Abstract
Purpose
Radical surgery is the most important treatment modality in gastric cancer. Preoperative or postoperative radiation therapy (RT) and perioperative chemotherapy are the treatment options that should be added to surgery. This study aimed to evaluate the overall survival (OS) and recurrence patterns by machine learning in gastric cancer cases undergoing RT.
Methods and Materials
Between 2012 and 2019, the OS and recurrence patterns of 75 gastric cancer cases receiving RT ± chemotherapy at the Department of Radiation Oncology were evaluated by machine learning. Logistic regression, multilayer perceptron, XGBoost, support vector classification, random forest, and Gaussian Naive Bayes (GNB) algorithms were used to predict OS, hematogenous distant metastases, and peritoneal metastases. After the correlation analysis, the backward feature selection was performed as the variable selection method, and the variables with P values less than .005 were selected.
Results
Over the median 23-month follow-up, recurrence was seen in 33 cases, and 36 patients died. The median OS was 23 (min: 7; max: 82) months, and the disease-free survival was 18 (min: 5, max: 80) months. The most common recurrence pattern was hematogenous distant metastasis, followed by peritoneal metastasis. In this study, the most successful algorithms in the prediction of OS, distant metastases, and peritoneal metastases were found to be GNB with an accuracy of 81% (95% confidence interval [CI], 0.65-0.97, area under the curve [AUC]: 0.89), XGBoost with 86% accuracy (95% CI, 0.74-0.97, AUC: 0.86), and random forest with 97% accuracy (95% CI, 0.92-1.00, AUC: 0.97), respectively.
Conclusions
In gastric cancer, GNB, XGBoost, and random forest algorithms were determined to be the most successful algorithms for predicting OS, distant metastases, and peritoneal metastases, respectively. To determine the most accurate algorithm and perhaps make personalized treatments applicable, more precise machine learning studies are needed with an increased number of cases in the coming years.
Introduction
Gastric cancer is the fifth most common malignant cancer worldwide and the third leading cause of cancer-related deaths.1 Radical surgery with lymph node dissection is considered to be the most important treatment strategy for gastric carcinoma. However, even after radical resection, locoregional recurrence, peritoneal spread, or distant metastasis (DM) may be observed, leading to an unfavorable prognosis.2 The available treatment options are postoperative or preoperative radiation therapy (RT) plus chemotherapy (CT) and perioperative CT.3
In the literature, recurrence rates in gastric cancer vary between 14% and 60%.4,5 The most important prognostic factor is the size and spread of tumor. Lymph node metastasis is another important prognostic factor. Other factors include tumor grade and histopathology, surgical margin, tumor location, and patient performance.6
In radiation oncology, the most common questions are, “Which patients have the highest risk of toxicity?” and “What are the rates of local control and overall survival (OS)?” Although clinical trials are accepted as the gold standard to answer these questions, such studies are expensive and progress slowly. Creating models with important parameters using the available data will be useful in better planning future clinical studies.7 Evidence-based medicine relies on randomized controlled trials designed for a large patient population. However, increasing the number of clinical and biological parameters that need to be investigated makes it difficult to design specific studies. To achieve accurate results, it is important to integrate such a large and heterogeneous amount of data and produce the right models.8
Recently, there has been an increasing interest in the use of machine learning (ML) algorithms to predict RT results such as toxicity, survival, and recurrence patterns. The review of the literature shows that there is still no consensus on an optimal classification algorithm. Researchers select algorithms for various reasons, such as experience, frequency of use in the literature, data characteristics, and usability of applications.9
The current study aimed to predict the OS and recurrence patterns by ML in 75 patients who received diagnoses of gastric cancer undergoing RT ± CT and to determine the optimum algorithm for this purpose.
Methods and Materials
Patient characteristics
Between 2012 and 2019, 75 cases of patients with gastric cancer undergoing RT ± CT at the Department of Radiation Oncology of Eskisehir Osmangazi University Faculty of Medicine were retrospectively evaluated. Patients who received a histopathological diagnosis of a gastric adenocarcinoma without DM, who had regular follow-up, and who had a Karnofsky Performance Scale (KPS) score of ≥70 were included in the study. TNM staging was performed according to the eighth edition of the American Joint Committee on Cancer staging system. T1-2N0M0 cases were not included in the study. After the diagnosis process, all patients were evaluated by the oncology council at the university, and treatment decisions were made in a multidisciplinary manner. The diagnosis of recurrence and planning of postrecurrence treatment were also undertaken in a multidisciplinary way by the same council.
Treatment characteristics
The patients’ lymph node dissection status and resection type were determined from the surgical notes and pathology reports. RT was applied to all cases as an adjuvant after surgery. Considering the patients’ KPS score, age, and comorbidities, a concurrent CT evaluation was performed, and the CT chart was obtained. As concurrent CT, FUFA (fluorouracil 400 mg/m2 + leucovorin 20 mg/m2 on days 1-4) or capecitabine (825 mg/m2 throughout RT 7 days a week for 5 weeks) was used. During the treatment, at least once a week in the outpatient clinic, the cases were evaluated based on complete blood count and blood biochemistry tests and examination findings. They were also closely monitored in terms of toxicity and weight, and their nutrition was supported orally or intravenously.
All RT patients were immobilized with a T-bar/wingboard in the supine position with the arms up, and planning computed tomography was taken with a 5-mm cross section. The lung, heart, liver kidneys, esophagus, medulla spinalis, and small intestines were contoured as organs at risk. The target volume was determined according to the location of the tumor in preoperative examinations and planned to cover the whole stomach, anastomoses, regional lymph nodes (right and left gastric, right and left gastroepiploic, celiac, porta hepatis, subpyloric, paraortic, gastroduodenal, and subpancreatic) and tumor bed postoperatively. Splenic hilus lymphatics (in proximal tumors) and retropancreaticodoudenal lymphatics (in distal tumors) were included in the target volume according to location. The clinical target volume was created by applying a 1-cm margin to these regions. According to the RT technique, planning target volume margin was determined as 0.5 to 1 cm. The RT dose was determined according to the resection status, being applied as 45 Gy in cases with R0 resection and 50.4 to 54 Gy in those with R1 and R2 resections considering the doses indicated for organs at risk. RT was applied to the patients at a daily dose of 1.8 Gy.
Posttreatment follow-up
After the adjuvant treatments were completed, the initial follow-up of the cases was undertaken in the first month. Then, the follow-up visits were arranged every 3 months for 2 years, followed by every 6 months up to 5 years, and annually from there on. At each follow-up session, anamnesis was obtained and a physical examination was performed. The abdominal tomographies were obtained within 4 to 12 weeks after RT, followed by annual tomography follow-up. The endoscopic examination of the upper gastrointestinal tract was carried out annually for up to 5 years, and biopsies were taken from areas suspected of recurrence. A positron emission tomography scan was requested in cases with suspected recurrence according to computed tomography.
Machine learning
In this study, the ML methods of logistic regression, multilayer perceptron (MLP), XGBoost, support vector classification, random forest, and Gaussian Naive Bayes (GNB) were used in the assessment of the OS, DM, and peritoneal recurrence (PR) prediction. Logistic regression predicts the probability that a result will have only 2 values. Logistic regression produces a logistic curve limited to values between 0 and 1. Logistic regression is produced using the natural logarithm of the probabilities of the target variable.10 Artificial neural networks-MLP are developed based on the biological neural networks of the human brain and an information processing system designed to perform the functions of these networks. Artificial neural networks collect their knowledge by detecting patterns and relationships in the data and learn by experience.11 XGBoost is an application of gradient-supported decision trees designed for speed and performance and structured on classification and regression predictive modeling problems or dominates data sets in tabular form.12 Support vector machine (SVM) predicts and generalizes new data by learning on data with unknown distribution. Support vector classification is an SVM-based classifier which is capable of performing binary and multiclass classification on a data set.13 In the random forest classification method, owing to the different data and variables in each tree, there is no overcompliance problem. It can be used easily in cases where there is missing data and in very large data sets, and high success rates can be obtained.14 GNB is a common technique used to process continuous values and can significantly improve the accuracy of the classifier.15
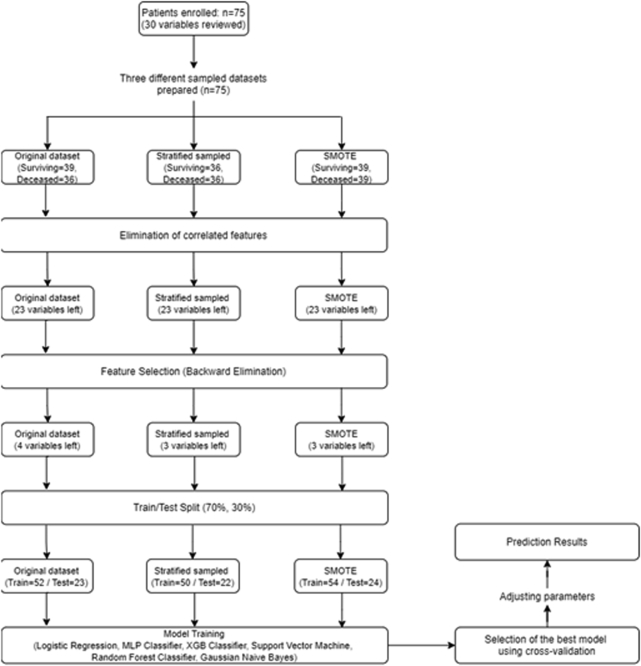
Age, sex, KPS score, tumor location, resection type, lymph node dissection type (D0/D1/D2), total number of lymph nodes removed, number of metastatic lymph nodes, lymph node ratio, T stage, N stage, TNM stage, tumor grade and size, lymphatic invasion, vascular invasion, perineural invasion, surgical margin (R0/R1/R2), neoadjuvant CT history, concurrent CT history, weight, body mass index, and pretreatment albumin, hemoglobin, neutrophil, lymphocyte, platelet, neutrophil/lymphocyte, and platelet/lymphocyte values were evaluated. The data set was divided into 2 parts: 70% for algorithm training and 30% for prediction. The models were constructed using the training set and validated using the prediction set. The optimal model was identified according to the receiver operating characteristic (ROC) curves.
In the data set, there was an imbalance concerning the number of cases for the prediction of the OS (mortality: 36, survival: 39), DM (present: 16, absent: 59), and PR (present: 13, absent: 62). In unbalanced data sets, the model performs predictions in favor of the group with a higher amount of data, leading to the problem of overfitting. In statistics, “overfitting” refers to a generated analysis being extremely adaptable to a certain data set (memorization), which causes an inability to adapt to new data that were not originally included in the data set. To overcome this situation, a balanced data set should be used.16 To create a balanced data set for the prediction of OS, stratified sampling was used. For the prediction of DM and PR, synthetic minority oversampling technique (SMOTE) was used.
Stratified sampling is the method of separating a population (data set) into homogeneous layers. It is used in cases where there are substrates or subunit groups in a data set with determined boundaries.17
In SMOTE, the type of class with unbalanced data distribution is replicated artificially to achieve a balance. In the prediction of DM and PR, the samples of the minority class type were replicated by SMOTE.18
Statistics and application
The confusion matrix contains information about real and predicted classifications performed by a classification system. The performance of such systems is generally evaluated using the data in the matrix.19
In the current study, the accuracy rate method was used. The accuracy method involves the calculation of the ratio of a system’s correctly classified instances (true positive and true negative) to the total number of instances. The error rate refers to the ratio of the number of incorrectly calculated instances (false positive and false negative) to the total number of instances.20 The success rates calculated using the confusion matrix are presented in Appendix E1.
An ROC curve is a graph showing the performance of a classification model at all classification thresholds. The area under the curve (AUC) represents the classification performance of the constructed model and takes a value between 0 and 1. An AUC value close to 1 means that the classification performance of the model is high.21
Statistical analyses and ML algorithms were carried out using Python software (Python Software Foundation. Python Language Reference, version 3.5. Available at http://www.python.org) and Scikit-Learn library.22 After correlation analysis in the variable selection process, the χ2 test was conducted for categorical variables and the backward test for noncategorical variables, and those with P values less than .05 were considered statistically significant. Wrapping methods evaluate the importance of each feature. The most notable wrapping methods are forward, backward, and stepwise selection. The backward selection starts with all the features in the data set. It then runs a model and calculates a P value associated with the model’s t test or F-test for each feature. The feature with the larger trivial P value is removed from the model and the process starts again. This continues until all features with insignificant P values are removed from the model.23
OS was also evaluated by traditional statistical methods. Kaplan–Meier test and Cox regression analyses were performed. P < .05 was considered statistically significant.
Results
Patient, tumor, and treatment characteristics
The median age was 60 years. The patient and tumor characteristics are summarized in Table 1. Concerning the surgical margin, the resection rates were 76%, 18.7%, and 5.3% for R0, R1, and R2, respectively. The median RT dose was 45 Gy. The treatment characteristics are summarized in Table 2.
Table 1.
Patient and tumor characteristics
| Variable | Number of patients (%)/(min-max) |
|---|---|
| Age | Median: 60 (22-78) |
| Sex | |
| Female | 16 (21.3) |
| Male | 59 (78.7) |
| Karnofsky Performance Scale score | Median: 90 (70-100) |
| Tumor location | |
| Proximal | 17 (22.7) |
| Middle | 25 (33.3) |
| Distal | 33 (44) |
| T stage | |
| Ia | 2 (2.7) |
| Ib | 1 (1.3) |
| II | 4 (5.3) |
| III | 43 (57.3) |
| IVa | 24 (32) |
| IVb | 1 (1.3) |
| N stage | |
| N0 | 11 (14.7) |
| N1 | 17 (22) |
| N2 | 21 (28) |
| N3a | 16 (21.3) |
| N3b | 10 (13.3) |
| TNM stage | |
| IB | 1 (1.3) |
| IIA | 10 (13.3) |
| IIB | 17 (22.7) |
| IIIA | 20 (26.7) |
| IIIB | 14 (18.7) |
| IIIC | 13 (17.3) |
| Tumor grade | |
| I (well-differentiated) | 6 (8) |
| II (moderately differentiated) | 31 (41.3) |
| III (poorly differentiated) | 38 (50.7) |
| Lymphatic invasion | |
| Positive | 47 (62.7) |
| Negative | 28 (37.7) |
| Vascular invasion | |
| Positive | 44 (58.7) |
| Negative | 31 (41.3) |
| Perineural invasion | |
| Positive | 46 (61.3) |
| Negative | 29 (38.7) |
| Tumor size, mm | Median: 55 (10-150) |
Table 2.
Treatment characteristics
| Clinical characteristics | Number of patients (%)/(min-max) |
|---|---|
| Radiation therapy dose, Gy | Median: 45 (45-54) |
| Resection type | |
| Total gastrectomy | 56 (74.4) |
| Subtotal gastrectomy | 19 (25.3) |
| Lymph node dissection | |
| D1 | 37 (49.3) |
| D2 | 38 (50.7) |
| Number of dissected lymph nodes | Median: 25 (6-82) |
| Number of metastatic lymph nodes | Median: 4 (0-52) |
| Number of metastatic lymph nodes/number of dissected lymph nodes | Median: 3 (0-5) |
| Surgical margin | |
| R0 | 57 (76) |
| R1 | 14 (18.7) |
| R2 | 4 (5.3) |
| Neoadjuvant chemotherapy | |
| Yes | 12 (16) |
| No | 63 (84) |
| Concurrent chemotherapy | |
| Yes | 63 (84) |
| No | 12 (16) |
| Concurrent chemotherapy regime | |
| FUFA | 38 (50.7) |
| Capecitabine | 25 (33.3) |
| None | 12 (16) |
Recurrence patterns
Over the median 23 months of follow-up, recurrence was seen in 33 cases, and 36 patients died. The median OS time was 23 (min: 7, max: 82) months, and the median disease-free survival time was 18 (min: 5 max 80) months. The most common recurrence pattern was hematogenous DM, followed by peritoneal metastasis. DM occurred in 16 cases and PR in 13 cases. The recurrence patterns are given in Figure 1.
Figure 1.
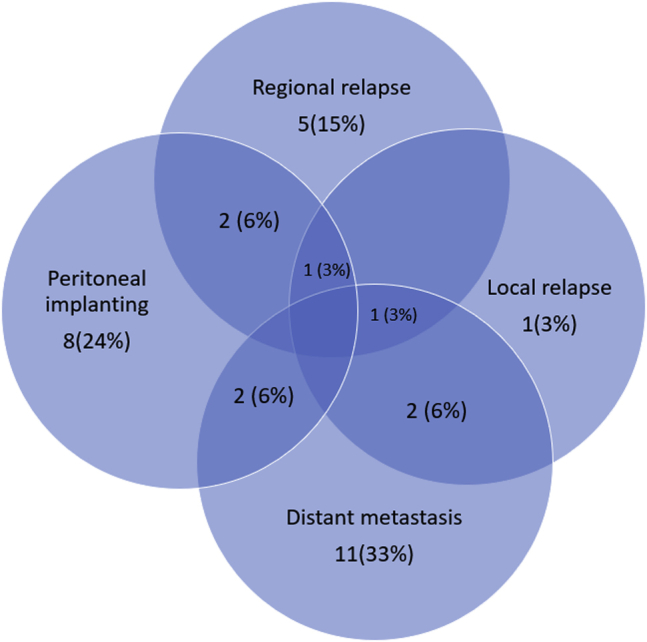
Recurrence patterns.
Machine learning algorithms
The ML workflow for OS prediction is presented in Appendix E2. Significant variables were determined as KPS score, resection type, and pretreatment platelet values for OS; age, sex, KPS score, tumor grade, tumor location, T stage, and N stage for DM; and KPS score, lymph node dissection type, tumor size, lymphatic invasion, pretreatment albumin and lymphocyte, tumor location, T stage, N stage, resection type, and concurrent CT for PR.
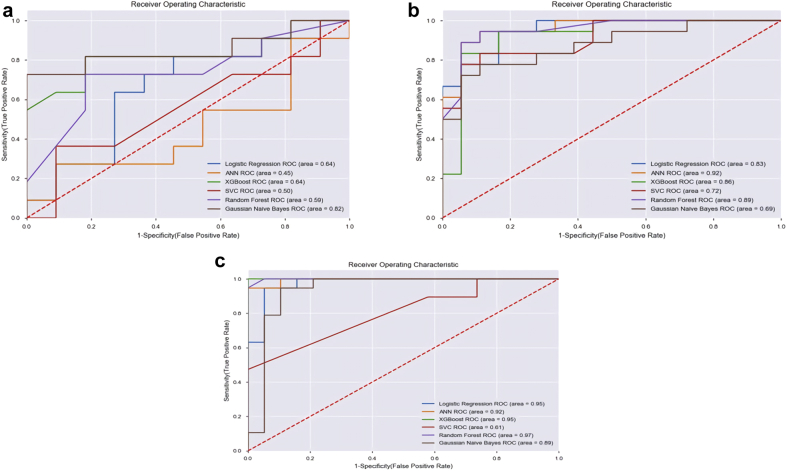
To create a balanced data set in the modeling of the OS, considering that 36 cases died, the data of 36 surviving cases were selected from the data set. Seventy percent of the cases (n = 51; 25 surviving, 26 deceased) were allocated to the training set and 30% (n = 21 cases; 11 surviving and 10 deceased) were used in the test. The best result for the prediction of OS was obtained from the GNB algorithm with an accuracy of 81.8% (95% CI, 0.65-0.97). The algorithm correctly predicted 9 out of 11 surviving cases and 9 out of 10 deceased cases (sensitivity: 81%, specificity: 81%, AUC: 0.89). The results of the evaluated algorithms in terms of the prediction of OS are given in Table 3, their confusion matrix in Table 4, and the ROC curve graph in Figure 2a.
Table 3.
Machine learning algorithms
| Algorithm | Accuracy rate |
Sensitivity |
Specificity |
AUC |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (CI) | ||||||||||||
| OS | DM | PR | OS | DM | PR | OS | DM | PR | OS | DM | PR | |
| Logistic regression | 0.63 | 0.83 | 0.94 | 0.71 | 0.80 | 0.94 | 0.60 | 0.87 | 0.94 | 0.64 | 0.83 | 0.95 |
| (0.43-0.93) | (0.71-0.95) | (0.87-1) | ||||||||||
| MLP | 0.45 | 0.91 | 0.92 | 0.46 | 0.89 | 0.94 | 0.42 | 0.94 | 0.90 | 0.45 | 0.92 | 0.92 |
| (0.24-0.66) | (0.82-1) | (0.83-1) | ||||||||||
| XGBoost | 0.63 | 0.86 | 0.94 | 0.71 | 0.93 | 1 | 0.60 | 0.80 | 0.90 | 0.64 | 0.86 | 0.95 |
| (0.43-0.83) | (0.74-0.97) | (0.87-1) | ||||||||||
| SVC | 0.50 | 0.72 | 0.60 | 0.50 | 0.83 | 0.55 | 0.60 | 0.66 | 1 | 0.50 | 0.72 | 0.61 |
| (0.29-0.70) | (0.57-0.86) | (0.45-0.76) | ||||||||||
| Random forest | 0.59 | 0.89 | 0.97 | 0.62 | 0.89 | 1 | 0.57 | 0.89 | 0.95 | 0.59 | 0.89 | 0.97 |
| (0.38-0.79) | (0.78-0.99) | (0.92-1) | ||||||||||
| GNB | 0.81 | 0.69 | 0.80 | 0.81 | 0.81 | 0.94 | 0.81 | 0.64 | 0.85 | 0.82 | 0.69 | 0.89 |
| (0.65-0.97) | (0.54-0.84) | (0.79-0.99) | ||||||||||
Abbreviations: AUC = area under the curve; CI = confidence interval; DM = distant metastasis; GNB = Gaussian Naive Bayes; MLP = multilayer perceptron; OS = overall survival; PR = peritoneal recurrence; SVC = support vector classification.
Table 4.
Confusion matrix for overall survival
| Outcome | Gaussian Naive Bayes |
||
|---|---|---|---|
| Surviving | Deceased | Accuracy, % | |
| Surviving | 9 | 2 | 81.8 |
| Deceased | 1 | 9 | 81.8 |
| Accuracy, % | 81.8 | ||
Figure 2.
Receiver operating characteristic curve graphs of (a) overall survival, (b) distant metastasis, and (c) peritoneal recurrence.
Owing to the presence of DM in 16 cases, the data were replicated with the SMOTE method. The data of a total of 118 cases (16 DM, 59 non-DM, and 43 SMOTE-replicated DM) were used. Eighty-two cases (41 DM and 41 non-DM) were allocated to the training set, and 36 (18 DM and 18 non-DM) were used in the test. The best result was obtained from the XGBoost algorithm with an accuracy rate of 86% (95% CI, 0.74-0.97). The algorithm correctly predicted 17 of 18 DM cases and 14 of 18 patients without DM (sensitivity: 93%, specificity: 80%, AUC: 0.86). The results of the evaluated algorithms are presented in Table 3, the confusion matrix in Table 5, and the ROC curve graph in Figure 2b.
Table 5.
Confusion matrix for distant metastasis
| Distant metastasis | XGBoost |
||
|---|---|---|---|
| Absent | Present | Accuracy, % | |
| Absent | 17 | 1 | 94 |
| Present | 2 | 16 | 89 |
| Accuracy, % | 91 | ||
Because there were 13 cases with PR, the data were replicated with the SMOTE method. The data belonging to a total of 124 cases (13 PR, 62 non-PR, and 49 SMOTE-replicated PR) were used. Eighty-six cases (43 PR and 43 non-PR) were used in training and 38 (19 PR and 19 non-PR) in the test. For the prediction of PR, the highest accuracy rate (97%) was seen in the random forest algorithm (95% CI, 0.92-1.00), which correctly predicted 18 of 19 PR cases and all 19 non-PR cases (sensitivity: 100%, specificity: 95%, AUC: 0.97). The results of the evaluated algorithms are shown in Table 3, the confusion matrix in Table 6, and the ROC curve chart in Figure 2c.
Table 6.
Confusion matrix for peritoneal recurrence
| Peritoneal recurrence | Random forest |
||
|---|---|---|---|
| Absent | Present | Accuracy, % | |
| Absent | 18 | 1 | 94 |
| Present | 0 | 19 | 100 |
| Accuracy, % | 97 | ||
Statistical analysis results
In terms of OS, univariate analysis showed that KPS, surgical margin, and neoadjuvant CT history were associated with OS. In turn, the multivariate analysis showed that KPS, total number of lymph nodes removed, number of metastatic lymph nodes, lymph node ratio, and neoadjuvant CT history were associated with OS. The P value of the pretreatment platelet variable remained at 0.072 (Table 7).
Table 7.
Cox regression analysis: overall survival
| Variables | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% CI | |
| KPS score | .031 | 0.965 | 0.935-0.997 | .004 | 0.944 | 0.908-1.000 |
| Total number of lymph nodes removed | .650 | 1.005 | 0.983-1.028 | .003 | 1.071 | 1.024-1.120 |
| Number of metastatic lymph nodes | .412 | 1.010 | 0.986-1.034 | .004 | 0.893 | 0.827-0.965 |
| Lymph node ratio | .105 | 2.257 | 0.843-6.042 | .002 | 54.151 | 4.500-651.567 |
| Surgical margin | .046 | 0.496 | 0.249-0.988 | .282 | 0.626 | 0.267-1.469 |
| Neoadjuvant CT history | .001 | 0.266 | 0.119-0.596 | .001 | 0.223 | 0.088-0.561 |
| Pretreatment platelet | .271 | 0.998 | 0.995-1.002 | .072 | 0.996 | 0.993-1.000 |
Bold indicates statistical significance.
Discussion
Despite multimodal treatments, recurrence rates in gastric cancer vary between 14% and 60%.4,5 The results of these treatments are difficult to compare due to different inclusion criteria, variations in treatment regimens, and imbalance in patient populations. Our results confirm those of previous studies showing that DM was the most common recurrence in gastric cancer cases after curative treatment (multimodal therapy).5,24 Spolverato et al reported that DM was seen in at least 3 of every 4 cases with recurrence.5
In cases with DM, survival is worse than in those with locoregional recurrence.25 If hematogenous or transperitoneal spread is present, oncological outcomes are poor despite treatment. Progressive tumor spread within or distant from gastric wall decreases survival rates.26 The size and spread of the tumor and the number of lymph nodes involved have also been associated with OS.27,28 In the current study, T stage and N stage were determined as important variables for hematogenous DM and PR. Recently, the relationship between inflammation and malignant tumors has been extensively investigated in many studies.29,30 Numerous researchers have shown that the inflammatory response, including neutrophil/lymphocyte ratio and platelet/lymphocyte ratio, is associated with poor prognosis in cancer.31,32 In addition, the number of platelets has been examined in terms of its prognostic role. It has been reported that increased platelet count or pretreatment thrombocytosis may be associated with poor prognosis in cancer.33, 34, 35 Similarly, in the current study, the number of platelets before treatment was among the important variables for the prediction of OS. KPS score is another important variable in predicting OS, DM, and PR. In previous studies on gastric cancer, KPS score was found to be associated with prognosis.36 Some studies suggested that gastric cardia tumors might have different epidemiologic factors and exhibited a different tumor biology than distal gastric cancers. In these studies, prognosis was found to be worse in cardiac lesions.37,38 In the current study, tumor location was found to be associated with DM, which is consistent with the literature.
In gastric cancer, postoperative RT and CT have a potential effect on locoregional control. Yang et al investigated the survival and recurrence patterns in cases undergoing adjuvant chemoradiotherapy after D2 dissection and reported that the most common recurrence pattern was PR in this patient group.39 In the current study, the type of lymph node dissection (D0/D1/D2) was also identified as an important variable in predicting PR. In a study conducted with 699 cases diagnosed with gastric cancer, Lee et al found lymphovascular invasion as a poor prognostic factor for recurrence-free survival,40 which is in agreement with our results.
To the best of our knowledge, there is no other study predicting OS prognosis and recurrence patterns in gastric cancer by ML. In the literature, ML algorithms are becoming more popular for the predicting of patients’ response to RT.41,42 However, there is currently no consensus on an optimal algorithm to predict RT results (eg, survival, treatment failure, and toxicity) by ML. Therefore, researchers select algorithms based on previous use in the literature, data characteristics and quality, usability of applications, and interpretability of models.9 As available studies on this subject increase, optimum algorithms can be determined. In the current study, the best algorithms with the highest accuracy for the prediction of OS, DM and PR were found to be GNB, XGBoost, and random forest, respectively. Significant variables were determined as KPS score, resection type, and pretreatment platelet values for OS; age, sex, KPS score, tumor grade, tumor location, T stage, and N stage for DM; and KPS score, lymph node dissection type, tumor size, lymphatic invasion, pretreatment albumin and lymphocyte, tumor location, T stage, N stage, surgical margin, and concurrent CT for PR.
There are studies in the literature that perform recurrence predictions by ML in cancer types other than gastric cancer. In their study with 86 oral cavity cancer cases, Exarchos et al used clinical, radiologic, tissue, and blood genomics. In the follow-up, recurrence was seen in 13 cases, and the most successful prediction with 100% accuracy was obtained from the Bayesian networks algorithm.43 In another recurrence prediction study conducted with 679 cases with a diagnosis of breast cancer, Kim et al reported that the SVM algorithm was the best algorithm with an accuracy rate of 89%.44 In other recurrence prediction studies on cervical and breast cancer, the SVM algorithm was identified as the best algorithm with an accuracy rate of 68% and 95%, respectively.45,46 The current study has a disadvantage of low number of patients. In future work, it is planned to evaluate the accuracy rates of ML algorithms by increasing the number of cases.
With each passing day, the parameters that need to be evaluated in clinical studies are increasing. The growth and sharing of data, increased computing power, and developments in ML have initiated a transformation in health care. Advances in radiation oncology have generated substantial data that must be integrated with the data obtained from computed tomography imaging, dosimetry, and other modalities performed before each fraction. Integrating such large and heterogeneous data results in a problem that needs to be overcome to produce the right models. Using ML, the right models can be created with appropriate algorithms that can guide treatment and increase the workflow efficiency based on existing big data.
The use of ML techniques for the prediction of response and survival in RT patients offers an important opportunity to further improve decision support systems and provide an objective assessment of the relative benefits of various treatment options for each case. By determining certain factors by ML and using related algorithms, the concept personalized treatment can finally be implemented.
Conclusions
According to the results, 82% success rate in OS estimation was achieved with GNB algorithm, 92% success rate in DM estimation was achieved with MLP algorithm, and 97% success rate in peritoneal recurrence estimation with random forest algorithm. Algorithm performances vary according to the data structures. To improve long-term prognosis, it is important to predict the overall survival and recurrence patterns of patients receiving multimodal treatment with a diagnosis of gastric cancer. With the evaluation of clinical, radiologic, genetic, dosimetric, and epidemiologic factors using ML, it is possible to perform accurate predictions to achieve personalized treatment. Further ML studies with a larger number of patients are needed to determine the optimum algorithm and support the decision-making process for personalized treatment.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: The authors declare they have no conflict of interest.
All data generated and analyzed during this study are included in this article (and its supplementary information files).
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.07.007.
Supplementary Data
Appendix E1.
Appendix E2.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2017;70:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chang J.S., Lim J.S., Noh S.H. Patterns of regional recurrence after curative D2 resection for stage III (N3) gastric cancer: Implications for postoperative radiotherapy. Radiother Oncol. 2012;104:367–373. doi: 10.1016/j.radonc.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson L.L., Tepper J.E. 4th ed. Elsevier; 2016. Clinical Radiation Oncology. [Google Scholar]
- 4.Seyfried F., von Rahden B.H., Miras A.D. Incidence, time course and independent risk factors for metachronous peritoneal carcinomatosis of gastric origin—a longitudinal experience from a prospectively collected database of 1108 patients. BMC Cancer. 2015;15:73. doi: 10.1186/s12885-015-1081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spolverato G., Ejaz A., Kim Y. Rates and patterns of recurrence after curative intent resection for gastric cancer: A United States multi-institutional analysis. J Am Coll Surg. 2014;219:664–675. doi: 10.1016/j.jamcollsurg.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 6.Yu C.C., Levison D.A., Dunn J.A. Pathological prognostic factors in the second British Stomach Cancer Group trial of adjuvant therapy in resec;006C gastric cancer. Br J Cancer. 1995;71:1106–1110. doi: 10.1038/bjc.1995.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang J., Schwartz R., Flicking J. Machine learning approaches for predicting radiation therapy outcomes: A clinician’s perspective. Int J Radiation Oncol Biol Phys. 2015;93:1127–1135. doi: 10.1016/j.ijrobp.2015.07.2286. [DOI] [PubMed] [Google Scholar]
- 8.Chen C., He M., Zhu Y. Five critical elements to ensure the precision medicine. Cancer Metastasis Rev. 2015;34:313–318. doi: 10.1007/s10555-015-9555-3. [DOI] [PubMed] [Google Scholar]
- 9.Deist T.M., Dankers F.J., Valdes G. Machine learning algorithms for outcome prediction in (chemo)radiotherapy: An empirical comparison of classifiers. Med Phys. 2018;45:3449–3459. doi: 10.1002/mp.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osmanoglu U.O., Atak O.N., Caglar K., Kayhan H., Can T.C. Sentiment analysis for distance education course materials: A machine learning approach. J Educ Technol Online Learning. 2020;3:31–48. [Google Scholar]
- 11.Beale H.D., Demuth H.B., Hagan M.T. PWS Publishing Co.; Boston: 1996. Neural Network Design. [Google Scholar]
- 12.Chen T., Guestrin C. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (KDD ’16) Association for Computing Machinery; New York, NY: 2016. XGBoost: A scalable tree boosting system; pp. 785–794. [Google Scholar]
- 13.Suykens J.A., Vandewalle J. Least squares support vector machine classifiers. Neural Processing Letters. 1999;9:293–300. [Google Scholar]
- 14.Breiman L. Manual-setting up, using, and understanding random forests. https://www.stat.berkeley.edu/∼breiman/Using_random_forests_V3.1.pdf Available at:
- 15.John G.H., Langley P. arXiv preprint; 2013. Estimating continuous distributions in Bayesian classifiers. [Google Scholar]
- 16.Sun Y., Kamel M.S., Wong A.K. Cost-sensitive boosting for classification of imbalanced data. Pattern Recognition. 2007;40:3358–3378. [Google Scholar]
- 17.Kirkwood B.R., Sterne J.A.C. Essential Medical Statistics. 2nd ed. Blackwell Science; Hoboken, NJ: 2003. Linking analysis to study design: summary of methods; pp. 409–410. [Google Scholar]
- 18.Han H, Wang WY, Mao BH. Borderline-SMOTE: A new over-sampling method in imbalanced data sets learning. In: Huang DS, Zhang XP, Huang GB, eds. Advances in Intelligent Computing. ICIC 2005. Lecture Notes in Computer Science. Berlin, Heidelberg: Springer; 2005:3644.
- 19.Provost F., Kohavi R. Glossary of terms. Mach Lear. 1998;30:271–274. [Google Scholar]
- 20.Powers D. Evaluation: from precision, recall and F-measure to ROC, informedness, markedness and correlation. J Mach Learn Technol. 2011:2229–3981. [Google Scholar]
- 21.Celik O., Osmanoglu U.O. Comparing to techniques used in customer churn analysis. J Multidiscip Dev. 2019;4:30–38. [Google Scholar]
- 22.Pedregosa F., Varoquaux G., Gramfort A. Scikit-learn: Machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 23.Ozer C. Examination of feature selection methods and an application. Am J Eng Res. 2020;9:33–40. [Google Scholar]
- 24.D’Angelica M., Gonen M., Brennan M.F. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokadem I., Dijksterhuis W.P.M., Putten M. Recurrence after preoperative chemotherapy and surgery for gastric adenocarcinoma: A multicenter study. Gastric Cancer. 2019;22:1263–1273. doi: 10.1007/s10120-019-00956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers W.C., Damiano R.J., Jr., Rotolo F.S. Adenocarcinoma of the stomach. Changing patterns over the last 4 decades. Ann Surg. 1987;205:1–8. doi: 10.1097/00000658-198701000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunderson L.L., Sosin H. Adenocarcinoma of the stomach: Areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 28.Nagatomo T., Murakami E., Kondo K. Histologic criteria of serosal rupture and prognosis in gastric carcinoma. Cancer. 1972;29:180–190. doi: 10.1002/1097-0142(197201)29:1<180::aid-cncr2820290128>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Li Q.Q., Lu Z.H., Yang L. Neutrophil count and the inflammation-based Glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945–950. doi: 10.7314/apjcp.2014.15.2.945. [DOI] [PubMed] [Google Scholar]
- 30.Mimatsu K., Mimatsu K., Fukino N. Utility of inflammatory marker- and nutritional status-based prognostic factors for predicting the prognosis of stage IV gastric cancer patients undergoing non-curative surgery. Anticancer Res. 2017;37:4215–4222. doi: 10.21873/anticanres.11812. [DOI] [PubMed] [Google Scholar]
- 31.Min G.T., Wang Y.H., Yao N. The prognostic role of pretreatment platelet-to-lymphocyte ratio as predictors in patients with colorectal cancer: A meta-analysis. Biomarkers Med. 2017;11:87–97. doi: 10.2217/bmm-2016-0181. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z.G., Ye C.J., Liu L.X. The pretransplant neutrophil-lymphocyte ratio as a new prognostic predictor after liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. Biomarkers Med. 2018;12:189–199. doi: 10.2217/bmm-2017-0307. [DOI] [PubMed] [Google Scholar]
- 33.Al-Saeed E.F., Tunio M.A., Al-Obaid O. Correlation of pretreatment hemoglobin and platelet counts with clinicopathological features in colorectal cancer in Saudi population. Saudi J Gastroenterol. 2014;20:134–138. doi: 10.4103/1319-3767.129479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai K., Kitayama J., Tsuno N.H. Thrombocytosis before pre-operative chemoradiotherapy predicts poor response and shorter local recurrence-free survival in rectal cancer. Int. J. Colorectal Dis. 2013;28:527–535. doi: 10.1007/s00384-012-1594-4. [DOI] [PubMed] [Google Scholar]
- 35.Kim H.J., Choi G.S., Park J.S. Clinical significance of thrombocytosis before preoperative chemoradiotherapy in rectal cancer: Predicting pathologic tumor response and oncologic outcome. Ann Surg Oncol. 2015;22:513–519. doi: 10.1245/s10434-014-3988-8. [DOI] [PubMed] [Google Scholar]
- 36.Chau I., Norman A.R., Cunningham D. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 37.Fein R., Kelsen D.P., Geller N. Adenocarcinoma of the esophagus and gastroesophageal junction: Prognostic factors and results of therapy. Cancer. 1985;56:2512–2518. doi: 10.1002/1097-0142(19851115)56:10<2512::aid-cncr2820561032>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Hartley L.C., Evans E., Windsor C.J. Factors influencing prognosis in gastric cancer. Aust NZ J Surg. 1987;57:5–9. doi: 10.1111/j.1445-2197.1987.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang W., Hu R., Li G.C. Survival outcomes and patterns of failure after D2 dissection and adjuvant chemoradiotherapy for locally advanced gastric cancer: a retrospective study. Br J Radiol. 2018;91 doi: 10.1259/bjr.20170594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J.H., Kim M.G., Jung M.S. Prognostic significance of lymphovascular invasion in node-negative gastric cancer. World J Surg. 2015;39:732–739. doi: 10.1007/s00268-014-2846-y. [DOI] [PubMed] [Google Scholar]
- 41.Lambin P., van Stiphout R.G.P.M., Starmans M.H.W. Predicting outcomes in radiation oncology—multifactorial decision support systems. Nat Rev Clin Oncol. 2013;10:27–40. doi: 10.1038/nrclinonc.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambin P., Roelofs E., Reymen B. Rapid learning health care in oncology – an approach towards decision support systems enabling customised radiotherapy. Radiother Oncol. 2013;109:159–164. doi: 10.1016/j.radonc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Exarchos K.P., Goletsis Y., Fotiadis D.I. Multiparametric decision support system for the prediction of oral cancer reoccurrence. IEEE Trans Inf Technol Biomed. 2012;16:1127–1134. doi: 10.1109/TITB.2011.2165076. [DOI] [PubMed] [Google Scholar]
- 44.Kim W., Kim K.S., Lee J.E. Development of novel breast cancer recurrence prediction model using support vector machine. J Breast Cancer. 2012;15:230–238. doi: 10.4048/jbc.2012.15.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseng C.-J., Lu C.-J., Chang C.-C. Application of machine learning to predict the recurrence-proneness for cervical cancer. Neural Comput Applic. 2014;24:1311–1316. [Google Scholar]
- 46.Eshlaghy A.T., Poorebrahimi A., Ebrahimi M. Using three machine learning techniques for predicting breast cancer recurrence. J Health Med Inform. 2013;4:124. [Google Scholar]