Abstract
Purpose
Pulmonary metastases are common in many pediatric solid tumors; however, little is known about safety and efficacy of lung stereotactic body radiation therapy (SBRT) for pediatric patients. We conducted a phase I/II study to investigate the minimum effective dose level of SBRT with an acceptable safety profile in pediatric patients.
Methods and Materials
Patients with sarcoma and metastatic pulmonary lesions ≤3 cm in diameter and ≤21 years of age were enrolled. Dose levels 1, 2, and 3 were 24, 30, and 36 Gy in 3 fractions, respectively. Enrolled patients with metastases from primary renal tumors and sarcoma histologies were to begin at dose level 1 and 2, respectively. Exclusion criteria included receipt of whole-lung/hemi-thorax irradiation >12 Gy within 6 months of consent. Primary endpoints were tolerability and safety per Common Terminology Criteria for Adverse Events grading and disease response at 6 weeks post-SBRT per response evaluation criteria in solid tumors (RECIST) 1.1 criteria. Secondary endpoints included rates of local control and distant failure within the lung, but outside of the treatment volume.
Results
Five patients with median age of 13 years (range, 7-21) received SBRT at dose level 2. Primary tumor histologies included Ewing sarcoma (n = 3), anaplastic chordoma (n = 1), and osteosarcoma (n = 1). No grade ≥3 adverse events were observed. At 6 weeks after SBRT, 7/8 (87.5%) lesions achieved partial response. With median follow-up of 2.1 years (range, 1.4-2.5), 2-year local control and distant failure-free survival were 60% (n = 8) and 40% (n = 5), respectively. One patient developed widespread metastases and succumbed to disease 1.4 years after SBRT.
Conclusions
SBRT for pulmonary metastases produces responses in pediatric patients with sarcoma at 6 weeks with acceptable toxicity; however, patients remain at risk of local and distant failure within the lung. Future prospective studies are needed to investigate whether higher doses of SBRT, possibly in combination with other therapies, are safe and provide more durable response.
Introduction
Pulmonary metastases are common in many pediatric solid tumors, including bone and soft-tissue sarcomas and Wilms tumor.1 Whole lung irradiation is frequently used to treat pulmonary metastases in Wilms tumor and Ewing sarcoma, and many studies describe long-term outcomes after pulmonary metastasectomy in pediatric patients with sarcoma and Wilms tumor.1, 2, 3, 4, 5, 6 Stereotactic body radiation therapy (SBRT) delivers highly conformal ablative doses of radiation in ≤5 fractions, and can produce excellent local control (LC) for metastatic lung lesions in adult patients.7,8 Within the pediatric population, few retrospective case reports and series have described SBRT for pulmonary metastases.9, 10, 11, 12 We aimed to prospectively investigate the lowest dose of SBRT with an acceptable safety profile and efficacy in pediatric patients with metastatic pulmonary lesions.
Methods and Materials
The study (NCTG1600058) was approved by the institutional review board with an investigational device exemption from the U.S. Food and Drug Administration. Patients ≤21 years of age with a diagnosis of sarcoma or primary renal tumor and pulmonary metastases found at time of relapse were enrolled from 2017 to 2018. Other inclusion and exclusion criteria are included in Table 1. Patients who required sedation for planning and treatment were excluded because these patients may have been too young to cooperate adequately when performing pulmonary function tests, which limits the ability to assess early toxicity. Patients could receive concurrent immunotherapy. Patients were stratified by primary histology: renal tumor (stratum I) and sarcoma (stratum II).
Table 1.
Inclusion and exclusion criteria for the study
| Inclusion criteria |
|
| Exclusion criteria |
|
Abbreviations: FEV1 = forced expiratory volume; WLI = whole lung irradiation.
Patients underwent simulation in a standard stereotactic immobilization device with 4-dimensional computed tomography (CT) to account for respiratory motion. A free-breathing CT scan was also performed. Abdominal compression was used if the tumor moved >1 cm with respiratory motion. Internal target volume was defined as gross tumor plus margin for internal motion. Planning target volume was defined as internal target volume plus 5 mm. Organs at risk and dose constraints were defined as previously described.13,14
Treatment was delivered over 1 to 2 weeks with the following dose levels in 3 fractions: 1 = 24 Gy, 2 = 30 Gy, and 3 = 36 Gy. Stratum I and II started at dose levels 1 and 2, respectively. Dose levels were calculated with an α/β ratio of 10 for the tumor. For the renal cohort, we used 24 Gy in 3 fractions with a biologically effective dose (BED) of 43.2 Gy and equivalent dose delivered in 2 Gy fractions (EQD2) of 36 Gy. This dose was determined based on prior studies of Wilms tumor. Radiation with doses of 21.6 Gy in 1.8 Gy fractions or 21 Gy in 1.5 Gy fractions are frequently used in treatment of gross disease in Wilms tumor or pediatric clear cell carcinoma of the kidney.15 BED and EQD2 for 21.6 Gy in 1.8 Gy fractions are approximately 25.5 Gy and 21.2 Gy, respectively. A recent study for metastatic Wilms tumor with high-risk histologies explored the use of 25.2 Gy in 1.8 Gy fractions with a 10 Gy boost for gross residual disease or involved lymph nodes.16 BED and EQD2 for 25.2 Gy in 1.8 Gy fractions with a 10 Gy boost are approximately 41.7 Gy and 34.8 Gy, respectively. For the sarcoma cohort, we used 30 Gy in 3 fractions for a BED of 60.0 Gy and EQD2 of 50.0 Gy as the starting dose level. Definitive radiation with 55.8 Gy in 1.8 Gy fractions for Ewing sarcoma produces a BED of 65.8 Gy and EQD2 of 54.9 Gy, while 50.4 Gy in 1.8 Gy fractions is used for definitive treatment of rhabdomyosarcoma and produces a BED of 59.5 Gy and EQD2 of 49.6 Gy.17,18
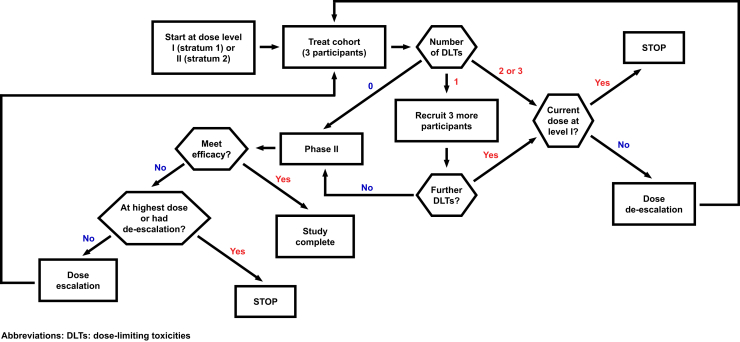
The phase I and II trial designs were employed sequentially (Fig 1). If a dose level was found to be safe in phase I, it was tested in phase II before dose escalation back in phase I. A 3+ 3 study design was used in the phase I portion. Dose-limiting toxicites (DLTs) were defined by Common Terminology Criteria for Adverse Events v4.0. If 0/3 or 1/6 patients experienced a DLT in phase I, that dose level was deemed safe and would be evaluated for efficacy in the phase II portion. A 2-stage design was used in the phase II study. The first stage aimed to accrue 7 patients including patients enrolled in the phase I portion at the particular dose level. If at least 2 responses were observed, 8 additional patients would be enrolled. If ≥5 responses were observed among the 15 patients, this dose level would be considered tolerable and effective for this patient population and the study would be closed. With this design, the phase II portion has 90% power to distinguish the response rate of 50% versus 20% based on a 0.14 level 1-sided exact binomial test. If a dose level examined in the phase II portion does not meet the prespecified criteria for efficacy, the next higher dose level would start in the phase I portion until a dose level met both phase I and phase II criteria for safety and efficacy.
Figure 1.
Schematic of the phase I/II trial design.
Patients were followed with physical examinations and CT chest imaging at 6 weeks after SBRT and every 3 months thereafter for the first year, every 6 months during the second year, and annually up to 5 years of follow-up.
Primary aims of the phase I and II studies were to determine the safety of SBRT for pulmonary metastases in pediatric patients with sarcoma or primary renal tumors and evaluate disease response at 6 weeks after SBRT defined according to response evaluation criteria in solid tumors (RECIST) 1.1 criteria, respectively.19 Secondary objectives included LC rate defined by RECIST, distant lung failure-free survival, best overall response, and changes in pulmonary function tests (PFTs) after SBRT.
Patient and treatment characteristics were reported descriptively with medians and ranges, and PFT results were reported descriptively with values for forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio. The Kaplan-Meier method was used to analyze time-to-event endpoints.
Results
Five patients with median age of 13 years (range, 7-21) received SBRT for 8 metastatic pulmonary lesions at dose level 2 with 3 patients receiving SBRT to 2 lesions. Two patients were female. Primary tumor histologies included Ewing sarcoma (n = 3), anaplastic chordoma (n = 1), and osteosarcoma (n = 1). No patients with primary renal tumors were enrolled. One patient completed prior whole lung irradiation with 12 Gy in 8 fractions 2 years before SBRT. Median size of the pulmonary lesion before SBRT was 10.8 mm (range, 5.4-26.9). No patients had central lung lesions, previously defined as a lesion within 2 cm of the proximal bronchial tree.20 Median distance from the proximal bronchial tree was 4.7 cm (range, 2.4-8.8). One patient received concurrent nivolumab. Median clinical and imaging follow-up were 2.4 years (range, 1.4-2.6) and 2.3 years (range, 1.2-2.6), respectively.
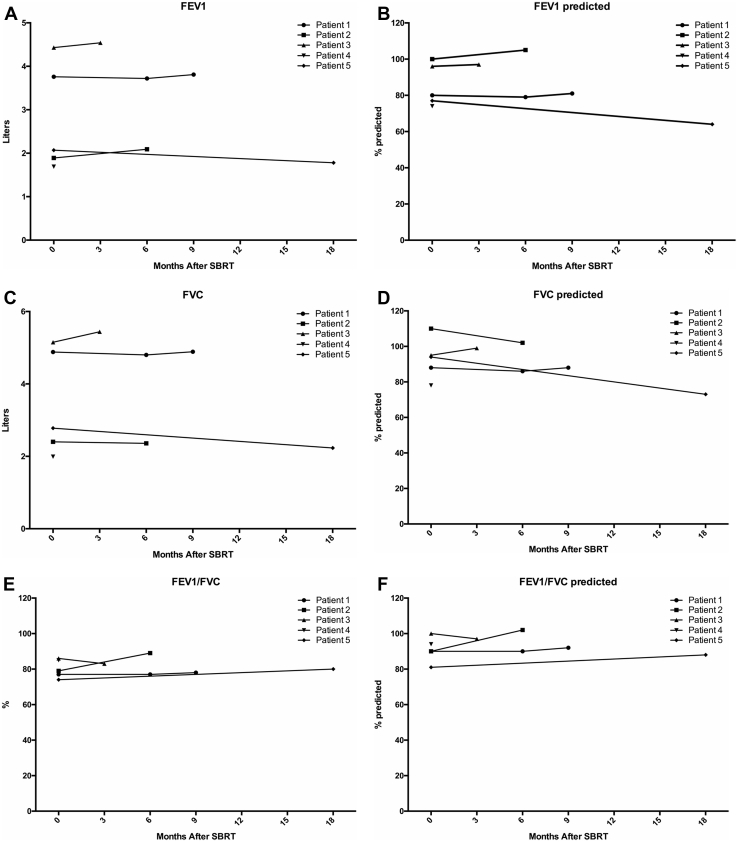
No patients were enrolled in stratum I. Three patients were enrolled in the phase I study for the sarcoma stratum at dose level 2, and no DLTs were observed. The stratum II cohort proceeded to the phase II study, and 2 additional patients were enrolled at dose level 2. Dosimetric characteristics are available in Table 2. For 1 patient, the coverage of the planning target volume for a right lower lobe lesion was slightly lower owing to proximity of the lesion to the heart and to respect the dose constraint for the heart. Radiation plans for all other lesions met the dose constraints for organs at risk. No grade ≥3 adverse events were observed. One patient who received concurrent nivolumab developed grade 2 pneumonitis 4 months after SBRT with improvement after discontinuing nivolumab and a 10-month slow steroid taper. PFTs were largely stable after SBRT, with only patient 5 experiencing a slight decline (Fig 2).
Table 2.
Dosimetric characteristics of SBRT treatments
| Dosimetric parameter | Median (range) |
|---|---|
| PTV V100%Rx (n = 8) | 95% (range, 95-99.5) |
| PTV minimum (n = 8) | 90.5%Rx (range, 85.6-95) |
| Chest wall V30 Gy (n = 8) | 0.2% (range, 0.0-1.2) |
| Bronchial tree/trachea V15 Gy (n = 3) | 0.0% (range, 0.01-0.3) |
| Bronchial tree/trachea D0.035cc (n = 3) | 5.1 Gy (range, 3.2-14.8) |
| Spinal cord D0.035cc (n = 8) | 2.8 Gy (range, 2-4.9) |
| Esophagus/stomach D0.035cc (n = 8) | 3.3 Gy (range, 0.4-22) |
| Heart D0.035cc (n = 8) | 3.1 Gy (range, 0.2-31.4) |
| Lungs V5 Gy (n = 8) | 6.8% (range, 1.5-16.7) |
| Lungs V10 Gy (n = 8) | 2.8% (range, 0.7-4.1) |
| Lungs V20 Gy (n = 8) | 1.0% (range, 0.3-1.2) |
| Lungs mean dose (n = 8) | 1.3 Gy (range, 0.3-2.3) |
| Ribs (uninvolved) D0.035cc (n = 8) | 30.5 Gy (range, 4.1-32.4) |
| Skin D0.035cc (n = 8) | 11.3 Gy (range, 8.0-21.4) |
Abbreviations: PTV = planning target volume; SBRT = stereotactic body radiation therapy.
Figure 2.
Pulmonary function tests before and after stereotactic body radiation therapy. (A) Forced expiratory volume in one second (FEV1), (B) FEVI% predicted, (C) forced vital capacity (FVC), (D) FVC% predicted, (E) FEV1/FVC ratio, (F) FEV1/FVC ratio % predicted, (G) DLCO, and (H) DLCO% predicted for all 5 patients before and after stereotactic body radiation therapy. Abbreviations: DLCO = diffusing capacity of lung for carbon monoxide; FEV1 = forced expiratory volume in one second.
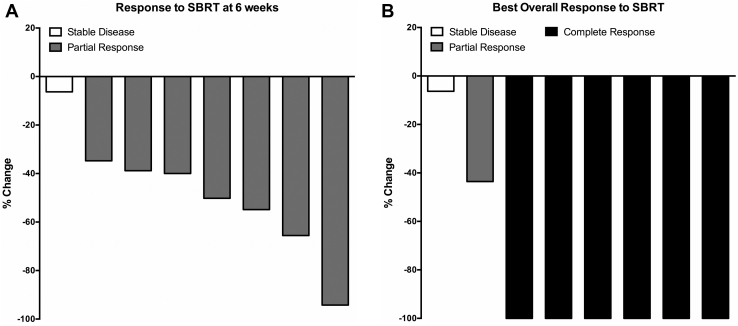
At 6 weeks after SBRT, 7 lesions (87.5%) achieved partial response (PR) (Fig 3A). We observed a PR in pulmonary metastases for all 5 patients (100%) at 6 weeks, meeting criteria to conclude efficacy. Best overall response was complete response (CR) in 6 lesions (75.0%) (Fig 3B).
Figure 3.
Response of pulmonary lesions after stereotactic body radiation therapy (SBRT). (A) Response of pulmonary lesions at 6 weeks after SBRT. (B) Best overall response of pulmonary lesions after SBRT.
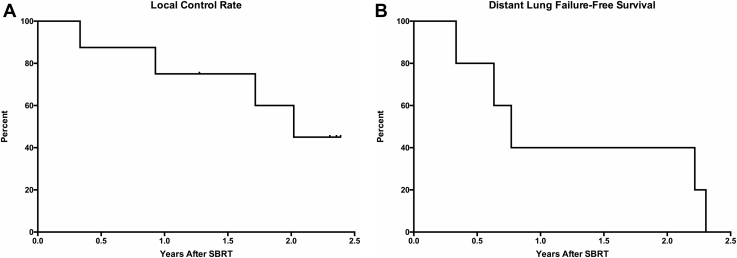
Two-year LC rate and distant lung failure-free survival were 60% (n = 8) and 40% (n = 5) (Fig 4A-B). The patient who received concurrent nivolumab had distant lung recurrence 2.2 years after completing lung SBRT. Two patients received another course of SBRT for additional pulmonary metastases and 1 patient developed widespread metastases and succumbed to disease 1.4 years after SBRT.
Figure 4.
Local and distant control of pulmonary lesions after stereotactic body radiation therapy (SBRT). (A) Local control rates for 8 lesions after SBRT (n = 8). (B) Distant lung failure-free survival for 5 patients after SBRT (n = 5).
Discussion
SBRT for metastatic pulmonary lesions can produce excellent LC rates in adult patients,7,8 but its efficacy and tolerability in pediatric patients remain not well characterized. Herein, we report that all 5 patients experienced PR after 30 Gy in 3 fractions at 6 weeks, meeting the predefined endpoint for closure of the phase II study, and there were no DLTs, with 1 patient developing grade II pneumonitis. To our knowledge, this is the first prospective study of SBRT for pulmonary metastases in pediatric patients.
A phase I/II dose-escalation trial in adult patients with metastatic lung lesions demonstrated that 54 Gy with heterogeneity correction in 3 fractions can produce a 2-year LC rate of 96% with limited toxicity.8 Retrospective case series have reported SBRT for metastatic lung lesions in pediatric patients using 30 to 50 Gy in 3 to 5 fractions with durable LC for some patients.9, 10, 11, 12 We found 30 Gy in 3 fractions achieved CR in 75% of lesions and produced 2-year LC rate of 60%. Four lesions (50%) treated with SBRT recurred or progressed at last follow-up, suggesting that this dose and fractionation regimen may not produce as durable responses as seen in adult patients with 60 Gy in 3 fractions.8 Furthermore, patients remained at risk of distant progression in the lung after SBRT, with all patients recurring outside of the SBRT field.
We also reported no dose-limiting toxicities with 30 Gy in 3 fractions. Previous retrospective reports reported a rib fracture and radiation pneumonitis in pediatric patients after lung SBRT.9,11 One patient who received concurrent nivolumab developed grade 2 pneumonitis, requiring prolonged steroid taper. In adult patients, trials investigating concurrent checkpoint inhibitors and SBRT have noted pneumonitis21,22; however, the rate of grade ≥3 pulmonary events appeared comparable to that of Radiation Therapy Oncology Group 0236, a phase II trial investigating SBRT alone for inoperable nonsmall cell lung cancer.22,23 The tolerability of concurrent radioimmunotherapy for pulmonary lesions for pediatric patients remains unknown.
Our study has several limitations, including a small sample size and a primary efficacy endpoint at a relatively short follow-up interval. Although CRs or PRs have been observed at 6 weeks after lung SBRT in adult patients,24 it is not known whether these initial responses correlate with long-term LC. Our trial design aimed to find a minimum effective dose, and thus, we wanted to limit treating pediatric patients at ineffective dose levels of lung SBRT. Thus, we opted for a primary endpoint of response at 6 weeks to allow for quicker dose escalation. Although we found that SBRT was well-tolerated and resulted in responses in all patients in the study, these responses were not sustained, which also contributed to closure of the trial. These data suggest that response at 6 weeks may not be strict enough and be clinically significant; however, our data demonstrating an LC rate of 60% at 2 years suggest that some patients had durable LC after lung SBRT with 30 Gy in 3 fractions. All patients had peripheral lung lesions, thus our findings cannot be extrapolated to treat central lung lesions. Furthermore, we chose both primary renal histologies and sarcoma, as both pediatric populations commonly have lung metastases. However, no patients with primary kidney tumor histologies enrolled in the study owing to lack of eligible patients. Patients with primary renal tumors are often younger and require sedation, which is an exclusion criterion, and less frequently present with isolated lung metastases, making them poor candidates for lung SBRT. Thus, it remains unknown whether this dose is safe in younger patients and effective in patients with nonsarcoma histologies.
Future prospective studies with larger cohorts and multiple institutions are needed for pediatric patients with metastatic lesions to further characterize a safe lung SBRT dose that also produces a durable LC. Furthermore, given the risk of distant failure within the lung, systemic therapy, particularly with targeted agents or immunotherapy, will be important, and further investigation into the safety and efficacy of combining systemic therapy options with lung SBRT will be important.
Conclusions
Our study demonstrates that SBRT using 30 Gy in 3 fractions is well tolerated and produces responses in pediatric patients with sarcoma histologies at 6 weeks; patients are at risk for local and distant recurrences in the lung. Prospective studies with larger pediatric cohorts are needed to explore the durable efficacy and safety of higher doses of SBRT alone or with systemic therapies and in nonsarcoma histologies.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Heaton T.E., Davidoff A.M. Surgical treatment of pulmonary metastases in pediatric solid tumors. Semin Pediatr Surg. 2016;25:311–317. doi: 10.1053/j.sempedsurg.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirksen U., Brennan B., Le Deley M.C. High-dose chemotherapy compared with standard chemotherapy and lung radiation in ewing sarcoma with pulmonary metastases: Results of the European Ewing tumour working initiative of national groups, 99 trial and Ewing 2008. J Clin Oncol. 2019;37:3192–3202. doi: 10.1200/JCO.19.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meisel J.A., Guthrie K.A., Breslow N.E. Significance and management of computed tomography detected pulmonary nodules: A report from the National Wilms Tumor Study group. Int J Radiat Oncol Biol Phys. 1999;44:579–585. doi: 10.1016/s0360-3016(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 4.Briccoli A., Rocca M., Salone M. High grade osteosarcoma of the extremities metastatic to the lung: Long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol. 2010;19:193–199. doi: 10.1016/j.suronc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Harting M.T., Blakely M.L., Jaffe N. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2006;41:194–199. doi: 10.1016/j.jpedsurg.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 6.Warmann S.W., Furtwangler R., Blumenstock G. Tumor biology influences the prognosis of nephroblastoma patients with primary pulmonary metastases: Results from SIOP 93-01/GPOH and SIOP 2001/GPOH. Ann Surg. 2011;254:155–162. doi: 10.1097/SLA.0b013e318222015e. [DOI] [PubMed] [Google Scholar]
- 7.Rieber J., Streblow J., Uhlmann L. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases-a pooled analysis of the German working group “stereotactic radiotherapy.”. Lung Cancer. 2016;97:51–58. doi: 10.1016/j.lungcan.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Rusthoven K.E., Kavanagh B.D., Burri S.H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 9.Amsbaugh M., Bertke M., Cheerva A. Stereotactic body radiation therapy for lung metastases in a child with Ewing sarcoma. CHEST. 2014;146:662A. [Google Scholar]
- 10.Brown L.C., Lester R.A., Grams M.P. Stereotactic body radiotherapy for metastatic and recurrent Ewing sarcoma and osteosarcoma. Sarcoma. 2014;2014 doi: 10.1155/2014/418270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deck J., Eastwick G., Sima J. Efficacy and tolerability of stereotactic body radiotherapy for lung metastases in three patients with pediatric malignancies. Onco Targets Ther. 2019;12:3723–3727. doi: 10.2147/OTT.S194812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui F., Kunos C.A., Paulino A.C. Stereotactic body radiation therapy in head and neck, gynecologic, and pediatric malignancies. J Radiat Oncol. 2012;1:31–42. [Google Scholar]
- 13.Kong F.M., Ritter T., Quint D.J. Consideration of dose limits for organs at risk of thoracic radiotherapy: Atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys. 2011;81:1442–1457. doi: 10.1016/j.ijrobp.2010.07.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmerman R., Heinzerling J., Abdulrahman R. Stereotactic body radiation therapy for thoracic cancers: Recommendations for patient selection, setup and therapy. Front Radiat Ther Oncol. 2011;43:395–411. doi: 10.1159/000322503. [DOI] [PubMed] [Google Scholar]
- 15.Kalapurakal J.A., Green D.M., Haase G. Outcomes of children with favorable histology wilms tumor and peritoneal implants treated in national wilms tumor studies-4 and -5. Int J Radiat Oncol Biol Phys. 2010;77:554–558. doi: 10.1016/j.ijrobp.2009.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasqualini C., Furtwangler R., van Tinteren H. Outcome of patients with stage IV high-risk Wilms tumour treated according to the SIOP2001 protocol: A report of the SIOP Renal Tumour Study Group. Eur J Cancer. 2020;128:38–46. doi: 10.1016/j.ejca.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson S.S. Ewing sarcoma: Radiation dose and target volume. Pediatr Blood Cancer. 2004;42:471–476. doi: 10.1002/pbc.10472. [DOI] [PubMed] [Google Scholar]
- 18.Wolden S.L., Lyden E.R., Arndt C.A. Local control for intermediate-risk rhabdomyosarcoma: Results from d9803 according to histology, group, site, and size: A report from the children's oncology group. Int J Radiat Oncol Biol Phys. 2015;93:1071–1076. doi: 10.1016/j.ijrobp.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Timmerman R., McGarry R., Yiannoutsos C. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 21.Luke J.J., Lemons J.M., Karrison T.G. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36:1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma V., Cushman T.R., Selek U. Safety of combined immunotherapy and thoracic radiation therapy: Analysis of 3 single-institutional phase I/II trials. Int J Radiat Oncol Biol Phys. 2018;101:1141–1148. doi: 10.1016/j.ijrobp.2018.04.054. [DOI] [PubMed] [Google Scholar]
- 23.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed N., Grills I.S., Wong C.Y. Radiographic and metabolic response rates following image-guided stereotactic radiotherapy for lung tumors. Radiother Oncol. 2011;99:18–22. doi: 10.1016/j.radonc.2011.03.003. [DOI] [PubMed] [Google Scholar]