Abstract
Purpose
Our purpose was to study the effect of 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) positron emission tomography (PET)-computed tomography (CT) on staging/treatment recommendations of previously untreated prostate cancer. We report here results of a prospective single center single arm imaging trial within Veterans Affairs (Greater Los Angeles): the frequency of patients upstaged to M1 disease (primary endpoint) and the frequency of patients with change in treatment recommendations (secondary endpoint). This is the first report of prostate-specific membrane antigen PET-CT exclusive to U.S. veterans.
Methods and Materials
Veterans with Gleason ≥4 + 3, clinical stage ≥T2c, or prostate-specific antigen >10 ng/mL were eligible. Patients underwent conventional imaging (99mTc-methyl diphosphonate bone scan or 18F-NaF PET-CT; and pelvic CT or pelvic magnetic resonance imaging) in addition to 18F-DCFPyL PET-CT. The effect of 18F-DCFPyL PET-CT on treatment change was determined by applying prespecified treatment recommendations based on National Comprehensive Cancer Network guidelines and modern clinical practice.
Results
One hundred patients underwent 18F-DCFPyL PET-CT. Nineteen out of 84 (23%) patients initially thought to be nonmetastatic were upstaged to M1; 8/16 (50%) patients initially thought to have M1 disease were downstaged to M0. In total, 39/100 (39%) had a change in prespecified treatment recommendations, including change of radiation therapy volume/dose in 39/100 (39%) and starting abiraterone in 22/100 (22%).
Conclusions
Incorporation of 18F-DCFPyL PET-CT into the initial conventional imaging workup for prostate cancer can substantially affect staging/treatment recommendations.
Introduction
Treatment recommendations for patients with newly diagnosed prostate cancer have evolved considerably. Docetaxel, abiraterone acetate, apalutamide, and enzalutamide each individually improve survival in patients with castration-sensitive metastatic prostate cancer when added to long-term androgen suppression.1, 2, 3, 4, 5 Radiation therapy (RT) directed to the prostate improves survival in patients with low-volume castration-sensitive metastatic prostate cancer.6 RT and abiraterone acetate improve progression-free survival in N1 CSPC.3,7 Metastasis directed therapy (MDT), commonly delivered as stereotactic body RT, offers excellent local control of prostate cancer metastases with generally low toxicity.8 Phase II trials suggest a survival benefit to MDT9—a hypothesis currently being tested—but even absent phase III data, MDT is often offered to patients with oligometastatic disease.10 Given these significant developments in the treatment landscape of de novo prostate cancer, the need for accurate upfront staging is absolutely critical.
Prostate-specific membrane antigen (PSMA) ligands offer a strategy to develop prostate-specific positron emission tomography (PET) tracers, greatly improving accuracy of systemic prostate cancer imaging.11 68Ga-PSMA-11 has been the most studied, both in de novo12,13 and recurrent settings.13,14 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) is another PSMA PET tracer with published data largely in the recurrent setting.15, 16, 17 There are currently little published data on the effect of using 18F-DCFPyL tracer in PET-computed tomography (CT) in patients with de novo prostate cancer.
A 2018 study investigated the effect of 18F-DCFPyL PET-CT in 25 patients with high-risk or very high-risk prostate cancer preparing to undergo radical prostatectomy with pelvic lymphadenectomy.18 18F-DCFPyL PET-CT was found to have 71.4% sensitivity and positive predictive value (PPV), as well as 88.9% specificity and negative predictive value in detection of N1 disease. Three men (12%) had evidence of M1a disease. Preliminary results of the multicenter trial (NCT02981368) were reported in 93 patients (“Cohort B”) who were found to have radiologic evidence of recurrent or metastatic prostate cancer who subsequently underwent biopsy. Among the 3 central, blinded, and independent readers evaluating extrapelvic lesions on 18F-DCFPyL PET-CT scans, sensitivity ranged from 90.9% to 98.2% (lower bound of 95% confidence interval, 80.0%-89.0%), and PPV ranged from 83.1% to 86.2% (lower bound of 95% confidence interval, 74.0%-77.0%).19
This single-arm phase II clinical trial aimed to study the effect of adding 18F-DCFPyL PET-CT to conventional imaging on staging/management of veterans with de novo prostate cancer. The primary clinical endpoint was the rate of patients with prostate cancer identified to have M1 disease by 18F-DCFPyL PET-CT. The secondary endpoint was the rate of patients with changes in treatment recommendation. We report how incorporation of 18F-DCFPyL PET-CT affects treatment recommendations of radiation and systemic therapy.
Methods and Materials
This prospective single center, open label, single arm phase II imaging study was approved by the local Veterans Affairs hospital institutional review board (PCC 2018-100989), registered to clinicaltrials.gov (NCT03852654), and relied upon an investigational new drug application for 18F-DCFPyL (IND #IND #136007).
Eligibility criteria for trial enrollment included histologically confirmed prostate adenocarcinoma with prostate-specific antigen >10 ng/mL, Gleason ≥4 + 3, or clinical stage ≥T2c. All patients also underwent routine staging with conventional imaging: 99mTc-methyl diphosphonate bone scan or 18F-NaF PET-CT, and CT or magnetic resonance imaging of the pelvis. Exclusion criteria included prior local therapy for prostate cancer (ie, prostatectomy, RT, etc). Although previous use of androgen deprivation therapy (ADT) or antiandrogen was allowed if the patient had been off therapy for 3 months or more, no such patients actually ended up enrolling.
The 18F-DCFPyL PET-CT was evaluated by a board-certified nuclear medicine physician during clinical readout, at which time access to all medical information (including prior clinical imaging) was made available. On conventional imaging, pathologically enlarged lymph nodes were defined as >1 cm short axis diameter; on 18F-DCFPyL PET-CT, positivity was determined using PSMA Reporting and Data System criteria. Prespecified treatment recommendations (Table 1) were formulated based on National Comprehensive Cancer Network criteria and applied to patients before and after 18F-DCFPyL PET-CT staging based on the results of the imaging and clinical-pathologic features without regard for comorbidity or patient or physician preference. Low and high burden metastatic disease were defined by ChemoHormonal therapy versus Androgen Ablation Randomized Trial for Extensive Disease (CHAARTED) criteria.5 To simplify the analysis, RT was selected as the primary tumor treatment modality. MDT was recommended for low burden metastatic disease with up to 3 metastases.
Table 1.
Predefined treatment recommendations based on post-18F-DCFPyL PET-CT staging
| Risk group | Treatment recommendation (radiation target volumes) | Treatment recommendation (systemic therapy) |
|---|---|---|
| Unfavorable intermediate | RT (prostate and SVs) | Short course ADT (4-6 months) |
| High | RT (prostate, SVs, pelvic lymph nodes) | Long-course ADT (18-24 months) |
| Node-positive | RT (prostate, SVs, pelvic lymph nodes including boost) | 24 months ADT + abiraterone |
| M1 (“low burden” by CHAARTED criteria) | RT to prostate; metastasis-directed therapy up to 3 metastases | Indefinite ADT + abiraterone |
| M1 (“high burden” by CHAARTED criteria) | None | Indefinite ADT + abiraterone |
Abbreviations: ADT = androgen deprivation therapy; 18F-DCFPyL = 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid; M1 = metastatic; PET-CT = positron emission tomography–computed tomography; RT = radiation therapy; SV = seminal vesicle.
Risk group definitions:
-
-2 or 3 intermediate risk factors (IRFs): T2b-T2c, grade group 2 or 3, prostate-specific antigen (PSA) 10 to 20 ng/mL
-
-Grade group 3
-
-≥50% biopsy cores positive
-
-T3a or higher
-
-Grade group 4 or 5
-
-PSA >20 ng/mL
Node-positive21: pelvic, obturator, internal iliac (hypogastric), external iliac, and sacral (lateral, presacral, or promontory) lymph nodes [Note: common iliac lymph nodes are excluded from this group and considered to constitute M1a disease]
M1 (low burden)5: patient with M1 disease that does not qualify as “high burden” disease (as below)
M1 (high burden)5: presence of visceral metastases or ≥ 4 bone lesions with ≥ 1 beyond the vertebral bodies and pelvis
Results
One hundred patients have enrolled since July 2018 (total accrual will be n = 170). Baseline characteristics of patients are found in Table 2.
Table 2.
Baseline characteristics of enrolled patients
| Variable | Number (%) |
|---|---|
| Age | |
| Median | 70.4 |
| Mean | 69.4 |
| Range | 49.6-86.4 |
| Race | |
| White | 47 (47%) |
| Hispanic | 8 (8%) |
| Black | 41 (41%) |
| Asian | 2 (2%) |
| Unknown | 1 (1%) |
| PSA | |
| Median | 13.3 |
| Mean | 19.0 |
| Range | 0.61-167.92 |
| Initial imaging | |
| CT abdomen/pelvis | 62 (62%) |
| MRI | 77 (77%) |
| 99mTc-MDP bone scan | 29 (29%) |
| 18F-NaF PET-CT | 74 (74%) |
| CT chest | 7 (7%) |
| Initial Gleason score | |
| 3 + 3 | 8 (8%) |
| 3 + 4 | 26 (26%) |
| 4 + 3 | 25 (25%) |
| 4 + 4 | 21 (21%) |
| 9-10 | 19 (19%) |
Abbreviations: CT = computed tomography; MDP = methyl diphosphonate; MRI = magnetic resonance imaging; PET = positron emission tomography; PSA = prostate-specific antigen.
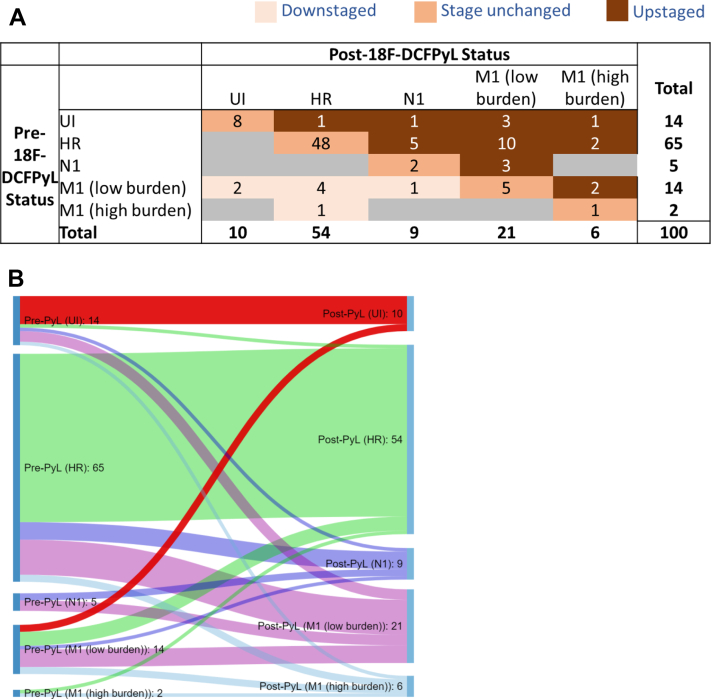
18F-DCFPyL PET-CT identified M1 disease in 27 out of 100 patients. Complete change in staging after 18F-DCFPyL PET-CT is shown in Figure 1. Of 100 patients, 28 were upstaged, 8 downstaged, and 64 unchanged. Example of upstaging is shown in Figure 2. Of patients with unfavorable-intermediate disease, 6 out of 14 (43%) were upstaged including 4 (29%) with M1 disease. In high-risk patients, 17 out of 65 (26%) were upstaged including 12 (18%) with M1 disease. In N1 patients, 3 out of 5 (60%) were upstaged to M1 disease. Of the 19 patients upstaged from M0 to M1, 16 were upstaged to low burden M1 disease, and of these patients, 6/16 were found to have distant nodal disease only. Of 14 patients initially thought to have low burden M1 disease, 7 (50%) were downstaged to M0 by 18F-DCFPyL PET-CT. Metastatic disease was found on 18F-DCFPyL PET-CT in 24%, 24%, 29%, and 50% of patients with prostate-specific antigen <10 ng/mL (n = 33), 10 to 20 ng/mL (n = 37), 20 to 40 ng/mL (n = 21), and >40 ng/mL (n = 8), respectively. Metastatic disease was found on 18F-DCFPyL PET-CT in 0%, 31%, 24%, 43%, and 21% of patients with Gleason grade group 1 (n = 8), group 2 (n = 26), group 3 (n = 25), group 4 (n = 21), and group 5 (n = 19), respectively.
Figure 1.
(A) Change in staging before and after 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid positron emission tomography–computed tomography scan, reflected in 2-dimensional matrix. Abbreviations: HR = high-risk; M1 = metastatic; N1 = pelvic node positive; UI = unfavorable intermediate risk. (B) Change in staging before and after 18F-DCFPyL PET-CT scan, reflected in Sankey diagram.
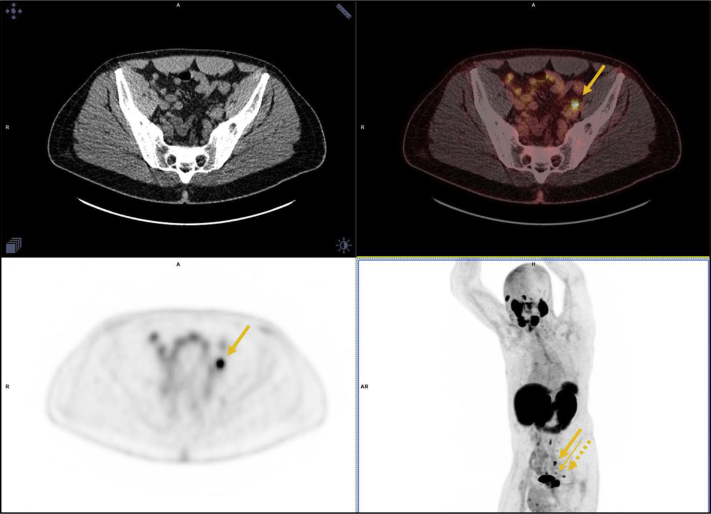
Figure 2.
A 65-year old man with iPSA = 78.52, bG3 + 4, and iT3aN0 on conventional imaging was found to have metastatic (M1a) disease on 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid positron emission tomography–computed tomography scan. Left common iliac node (thick arrow), left external iliac node (thin arrow), and inferior presacral node (dashed arrow). Abbreviation: iPSA = intact PSA.
Overall, 39 out of 100 patients had a change in prespecified treatment recommendation if 18F-DCFPyL PET-CT was incorporated. These include a recommendation to start abiraterone in 22 out of 100 patients and to increase duration in 3 out of 100 patients who already would have been recommended abiraterone. ADT duration would be increased in 26 out of 100 patients. Abiraterone would be avoided in 7 out of 100 patients and decreased in duration in 1 patient, and duration of ADT decreased in 8 out of 100 patients. Increased RT dose to pelvic nodes would be prescribed to 13 out of 100 patients owing to the identification of gross disease, MDT offered to 15 out of 100 patients, and modified MDT targets in 3 out of 100 patients. MDT and RT to the primary would have been avoided in 8 out of 100 and 5 out of 100 patients, respectively.
Discussion
Location of detected disease by 18F-DCFPyL PET-CT differed from conventional imaging in 39% of patients. If incorporated into routine treatment recommendations, this results in a substantial change in both tumor-directed and systemic disease management.
Notably, this is the first trial of PSMA PET-CT exclusive to U.S. veterans. Prostate cancer comprises almost a third of all cancers among U.S. veterans and the unique environmental exposure history in this population may affect both the incidence and severity of disease.22,23
For localized prostate cancer, RT and surgery are both curative options. To simplify analysis, the predefined recommendations assumed primary RT was selected. However, a similar analysis could be done based on an initial surgical strategy. For added simplicity, escalated systemic therapy was limited to abiraterone. The predefined treatment recommendations enabled an unbiased assessment of management change resulting exclusively from 18F-DCFPyL PET-CT. However, this does not necessarily reflect the treatments actually received (ie, primary surgical approach selected, effect of comorbid disease, enrollment in a therapeutic clinical trial, patient or physician preference, etc). Two limitations of this study include the lack of biopsy-confirmed tissue diagnosis to corroborate findings found on 18F-DCFPyL PET-CT, and the lack of a second reviewer confirming reads established by the nuclear medicine physician.
This study raises several questions. Does integration of 18F-DCFPyL PET-CT into treatment decision making improve long-term outcomes, and what is its comparative efficacy compared with other prostate-specific tracers? Further, what is the optimal utilization of 18F-DCFPyL PET-CT in de novo prostate cancer (eg, unfavorable intermediate-risk and above or a different subset)?
Finally, most current treatment paradigms rely on outcomes from trials that used conventional imaging. It remains to be seen if these paradigms will shift, or remain, when PSMA PET-CT is widely available. Notably, the high specificity and PPV of PSMA PET-CT makes findings difficult to ignore.
Conclusions
For veterans with de novo prostate cancer, adding 18F-DCFPyL PET-CT to conventional imaging substantially affects staging. In this study, 19/84 (23%) patients initially thought to have M0 disease were upstaged to M1 disease by 18F-DCFPyL PET-CT, whereas 8/16 (50%) patients initially thought to have M1 disease were downstaged to M0 disease. If incorporated into standard treatment recommendations, 18F-DCFPyL PET-CT could result in modification of treatment in at least 1 in every 3 veterans.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Kishan reports other from Varian Medical Systems, Inc, grants and other from ViewRay, Inc, other from Intelligent Automation, Inc, and other from Janssen outside the submitted work. Dr Rettig reports grants from Progenics, during the conduct of the study, personal fees from Bayer, personal fees from Janssen, personal fees from Ambrx, personal fees from Amgen, other from Constellation, nonfinancial support from Astellas, nonfinancial support from Pfizer, and nonfinancial support from Merck outside the submitted work. In addition, Dr Rettig has a patent, “Inhibitors of the AR N-terminal domain,” pending. Dr Nickols reports grants from Progenics during the conduct of the study, grants from Janssen, personal fees from Vividion, and other from Bayer outside the submitted work.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Davis I.D., Martin A.J., Stockler M.R. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 2.Chi K.N., Agarwal N., Bjartell A. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 3.James N.D., de Bono J.S., Spears M.R. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fizazi K., Tran N., Fein L. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney C.J., Chen Y.H., Carducci M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker C.C., James N.D., Brawley C.D. Radiation therapy to the primary tumor for newly diagnosed, metastatic prostate cancer (stampede): A randomized controlled phase 3 trial. Lancet. 2018;392:2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James N.D., Spears M.R., Clarke N.W. Failure-free survival and radiation therapy in patients with newly diagnosed nonmetastatic prostate cancer: Data from patients in the control arm of the stampede trial. JAMA Oncol. 2016;2:348–357. doi: 10.1001/jamaoncol.2015.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ost P., Reynders D., Decaestecker K. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase ii trial. J Clin Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 9.Palma D.A., Olson R., Harrow S. Stereotactic ablative radiation therapy versus standard of care palliative treatment in patients with oligometastatic cancers (sabr-comet): A randomized, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 10.Zilli T., Ost P. Metastasis-directed therapy: A new standard for oligorecurrent prostate cancer? Oncotarget. 2018;9:34196–34197. doi: 10.18632/oncotarget.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calais J., Cao M., Nickols N.G. The utility of pet/ct in the planning of external radiation therapy for prostate cancer. J Nucl Med. 2018;59:557–567. doi: 10.2967/jnumed.117.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herlemann A., Wenter V., Kretschmer A. (68)Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions before lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70:553–557. doi: 10.1016/j.eururo.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 13.Roach P.J., Francis R., Emmett L. The impact of (68)Ga-PSMA PET/CT on management intent in prostate cancer: Results of an australian prospective multicenter study. J Nucl Med. 2018;59:82–88. doi: 10.2967/jnumed.117.197160. [DOI] [PubMed] [Google Scholar]
- 14.Morigi J.J., Stricker P.D., van Leeuwen P.J. Prospective comparison of 18F-fluoromethylcholine versus (68)Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 15.Rowe S.P., Campbell S.P., Mana-Ay M. Prospective evaluation of PSMA-targeted 18F-DCFPyL PET-CT in men with biochemical failure after radical prostatectomy for prostate cancer. J Nucl Med. 2020;61:58–61. doi: 10.2967/jnumed.119.226514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song H., Harrison C., Duan H. Prospective evaluation in an academic center of 18F-DCFPyL PET-CT in biochemically recurrent prostate cancer: A focus on localizing disease and changes in management. J Nucl Med. 2020;61:546–551. doi: 10.2967/jnumed.119.231654. [DOI] [PubMed] [Google Scholar]
- 17.Liu W., Zukotynski K., Emmett L. A prospective study of 18F-DCFPyL PSMA PET-CT restaging in recurrent prostate cancer following primary external beam radiation therapy or brachytherapy. Int J Radiat Oncol Biol Phys. 2020;106:546–555. doi: 10.1016/j.ijrobp.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Gorin M.A., Rowe S.P., Patel H.D. Prostate specific membrane antigen targeted 18F-DCFPyL positron emission tomography/computerized tomography for the preoperative staging of high-risk prostate cancer: Results of a prospective, phase II, single center study. J Urol. 2018;199:126–132. doi: 10.1016/j.juro.2017.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe S., Gorin M., Pienta K. Results from the osprey trial: A prospective phase 2/3 multicenter study of 18f-dcfpyl pet/ct imaging in patients with prostate cancer - examination of diagnostic accuracy. J Nucl Med. 2019;60:586. [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer (Version 2.2020). 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed October 3, 2020.
- 21.Buyyounouski M.K., Choyke P.L., McKenney J.K. Prostate cancer - major changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:245–253. doi: 10.3322/caac.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamie K., DeVere White R.W., Lee D. Agent orange exposure, Vietnam war veterans, and the risk of prostate cancer. Cancer. 2008;113:2464–2470. doi: 10.1002/cncr.23695. [DOI] [PubMed] [Google Scholar]
- 23.Zullig L.L., Sims K.J., McNeil R. Cancer incidence among patients of the U.S. Veterans affairs health care system: 2010 update. Mil Med. 2017;182:e1883–e1891. doi: 10.7205/MILMED-D-16-00371. [DOI] [PMC free article] [PubMed] [Google Scholar]