Abstract
Purpose
The purpose of this prospective study was to evaluate radiation-induced myocardial damage after mediastinal radiation therapy (RT) using late gadolinium-enhancement (LGE) magnetic resonance imaging (MRI).
Methods and Materials
We enrolled 19 patients with esophageal cancer who were expected to have long-term survival by definitive treatment. They underwent delayed contrast-enhanced MRI (19 patients before treatment, 19 patients 6 months after treatment, and 12 patients 1.5 years after treatment). Dose distribution of the left ventricle was made using computed tomography, and the dose volume histogram of the left ventricle was calculated. Myocardial signal intensities in individual MRIs were normalized by the mean values in regions receiving low doses (<5 Gy). Changes in the normalized signal intensities after mediastinal radiation therapy were compared among regions where irradiation doses were 0 to 10 Gy, 10 to 20 Gy, 20 to 30 Gy, 30 to 40 Gy, 40 to 50 Gy, and 50 to 60 Gy, and we investigated whether intensity change was detected in a dose-dependent manner.
Results
The registered patients were treated with concurrent chemoradiotherapy with a median total dose of 60 Gy (50.4-66 Gy). Chemotherapy consisting of cisplatin and 5-fluorouracil was administered. In the population-based dose-response curve, dose-dependent intensity changes progressively increased in regions receiving more than 30 Gy. The averages of relative intensity change at 6 months and 1.5 years after treatment were 1.1% and −1.9% at 20 to 30 Gy and 37.5% and 17.5% at 40 to 50 Gy, respectively. LGE in regions receiving more than 30 Gy was detected in 68% (13/19) of the patients.
Conclusions
A dose-dependent relationship for myocardial signal intensity change was found by using LGE MRI. It may be necessary to reduce the volume of the myocardium receiving more than 30 Gy.
Introduction
Mediastinal radiation therapy (RT) for thoracic malignancies has frequently included a part of the heart, and many studies have shown RT-induced heart disease (RIHD) from previous treatment.1, 2, 3, 4 RIHD has become an important concern in radiation oncology. There have been several reports of correlations between the frequency of RIHD and RT total dose.5, 6, 7
It is assumed that a part of the heart is subject to high-dose irradiation in patients with esophageal cancer. Some recent studies have demonstrated that the use of RT was associated with heart disease-related death in patients with esophageal cancer.8,9 Some studies have shown that outcomes of chemoradiotherapy (CRT) were comparable to those of surgery in patients with esophageal cancer10,11 and that outcomes of CRT for early stage esophageal cancer were favorable.12 Therefore, we should consider additional care for long-term survivors who have received mediastinal RT for esophageal cancer.
Past studies showed that nuclear medicine imaging such as I-123b-methyl iodophenyl pentadecanoic acid (BMIPP) was useful for detecting RT-induced myocardial damage.13, 14, 15, 16, 17, 18 Late gadolinium enhancement (LGE) magnetic resonance imaging (MRI) enables visualization of the myocardial scar in patients with ischemic and nonischemic myocardial diseases,19,20 and it has higher spatial resolution than that of scintigraphy.21 A previous study showed that positive LGE was correlated with the RT field for esophageal cancer. However, serial MR examinations to evaluate changes in MR findings before and after RT were not conducted in that study.22 A study on quantification of fibrosis in infarcted swine hearts by LGE MRI showed that the signal intensity of LGE was strong in the myocardium with a high density of fibrosis.23 Hardenberg et al reported dose-effect regional cardiac perfusion abnormalities in patients with left-sided breast cancer treated with RT who underwent cardiac perfusion imaging.24 Based on these results, we hypothesized that it would be possible to evaluate the RT dose dependency of myocardial damage by quantitative analysis of LGE before and after treatment. The purpose of this prospective study was to evaluate whether RT-induced myocardial damage depending on RT dose can be detected by using LGE MRI in patients with esophageal cancer.
Methods and Materials
Patients
This study was approved by a local institutional review board (2010-59), and all of the patients gave written informed consent before enrollment. The study was registered on UMIN Clinical Trials Registry (UMIN000032551, https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno = R000037126) and accepted by the International Committee of Medical Journal Editors. Between 2013 and 2015, we enrolled 19 patients with esophageal cancer who received curative-intended mediastinal RT. The eligibility criteria were as follows: age, 20 to 75 years; Eastern Cooperative Oncology Group performance status, 0 to 1; normal kidney function (estimated glomerular filtration rate <30 mL/min/1.73 m2); no history of cardiac disease excluding hypertension. Four patients had a history of hypertension and took an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker; cases in which the myocardium was expected to be included in the RT field; cases in which long-term survival could be expected by definitive treatment; no other active malignancy at the start of our study; and no history of mediastinal radiation therapy and chemotherapy.
Chemoradiotherapy
The CRT regimen and RT technique were described in our previous report.25 Generally, 2 cycles of concurrent chemotherapy (2-hour infusion of cisplatin at 70 mg/m2 on day 1 and continuous infusion of 5-fluorouracil at 700 mg/m2 during a 24-hour period on days 1-4) with a 4-week interval was performed during RT.
Three-dimensional conformal RT was delivered to all patients. The initial clinical target volume (CTV) was defined as the region from the supraclavicular to celiac lymph nodes. Boost CTV was defined as the primary tumor with a 20- to 30-mm craniocaudal margin and an approximately 5-mm radial margin and nodal metastasis. The planning target volume was defined as CTV plus a 5- to 15-mm margin. The left ventricle (LV) was contoured by one radiation oncologist by referring to Feng et al.26 The inner cavity of the LV was excluded. The whole heart (WH), left main artery, left anterior descending artery (LAD), left circumflex artery (LCX), and right coronary artery were also contoured.
The initial CTV dose was approximately 40 Gy using parallel-opposed anterior or posterior fields. The boost CTV received 20 Gy using parallel-oblique fields to avoid the spinal cord. We allowed the radiation oncologist to adjust the RT total dose and the range of the RT field according to the patient's condition and clinical stage. A median total dose was 60 Gy (range, 50.4-66 Gy). The dose distribution was determined by ECLIPSE Varian Medical Systems (Palo Alto, CA) with the analytical anisotropic algorithm. V10 Gy (the percentage of the volume receiving 10 Gy in the left ventricle), V20 Gy, V30 Gy, V40 Gy, V50 Gy, V60 Gy, mean dose of LV and WH were calculated using the dose volume histogram (DVH) of the LV. Only mean dose of the left main artery, LAD, LCX, and right coronary artery were calculated because of the small volume sizes.
MRI protocol
The MRIs were performed using a 3-T whole body scanner (MAGNETOM Trio A Tim System; Siemens Health care, Erlangen, Germany). LGE imaging was performed 10 minutes after injection of 0.15 mmol/kg (0.30 mL/kg) of Gd-DTPA (gadopentetate dimeglumine, Magnevist; Bayer, Osaka, Japan) contrast. Generally, LGE imaging of the left ventricle was acquired in 15 short axial sections in the diastolic phase. LGE imaging was performed using a phase-sensitive inversion recovery gradient echo sequence (repetition time/echo time/flip time, 750 ms/1.95 ms/20 degrees and voxel size, 1.4 × 1.8 × 8 mm).
To investigate the correlation between LGE and cardiac function, LV ejection fraction (LVEF) and cardiac index were measured by the Simpson technique using a commercially available workstation (ZIOSTATION2, Ziosoft, Tokyo, Japan). Cine images were acquired using a breath-hold electrocardiogram (ECG) gating a balanced steady-state free precession sequence (repetition time/echo time/flip time, 60.75 ms/1.08 ms/43 degrees and voxel size, 1.3 × 1.3 × 8 mm).
Other cardiac examinations
Changes in brain natriuretic peptide (BNP) value, ECG, and pericardial effusion were evaluated before and after RT in all patients. The presence of pericardial effusion was observed on CT scans or MRIs.
Evaluation of the dose-dependent response in intensity change of LGE after treatment
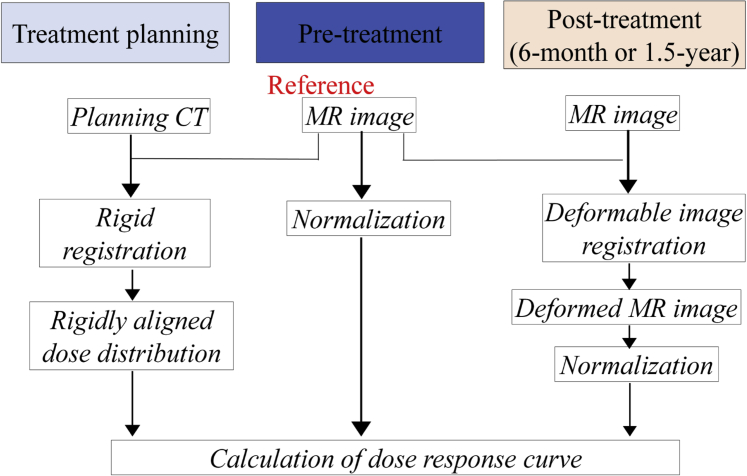
A flow chart illustrating the calculation of the dose-response curve after treatment is shown in Figure 1. First, we performed the rigid registration between the pretreatment MRI and the planning CT to create the rigidly aligned dose distribution using Mirada RTx (Mirada Medical, Oxford, UK). Second, we used the multimodel deformable image registration technique implemented in Mirada RTx to match posttreatment MRIs to pretreatment MRIs. In terms of deformable image registration accuracy, we confirmed that there were no large registration errors in all cases by visual inspection. At checking these registrations, a side-by-side reading was performed by 2 medical physicists. Third, the degree of signal intensity of LGE was normalized by the mean value in segments receiving low doses (<5 Gy); this was the method used to examine changes in pre-RT and post-RT MRI images because we assumed that radiation-induced damage was not present at this low dose. This method was conducted with reference to a previous report.17 The relative values of signal intensity of LGE to the normalized reference value in each dose region (0-10 Gy, 10-20 Gy, 20-30 Gy, 30-40 Gy, 40-50 Gy, and 50-60 Gy) were determined before and after treatment, and the change of relative values in each dose region was calculated for each patient. Finally, we calculated the population-based dose-response curve after treatment by aggregating those data.
Figure 1.
Flow chart illustrating the method of calculation of the dose-response curve after treatment. Abbreviations: CT = computed tomography; MR = magnetic resonance.
Evaluation of factors associated with RT-induced myocardial damage
We investigated which factors were related to RT-induced myocardial change as follows. As described above, we examined the signal intensity change in each dose region and set the dose threshold at which signal intensity changes became obvious. After that, cases in which signal change corresponding to the threshold dose was detected were defined as RT-induced signal intensity change (RT-induced signal intensive change [SIC]). If LGE outside the threshold region was observed, we examined whether the cause of LGE was an infarction caused by coronary artery stenosis due to irradiation. The dose distribution was confirmed by one radiation oncologist, and 2 radiologists evaluated the presence or absence of LGE. We examined the differences between patients with and without RT-induced SIC in RT-related and patient-related factors.
We also investigated whether cardiac abnormalities were more frequently observed in patients with RT-induced SIC than in patients without RT-induced SIC according to BNP level, ECG changes, and pericardial effusion. In evaluation of the ECG, the changes requiring treatment were defined as positive. In the evaluation of pericardial effusion, asymptomatic small to moderate effusion of G2 was regarded as positive according to Common Terminology Criteria for Adverse Events, version 4.0.
To assess the differences between patients with positive findings and those with negative findings, continuous and dichotomous variables were analyzed using the Mann-Whitney U test and Fisher’s test, respectively.
All statistical tests were 2-sided, and statistical significance was defined as a P value < .05. Statistical analysis was performed using JMP 10 (SAS Institute, Cary, NC).
Results
The registered patients underwent delayed contrast-enhanced MRI (19 patients before treatment, 19 patients at 6 months after treatment, and 12 patients at 1.5 years after treatment). The mean ± standard deviation (SD) of intervals between the completion of CRT and the acquisition of MRIs at the 6 month and 1.5-year follow-up were 191.3 ± 22.8 days and 559.6 ± 14.6 days, respectively. The patient characteristics are shown in Table 1 and in supplementary Table E1. Positron emission tomography or CT was performed to determine the stage in 17 patients. All patients received concurrent CRT. Only one patient underwent additional CRT after surgery owing to positive surgical margin. Three patients underwent CRT after endoscopic submucosal dissection owing to positive margin or recurrence. The range of the planned initial CTV was reduced in 5 patients. The scheduled CRT was completed in all patients, although only one cycle of concurrent chemotherapy was performed in 3 patients for various reasons such as myelosuppression. Adjuvant chemotherapy after CRT was not performed in any patients. Seven patients could not undergo MRI 1.5 years after treatment because of patient refusal of examination (2 patients), recurrence requiring cisplatin-based chemotherapy after CRT (1 patient), and lack of funds for research expenses (4 patients).
Table 1.
Patient and radiation therapy characteristics
| Characteristic | Mean ± SD | No. of Patient |
|---|---|---|
| Age at RT | 62.2 ± 5.6 y | |
| Primary site | ||
| Cervical: lower thoracic | 1 | |
| Middle thoracic | 11 | |
| Lower thoracic | 7 | |
| Stage (UICC 7th ed) | ||
| IA | 14 | |
| IB | 3 | |
| IIA | 1 | |
| IV | 1 | |
| Pathologic diagnosis | ||
| Squamous cell carcinoma | 19 | |
| Treatment before chemoradiotherapy | ||
| None | 15 | |
| Surgery | 1 | |
| Endoscopic submucosal dissection | 3 | |
| ECOG performance status | ||
| 0 | 15 | |
| 1 | 4 | |
| Hypertension | ||
| Yes | 4 | |
| No | 15 | |
| Hyperlipidemia | ||
| Yes | 6 | |
| No | 13 | |
| Habit of smoking | ||
| Yes | 12 | |
| No | 7 | |
| Body mass index | 21.0 ± 3.0 kg/m2 | |
| eGFR | 77.6 ± 11.9 mL/min/1.73m2 | |
| Coronary artery calcification | ||
| Yes | 11 | |
| No | 8 | |
| LV Dmean | 17.0 ± 6.1 Gy | |
| LV V10 Gy | 41.0% ± 15.1% | |
| LV V20 Gy | 34.6% ± 14.3% | |
| LV V30 Gy | 29.9% ± 13.7% | |
| LV V40 Gy | 17.8% ± 13.9% | |
| LV V50 Gy | 4.0% ± 5.9% | |
| LV V60 Gy | 1.2% ± 2.8% |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; eGFR = estimated glomerular filtration rate; LV10Gy = percentage of the volume receiving 10 Gy in the left ventricle; LV Dmean = mean dose in the left ventricle; RT = radiation therapy; SD = standard deviation; UICC = Union for International Cancer Control.
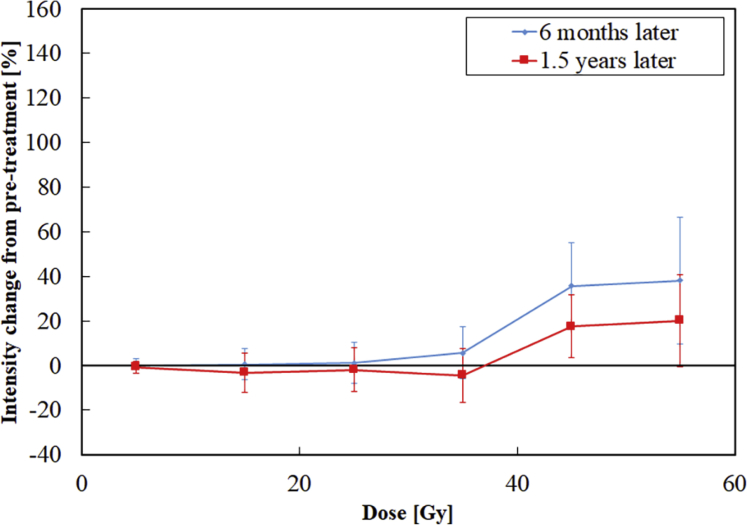
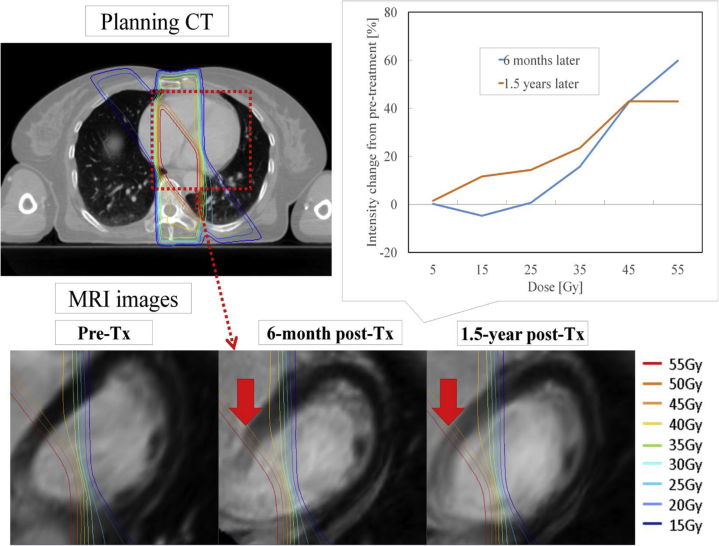
Population-based dose-response curves are shown in Figure 2. The average values of relative increase in intensity change at 6 months were −0.2%, 0.4%, 1.1%, 5.7%, 35.7%, and 38.1% at 0 to 10 Gy, 10 to 20 Gy, 20 to 30 Gy, 30 to 40 Gy, 40 to 50 Gy, and 50 to 60 Gy, respectively. The average values of relative increase in intensity change at 1.5 years were −0.8%, −3.2%, −1.9%, −4.4%, 17.5%, and 20.1%, respectively. An example case is shown in Figure 3. In the population-based dose-response curve, the dose-dependent signal progressively increased in regions receiving more than 30 Gy. Therefore, we determined that the threshold dose for the signal change was 30 Gy and defined a case in which LGE was observed in this region as positive. As a result, 13 of the 19 patients had RT-induced SIC. There were no patients in which LGE outside the 30 Gy dose line was detected.
Figure 2.
Population-based dose-response curves for signal intensity change of late gadolinium enhancement at 6 months and 1.5 years after treatment.
Figure 3.
Example case of magnetic resonance imaging after chemoradiotherapy for esophageal cancer. Dose-effect signal intensity changes of late gadolinium enhancement within the radiation field were clearly detected at 6 months and 1.5 years after treatment in this case. Abbreviations: CT = computed tomography; MRI = magnetic resonance image; Tx = treatment.
The results of analysis of radiation-related and patient-related factors for patients with and without RT-induced SIC are shown in Table 2 and Table E2. Although there were no significant differences in the DVH parameters of the LV between patients with and without RT-induced SIC, LV V10 Gy, LV V20 Gy, and LV V30 Gy tended to be higher in positive patients (P = .066, .053, and .066, respectively). There were significant differences in the DVH parameters of WH V10 Gy, WH V20 Gy, WH V30 Gy, LDA, and LCX (P = .020, .010, .049, .049, and .032). Although there were no significant differences in patient-related factors between patients with and without RT-induced SIC, sex, performance status, and hypertension tended to be significant factors (P = .059, .061, and .061, respectively).
Table 2.
Analysis of radiation-related and patient-related factors in patients with and without RT-induced SIC after treatment
| Factor | RT-induced SIC (+) | RT-induced SIC (−) |
|---|---|---|
| LV V10 Gy (%) | 45.6 ± 13.6 | 30.9 ± 14.1 |
| LV V20 Gy (%) | 39.0 ± 12.9 | 24.9 ± 12.9 |
| LV V30 Gy (%) | 34.2 ± 12.6 | 20.6 ± 11.9 |
| LV V40 Gy (%) | 20.1 ± 14.4 | 12.9 ± 7.9 |
| LV V50 Gy (%) | 3.5 ± 4.7 | 5.3 ± 8.3 |
| LV V60 Gy (%) | 0.7 ± 1.6 | 2.3 ± 4.5 |
| LV mean dose (Gy) | 18.6 ± 5.5 | 13.5 ± 6.4 |
| Age at RT (r) | 63.8 ± 4.1 | 58.5 ± 6.9 |
| Sex (n) | ||
| Male | 5 | 5 |
| Female | 8 | 1 |
| ECOG performance status (n) | ||
| 0 | 9 | 6 |
| 1 | 4 | 0 |
| Hypertension (n) | ||
| Yes | 4 | 0 |
| No | 9 | 6 |
| Hyperlipidemia (n) | ||
| Yes | 3 | 3 |
| No | 10 | 3 |
| Habit of smoking (n) | ||
| Yes | 7 | 5 |
| No | 6 | 1 |
| Body mass index (kg/m2) | 21.0 ± 3.4 | 20.9 ± 2.4 |
| eGFR (mL/min/1.73 m2) | 74.9±12.1 | 83.6±9.7 |
| Coronary artery calcification (n) | ||
| Yes | 8 | 3 |
| No | 5 | 3 |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; eGFR = estimated glomerular filtration rate; LV V10 Gy = percentage of the volume receiving 10 Gy in the left ventricle; RT = radiation therapy; SIC = signal intensity change.
The results of cardiac examinations after CRT are shown in Table 3. Although no patients had cardiac symptoms before and at 6 months after treatment, mild palpitation was observed in one patient with positive findings at 1.5 years after treatment. The mean ± SD of LVEF at pretreatment and 6 months and 1.5 years posttreatment in all patients was 60.4 ± 8.9, 62.8 ± 12.7 and 62.0 ± 10.4%, respectively. The mean ± SD deviation of cardiac index at pretreatment and 6 months and 1.5 years posttreatment in all patients were 2.7 ± 0.6, 2.9 ± 0.8, and 2.8 ± 0.9 L/min/m2, respectively. The mean ± SD of BNP value at pretreatment and 6 months and 1.5 years posttreatment in all patients were 25.9 ± 32.5, 40.3 ± 28.3, and 45.6 ± 28.9 pg/mL, respectively. There were no significant differences in LVEF, CI, and BNP between patients with and without RT-induced SIC. There were no positive findings in ECGs at pretreatment, at 6 months posttreatment and at 1.5 years posttreatment. pericardial effusion before RT was observed, but mild pericardial effusion was seen in some patients at 6 months and 1.5 years after treatment. There was no significant difference in the frequency of pericardial effusion between patients with and without RT-induced SIC.
Table 3.
Results of cardiac examinations in patients with and without RT-induced SIC at 6 months and 1.5 years after treatment
| Factor | 6 mo after treatment (n = 19) |
1.5 y after treatment (n = 12) |
||
|---|---|---|---|---|
| RT-induced SIC (+) | RT-induced SIC (−) | RT-induced SIC (+) | RT-induced SIC (−) | |
| LVEF (%) | 66.9 ± 11.3 | 53.9 ± 11.6 | 61.9 ± 8.2 | 62.5 ± 15.6 |
| CI (L/min/m2) | 2.9 ± 0.9 | 3.1 ± 0.4 | 2.7 ± 0.8 | 3.2 ± 1.0 |
| BNP (pg/mL) | 46.6 ± 31.1 | 27.7 ± 17.6 | 48.1 ± 31.4 | 40.6 ± 26.8 |
| ECG change (n) | ||||
| Yes | 0 | 0 | 0 | 0 |
| No | 11 | 4 | 8 | 4 |
| Pericardial effusion (n) | ||||
| Yes | 9 | 2 | 5 | 2 |
| No | 4 | 4 | 3 | 2 |
Abbreviations: BNP = brain natriuretic peptide; CI = cardiac index; ECG = electrocardiogram; LVEF = left ventricle ejection fraction; RT-induced SIC = radiation therapy-induced signal intensity change.
Continuous variables are presented as mean values ± standard deviation.
Four patients did not undergo ECG at 6 months after treatment.
Discussion
As expected, the signal intensity changes became stronger when the dose of radiation to the myocardium was increased. In a study by Hardenberg et al and our previous pilot study using BMIPP, dose-effect relationships for abnormal uptake similar to those found in the present study also tended to be observed at 6 months after RT.17,24 The signal intensity change was conspicuous in regions receiving 30 Gy or more. One of the risk factors of RIHD has been reported to be a high cumulative dose of RT (>30 Gy),27 and an autopsy study of patients irradiated with at least 35 Gy (mean dose of 56 Gy to the anterior heart surface) showed that 50% of the patients had myocardial fibrosis.28 The results of our study support those findings. Although there was no significant difference, LV V30 Gy tended to be higher in patients with RT-induced SIC in the present study. WH V10 Gy, V20 Gy, and V30 Gy were also significantly higher in patients with RT-induced SIC. Konski et al reported that the mean heart V20 (79.7% vs 67.2%), V30 (75.8% vs 61.9%), and V40 (69.2% vs 53.8%) were significantly higher in patients with symptomatic cardiac toxicity than those without.29 The results for WH parameters in their report are similar to our results. Therefore, it may be necessary to reduce the volume of the myocardium or heart receiving more than 30 Gy as much as possible. However, patients with RT-induced SIC had lower LV V50 and V60 than patients without RT-induced SIC. The reason for this is thought to be that the volume of high-dose irradiation in parallel-oblique fields for the boost CTV is small and it is affected by the regions receiving 30 Gy. In the present study, sex, PS, and hypertension were factors that tended to be related to RT-induced SIC. Some studies have shown that pre-existing cardiac disease, age, and sex were related to cardiac events after RT.4,8,29 Therefore, it might be better to decide the RT dose and the irradiation range with consideration of the patient's background.
In the present study, the evaluation of dose dependence was performed based on regions receiving less than 5 Gy. However, recent studies have shown that even low-dose irradiation affects the heart. Darby et al reported that the rate of major coronary events increased by 7.4% for every 1 Gy increase in mean dose to the heart in patients with breast cancer in which the mean dose to the whole heart was 4.9 Gy.5 RTOG 0617 also showed that heart V5 was a significant prognostic factor for overall survival.30 Thus, the whole heart is usually evaluated at the time of RT planning. However, we consider that it is not valid to simply evaluate the whole heart because the heart contains various structures, such as the myocardium, coronary arteries, valves, and pericardium. Contouring of each structure at the time of RT planning using CT seems to be difficult because of the small volume of each structure. We considered that the myocardium is more clearly visualized and easier to evaluate than other structures for detecting dose-dependent myocardial change. Furthermore, because we evaluated the dose-dependent relationship based on the method used in a past SPECT (Single Photon Emission Computed Tomography) study,31 we considered that our evaluation method was valid. We are currently conducting clinical trial in patients with esophageal cancer using intensity modulated radiation therapy to reduce the dose to the myocardium (UMIN000038147, https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000043481). We expect that regions of a low dose in the myocardium will increase, and we will report whether LGE in those regions can be detected by MRI.
In the present study, the dose to coronary arteries was also examined. Although the doses of the LAD and LCX were significantly higher in patients with RT-induced SIC, regions of LGE corresponding to RT field were detected in all of the patients with RT-induced SIC. Therefore, those findings might indicate a direct effect of RT on the myocardium. The signal intensity changes at 1.5 years after treatment were smaller than those at 6 months after treatment in the present study, although a temporal decrease of myocardial metabolism was observed after CRT in our previous study using BMIPP.18 Because a pathologic examination of the myocardium was not performed in the present study, it is difficult to explain determine the reason for that phenomenon in the present study. RT-induced myocardial damage has been reported to be associated with microvascular injury leading to inflammatory and thrombotic changes, capillary loss, focal ischemia, and interstitial fibrosis several months after RT.32,33 Based on this process, we suggest that the subacute myocardial inflammatory reaction caused by RT might be prolonged to 6 months after treatment and might be diminished at 1.5 years after treatment, leading to fibrosis due to focal ischemia.
In the present study, there was no significant difference in cardiac function between patients with and without RT-induced SIC. A difference in cardiac function might not be apparent because the doses delivered to the heart were relatively high in most patients. It has been reported that a decrease in local wall motion in the RT field was detected relatively early by echocardiography,34,35 although it was not performed in the present study. Therefore, there is a possibility that a decrease in wall motion corresponding to the LGE region was also observed in this study. We are therefore going to examine the effects of myocardial changes using various modalities in ongoing clinical trials. Thus, although there was no difference in the wall motion of the whole heart in the present study, BNP was relatively high and pericardial effusion was frequent in patients with RT-induced SIC. We previously reported that there was a significant increase of BNP values in patients with abnormal 18F-fluorodeoxyglucose accumulation in the irradiated myocardium after RT for esophageal cancer.36 Moreover, decreased myocardial metabolism was detected gradually from the start of RT even in our previous study using BMIPP.18 Although the effect of current practice by the results of our study has been limited at the moment, the effects of RT on the heart might appear gradually and eventually lead to a cardiac event. Therefore, we are going to carefully follow-up those patients.
There were some limitations in the present study. First, the number of enrolled patients in the present study was small. However, we think that we have provided sufficient information to support the results of previous study. Second, some patients took angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, which might have affected the results of our study because those drugs might attenuate RT-induced cardiac damage.37 Third, there were some difficulties in registering MRIs with the RT dose distribution because of the difference in modalities between MRIs and CT images at RT planning. Fourth, the regions receiving 50 Gy or more existed mainly at the base of the myocardium, and the base of the myocardium was susceptible to respiration in the present study. Fifth, there was no comparison with a nonirradiated control group. We are planning to conduct a clinical trial to reduce the dose to the myocardium using IMRT, and we will compare those results with the results of the present study. Sixth, there was no histologic validation of the LGE in the present study. Therefore, LGE in this study may not necessarily reflect myocardial fibrosis. However, an invasive procedure with possible significant complications should be avoided for asymptomatic patients.
Conclusions
A dose-effect relationship for myocardial signal intensity change using LGE MRI was observed, especially in regions receiving more than 30 Gy. We recommend careful consideration of high-dose irradiation to the myocardium.
Acknowledgments
The authors thank all the patients who participated in the present study and all of the staff of the Department of Radiation Oncology and Diagnostic Radiology in Tohoku University Hospital for support of the present study.
Footnotes
Sources of support: This work was supported in part by a grant from the Japan Society for the Promotion of Science (JSPS KAKENHI, grant number 26860973 and 24890018).
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.07.012.
Supplementary Materials
References
- 1.Aleman B.M., van den Belt-Dusebout A.W., De Bruin M.L. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 2.Lally B.E., Detterbeck F.C., Geiger A.M. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:911–917. doi: 10.1002/cncr.22845. [DOI] [PubMed] [Google Scholar]
- 3.Schellong G., Riepenhausen M., Bruch C. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55:1145–1152. doi: 10.1002/pbc.22664. [DOI] [PubMed] [Google Scholar]
- 4.Dess R.T., Sun Y., Matuszak M.M. Cardiac events after radiation therapy: Combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol. 2017;35:1395–1402. doi: 10.1200/JCO.2016.71.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 6.Wang K., Eblan M.J., Deal A.M. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hancock S.L., Tucker M.A., Hoppe R.T. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 8.Frandsen J., Boothe D., Gaffney D.K. Increased risk of death due to heart disease after radiotherapy for esophageal cancer. J Gastrointest Oncol. 2015;6:516–523. doi: 10.3978/j.issn.2078-6891.2015.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin S.H., Zhang N., Godby J. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer. 2016;122:917–928. doi: 10.1002/cncr.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariga H., Nemoto K., Miyazaki S. Prospective comparison of surgery alone and chemoradiotherapy with selective surgery in resectable squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2009;75:348–356. doi: 10.1016/j.ijrobp.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 11.Pottgen C., Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer: A meta-analysis of the randomized trials. Cancer Treat Rev. 2012;38:599–604. doi: 10.1016/j.ctrv.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Jingu K., Matsushita H., Takeda K. Results of chemoradiotherapy for stage I esophageal cancer in medically inoperable patients compared with results in operable patients. Dis Esophagus. 2013;26:522–527. doi: 10.1111/j.1442-2050.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 13.Marks L.B., Yu X., Prosnitz R.G. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Gayed I.W., Liu H.H., Yusuf S.W. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47:1756–1762. [PubMed] [Google Scholar]
- 15.Jingu K., Kaneta T., Nemoto K. The utility of 18F-fluorodeoxyglucose positron emission tomography for early diagnosis of radiation-induced myocardial damage. Int J Radiat Oncol Biol Phys. 2006;66:845–851. doi: 10.1016/j.ijrobp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Umezawa R., Takase K., Jingu K. Evaluation of radiation-induced myocardial damage using iodine-123 beta-methyl-iodophenyl pentadecanoic acid scintigraphy. J Radiat Res. 2013;54:882–889. doi: 10.1093/jrr/rrt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umezawa R., Takanami K., Kadoya N. Assessment of myocardial metabolic disorder associated with mediastinal radiotherapy for esophageal cancer -a pilot study. Radiat Oncol. 2015;10:96. doi: 10.1186/s13014-015-0410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takanami K., Arai A., Umezawa R. Association between radiation dose to the heart and myocardial fatty acid metabolic impairment due to chemoradiation-therapy: Prospective study using I-123 BMIPP SPECT/CT. Radiother Oncol. 2016;119:77–83. doi: 10.1016/j.radonc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Kim R.J., Wu E., Rafael A. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 20.Choudhury L., Mahrholdt H., Wagner A. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–2164. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 21.Wagner A., Mahrholdt H., Holly T.A. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: An imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 22.Umezawa R., Ota H., Takanami K. MRI findings of radiation-induced myocardial damage in patients with oesophageal cancer. Clin Radiol. 2014;69:1273–1279. doi: 10.1016/j.crad.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Pop M., Ghugre N.R., Ramanan V. Quantification of fibrosis in infarcted swine hearts by ex vivo late gadolinium-enhancement and diffusion-weighted MRI methods. Phys Med Biol. 2013;58:5009–5028. doi: 10.1088/0031-9155/58/15/5009. [DOI] [PubMed] [Google Scholar]
- 24.Hardenbergh P.H., Munley M.T., Bentel G.C. Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: Preliminary results. Int J Radiat Oncol Biol Phys. 2001;49:1023–1028. doi: 10.1016/s0360-3016(00)01531-5. [DOI] [PubMed] [Google Scholar]
- 25.Umezawa R., Jingu K., Matsushita H. Long-term results of chemoradiotherapy for stage II-III thoracic esophageal cancer in a single institution after 2000 -with a focus on comparison of three protocols. BMC Cancer. 2015;15:813. doi: 10.1186/s12885-015-1836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lancellotti P., Nkomo V.T., Badano L.P. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–1032. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Gladstone D.J., Flanagan M.F., Southworth J.B. Radiation-induced cardiomyopathy as a function of radiation beam gating to the cardiac cycle. Phys Med Biol. 2004;49:1475–1484. doi: 10.1088/0031-9155/49/8/007. [DOI] [PubMed] [Google Scholar]
- 29.Konski A., Li T., Christensen M. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol. 2012;104:72–77. doi: 10.1016/j.radonc.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradley J.D., Paulus R., Komaki R. Long-term results of NRG oncology RTOG 0617: Standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non–small-cell lung cancer. Lancet Oncol. 2015;16:187–199. doi: 10.1200/JCO.19.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seppenwoolde Y., Muller S.H., Theuws J.C. Radiation dose-effect relations and local recovery in perfusion for patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2000;47:681–690. doi: 10.1016/s0360-3016(00)00454-5. [DOI] [PubMed] [Google Scholar]
- 32.Darby S.C., Cutter D.J., Boerma M. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauk S., Kiszel Z., Buschmann J., Trott K.R. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–808. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 34.Erven K., Jurcut R., Weltens C. Acute radiation effects on cardiac function detected by strain rate imaging in breast cancer patients. Int J Radiat Oncol Biol Phys. 2011;79:1444–1451. doi: 10.1016/j.ijrobp.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Heggemann F., Grotz H., Welzel G. Cardiac function after multimodal breast cancer therapy assessed with functional magnetic resonance imaging and echocardiography imaging. Int J Radiat Oncol Biol Phys. 2015;93:836–844. doi: 10.1016/j.ijrobp.2015.07.2287. [DOI] [PubMed] [Google Scholar]
- 36.Jingu K., Nemoto K., Kaneta T. Temporal change in brain natriuretic peptide after radiotherapy for thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1417–1423. doi: 10.1016/j.ijrobp.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 37.van der Veen S.J., Ghobadi G., de Boer R.A. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 2015;114:96–103. doi: 10.1016/j.radonc.2014.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.