement.2 Now, with its most recent iteration, the GK Icon has the capability of treating patients in a frameless manner using a thermoplastic mask.3,4 Coupled with the thermoplastic mask is high definition motion management (HDMM) through the use of an infrared (IR) camera and a reflective marker placed on the patient’s nose.5 If motion exceeds a user-set threshold (typically 1-1.5 mm, but up to 3 mm), the treatment will pause. If the patient does not return to a position in tolerance within 30 seconds, the treatment will stop, the patient is taken out of the machine, and he or she is given a short break. These interruptions can lead to prolonged treatments and disruption of workflow. With these concepts in mind, we sought to review the records of our patients treated framelessly on the GK Icon and determine predictors of interruptions in treatment.
Methods
Patients
We retrospectively reviewed the records of patients treated framelessly on the GK Icon at our institution between May 2019 and April 2020. Owing to the quality assessment or improvement nature of this project, it was exempt from institution review board approval. Patients with any diagnosis that were treated framelessly were eligible. We tabulated baseline characteristics including age, sex, diagnosis, and Karnofsky performance status (KPS). Treatment planning and delivery details are outlined herein.
Treatment planning
All patients were treated on the GK Icon and plans were completed using Gammaplan treatment planning software (version 11.1.1.). All patients (except when noted) had a 1-mm slice thickness, contrast enhanced, volumetric axial magnetic resonance imaging (MRI) scan obtained within 1 week of stereotactic radiosurgery (SRS) for target delineation. Two patients were unable to get an MRI owing to a pacemaker or defibrillator and had a thin slice (1 mm) head computed tomography (CT) completed with contrast in diagnostic radiology for planning purposes. At time of mask fabrication a custom headrest is made on the GK Icon unit by a radiation therapist. The mask is formed to the patient’s face while pressure is applied over the forehead and chin. The nose is allowed to protrude from the mask, which allows for placement of a reflective marker. The mask cools and forms for 15 minutes, after which a cone beam CT (CBCT) is completed with a CT dose index of 6.3 mGy. This CBCT is used as a reference for patient localization before treatment and is coregistered or fused with the planning MRI or CT scan. For patients being treated for a gross tumor (schwannoma, glioma, meningioma, metastasis), the planning target volume was the gross target volume with no margin. For postoperative metastasis cases, the planning target volume was the resection bed with a 1-mm margin. Dosing was picked based on diagnosis and tumor size or volume.6,7 Planning was completed by a physicist in collaboration with a neurosurgeon and radiation oncologist. Planning was typically a combination of inverse and forward planning with a goal of target coverage of 99% to 100%.
Treatment delivery and motion management
At time of treatment the patient was immobilized using the custom mask and a 2.5-mGy CBCT was obtained to check for any shifts or change in dose distribution. If coverage was maintained at 99% to 100%, treatment was initiated. HDMM was used throughout treatment delivery by tracking the marker on the patient’s nose. Motion tolerance was set at 1.5 mm for fractionated cases and 1.0 mm for single fraction cases based on our training experience and our physicists’ recommendations. As described earlier, if motion exceeded the set threshold for over 30 seconds, treatment was interrupted, and the patient was given a short rest before resuming radiation. We recorded dosing, fractionation, total volume of target(s), treatment time per the plan, actual treatment time for the longest fraction, presence of any interruption, and maximum number of interruptions per course of treatment. We also recorded the shifts of the first fraction, which include rotational and translational x, y, and z, all in degrees or mm, respectively.
Statistics
We tabulated baseline characteristics for all patients. We performed a receiver operating characteristic curve analysis to determine a treatment time, shift in degrees/mm, and treatment volume, which correlated with treatment interruption to make these data categorical. Those values were 19 minutes, 0.47 degrees, 1.02 mm, and 5.36 mL, respectively. We performed a multivariable logistic regression to identify predictors of treatment interruption which included age, sex, treatment volume, diagnosis, KPS, treatment time, and average rotation and shift.
Results
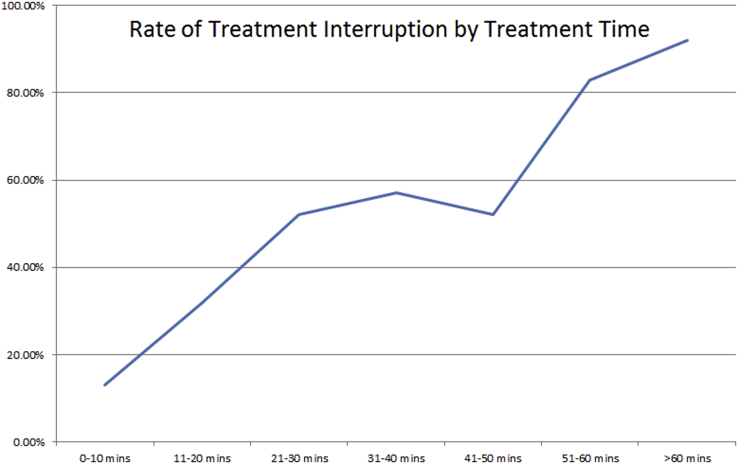
We identified 150 patients meeting eligibility. Most of the patients were female (55%) and the vast majority (82%) had brain metastases; other diagnoses included schwannoma, meningioma, and glioma. The median SRS dose was 27 Gy (16-30) in 3 (1-5) fractions. Full baseline characteristics are in Table 1. The median treatment time by plan was 23 minutes (range, 4-108). The median treatment time on day of treatment was 29 minutes (range, 4-106). Sixty-nine patients (46%) experienced at least one interruption. The median number of interruptions was 1 (range, 1-7). For patients with an interruption, the median increase in treatment time was 11 minutes (range, 2-110). By time, patients treated for less than ten minutes had a 13% interruption rate. When times exceeded 20 minutes, the rate of interruption was consistently above 50%, and as high as 92% for treatments exceeding 1 hour (Fig 1).
Table 1.
Patient characteristics (n = 150)
| Patient characteristic | n (% or range) |
|---|---|
| Sex | |
| Male | 67 (45) |
| Female | 83 (55) |
| Median age | 63 (30-87) |
| Diagnosis | |
| Metastases | 123 (82) |
| Meningioma | 10 (7) |
| Schwannoma | 7 (5) |
| Glioma | 10 (6) |
| KPS | 80 (60-100) |
| Dose (Gy) | 27 (16-30 Gy) |
| Isodose line (%) | 50 (46-90) |
| Volume (mL) | 2.83 (0.0048-57.757) |
| Fractions | 3 (1-5) |
| Treatment time by plan (min) | 23 (4-108) |
| Treatment time day of treatment (min) | 29 (4-106) |
| Treatment prolongation (min) | 3 (0-110) |
| No. of interruptions per fraction | 0 (0-7) |
| Average rotation/fx (degrees) | 0.63 (0.1-3.12) |
| Average shift/fx (mm) | 0.72 (0.08-3.18) |
Abbreviations: fx = fractions; KPS = Karnofsky performance status.
Figure 1.
Graph showing percent of patients with an interruption by time. If treatment time was less than 10 minutes, the rate was 13%. For any time over 20 minutes treatment, interruptions occurred more than 50% of the time and were as high as 92% for treatments exceeding 1 hour.
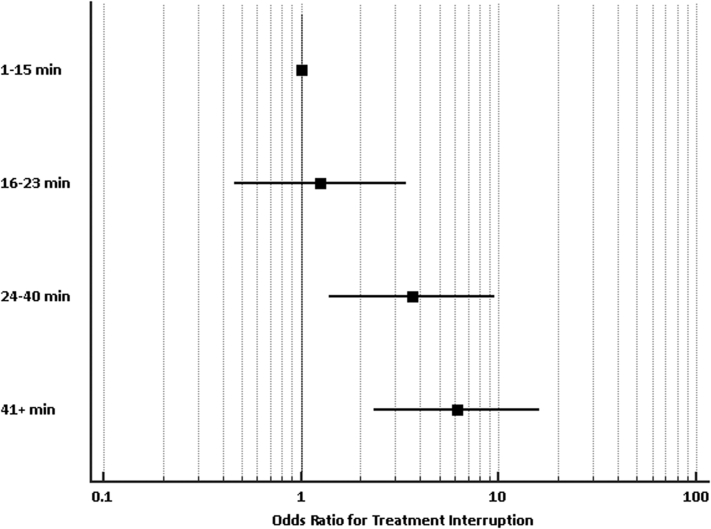
As described earlier, a receiver operating characteristic curve was generated to determine an a priori value associated with treatment interruption for treatment time, treatment volume, and average rotation or shift. These characteristics were entered into a logistic regression analysis along with age, sex, KPS, number of fractions, and diagnosis. Predictors of prolonged treatment time were average rotation >0.47 degrees (odds ratio [OR] 4.99; 95% confidence interval [CI], 1.97-12.67) and treatment greater than 19 minutes (OR 4.72; 95% CI, 1.75-12.71). Table 2 contains full list of odds ratios. Shift >1.02 mm, age, sex, KPS, fractions, HDMM threshold, and diagnosis did not predict for interruption. We also constructed a Forest plot using a univariate logistic regression to demonstrate odds ratios for interruption based on treatment time quartiles, with results further confirming increasing likelihood of interruption with longer times (Fig 2).
Table 2.
Odds ratios for likelihood of treatment interruption
| Characteristic | Odds ratio (95% CI) | P value |
|---|---|---|
| Age | ||
| ≤63 | Reference | |
| >63 | 1.25 (0.53-2.97) | .61 |
| Average shift, first fx | ||
| ≤1.02 mm | Reference | |
| >1.02 mm | 0.95 (0.38-2.36) | .91 |
| Average rotation, first fx | ||
| ≤0.47 degrees | Reference | |
| >0.47 degrees | 4.99 (1.97-12.67) | .0007 |
| KPS | ||
| KPS ≥70 | Reference | |
| KPS <70 | 3.66 (0.70-18.97) | .1227 |
| Sex | ||
| Male | Reference | |
| Female | 0.79 (0.37-1.68) | .5374 |
| Treatment time/fx | ||
| ≤19 min | Reference | |
| >19 min | 4.72 (1.75-12.71) | .0022 |
| Number of fractions | ||
| 1 | Reference | |
| 3 | 1.67 (0.50-5.62) | .66 |
| 5 | 0.69 (0.14-3.49) | .08 |
| Diagnosis | ||
| Metastases | Reference | |
| Meningioma | 0.93 (0.16-5.35) | .93 |
| Schwannoma | 0.07 (0.004-1.34) | .08 |
| Glioma | 3.56 (0.64-19.70) | .15 |
Abbreviations: CI = confidence interval; fx = fractions; KPS = Karnofsky performance status.
Bolding indicates statistical significance.
Figure 2.
Forest plot demonstrating odds ratios for interruption by treatment time as quartiles. Odds ratios for interruption for 16 to 23 minutes, 24 to 40 minutes, and ≥41 minutes were 1.25 (95% confidence interval, 0.46-3.38), 3.64 (95% confidence interval, 1.39-9.52), and 6.14 (2.33-16.18), respectively.
Discussion
The results presented here are one of the largest and among the first focusing specifically on predictors of interruption during frameless GK Icon cases. Not surprisingly, treatment time was one of the main predictors of interruption, as was average rotation at first fraction. Age, performance status, and diagnosis did not predict for interruption in this series, although most patients were in a similar performance status range and the vast majority were patients with metastases. These findings are clinically important because interruptions in treatment can lead to disruption in scheduling and workflow, in addition to prolonged treatment times. In addition, our results can perhaps help guide decision making as it relates to fractionation or utilization of a frame-based approach. Practitioners can also use these results to anticipate an interruption and consider scheduling a break for the patient to help maintain workflow and patient comfort. Another option could be to implement time remaining updates and encouragement to the patient throughout treatment, which may ease anxiety and help avoid interruption. We hope that these results can help guide patient selection and patient comfort, which are important for preserving outcomes.
The ability to treat patients framelessly on the GK Icon has increased eligibility for patients who may benefit from fractionation or are opposed to frame placement. An editorial from 2019 mentioned treatment time and non-anxious patients as key factors in deciding on frameless treatment.3 The group from Columbia presented their experience treating 100 patients framelessly on the GK Icon.5 Fifty-one percent of patients were treated in a single fraction and 42% of the patients had brain metastases. Thirty-one patients had more than one CBCT during treatment, which the authors reasonably used as a surrogate for a treatment interruption. Given the nature of their report, they did discuss the predictors of interruption. Regardless of the utility of the mask and its use in GK SRS, the traditional frame-based approach still has an integral role in SRS. It is important to note, that even with the frame, some motion or, worse, frame slippage is possible. One series in the literature reports on the use of CBCT to verify frame placement.8 The authors noted frame motion or slippage in 3 cases caught on CBCT, which thankfully allowed for adjustments before treatment.
The concept of intrafraction motion during frameless treatment has been studied in the past few years. A series from Carminucci et al compared GK treatment in 77 patients, of which 17% were treated using a mask.2 Patients had pre- and posttreatment CBCTs, and translational and rotational setup errors (ie, shifts) were recorded. Both groups had very small shifts, <1.0 mm, across the board. However, patients in the mask group had higher degree of shifts both rotationally and translationally. Of note, treatment times in that study were a bit longer in comparison, with an average length of 55 minutes. The number of treatment stoppages correlated with treatment time, but did not reach statistical significance, likely owing to small sample size. A large series from Sunnybrook examined motion during treatment across an astounding 1446 fractions, reporting a slightly lower rate of interruption of 29%.9 Not surprisingly, the motion relative to the CBCT increased with treatment time.
The group from the Mayo Clinic in Jacksonville recently published a similar series examining intrafraction displacement (ie, motion) in relation to the CBCT for 38 patients.10 It should be noted, the practice at that facility is to also use a bite block for immobilization in the vast majority of frameless cases, perhaps leading to better immobilization. Median treatment time was identical to ours at 23 minutes. The median displacement during treatment was 0.6 mm, with a maximum of 1.22 mm. Predictors for larger displacement in that study were male, with worse performance status, and malignant tumor. As part of the study, the authors also were able to document and record anxiolytic use and showed that it resulted in a significant reduction in displacement. Unfortunately, we did not have access to documentation of anxiolytic use in our cohort, so we were unable to include that in our analysis. One consideration could be use of anxiolytics for patients with treatment times extending beyond 19 minutes based on our data and the data from Mayo.
The limitations of this study include its retrospective nature and thus an inherent selection bias. Patients who physicians thought may be unable to tolerate a mask, or patients who refused treatment on the GK and would be more likely to have interruptions were not included and could have influenced the results. The majority of our patients had brain metastases, and thus extrapolation of the results to benign conditions may not be entirely reasonable. We were also unable to capture data as it related to medication use (steroids, anxiolytics) around time of GK SRS, body mass index, and other factors which may influence treatment times, intrafraction motion, and interruptions. Our center also used a rather strict or conservative motion threshold of 1 to 1.5 mm based on our training experience, newness to the technique, and physicists’ recommendation. Centers do have the option of allowing up to 3 mm of motion, which could reduce interruptions, but at least theoretically allow for geographic miss. It must also be mentioned that during the course of this study, our GK Icon was new with fresh cobalt sources leading to quicker treatment times. As such, centers with older units may be more prone to interruptions depending on the activity of their sources.
Conclusions
Prolonged overall treatment time (>19 minutes) may predict a higher rate of treatment interruption during frameless GK Icon SRS. Consideration of frame-based techniques, fractionation, or the expectation of a break are advised in those circumstances.
Footnotes
Sources of support: none.
Disclosures: none.
Patient level data from this study is not available for sharing.
References
- 1.Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316–319. [PubMed] [Google Scholar]
- 2.Carminucci A., Nie K., Weiner J., Hargreaves E., Danish S.F. Assessment of motion error for frame-based and noninvasive mask-based fixation using the Leksell Gamma Knife Icon radiosurgery system. J Neurosurg. 2018;129:133–139. doi: 10.3171/2018.7.GKS181516. [DOI] [PubMed] [Google Scholar]
- 3.Lunsford L.D., Niranjan A., Fallon K., Kim J.O. Frame versus frameless Leksell stereotactic radiosurgery. Prog Neurol Surg. 2019;34:19–27. doi: 10.1159/000493046. [DOI] [PubMed] [Google Scholar]
- 4.Li W., Cho Y.B., Ansell S. The use of cone beam computed tomography for image guided Gamma Knife stereotactic radiosurgery: Initial clinical evaluation. Int J Radiat Oncol Biol Phys. 2016;96:214–220. doi: 10.1016/j.ijrobp.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Vulpe H., Save A.V., Xu Y. Frameless stereotactic radiosurgery on the Gamma Knife icon: Early experience from 100 patients. Neurosurgery. 2020;86:509–516. doi: 10.1093/neuros/nyz227. [DOI] [PubMed] [Google Scholar]
- 6.Colaco R.J., Yu J.B., Bond J.S. A contemporary dose selection algorithm for stereotactic radiosurgery in the treatment of brain metastases: An initial report. J Radiosurg SBRT. 2016;4:43–52. [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw E., Scott C., Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 8.Peach M.S., Trifiletti D.M., Dutta S.W., Larner J.M., Schlesinger D.J., Sheehan J.P. Spatial shifts in frame-based Gamma Knife radiosurgery: A case for cone beam CT imaging as quality assurance using the Gamma Knife(R) Icon. J Radiosurg SBRT. 2018;5:315–322. [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald R.L., Lee Y., Schasfoort J., Soliman H., Sahgal A., Ruschin M. Real-time infrared motion tracking analysis for patients treated with gated frameless image guided stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2020;106:413–421. doi: 10.1016/j.ijrobp.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Seneviratne D.S., Vallow L.A., Hadley A. Intracranial motion during frameless Gamma-Knife stereotactic radiosurgery. J Radiosurg SBRT. 2020;6:277–285. [PMC free article] [PubMed] [Google Scholar]