Summary
Background
Seasonal malaria chemoprevention (SMC) aims to prevent malaria in children during the high malaria transmission season. The Achieving Catalytic Expansion of SMC in the Sahel (ACCESS-SMC) project sought to remove barriers to the scale-up of SMC in seven countries in 2015 and 2016. We evaluated the project, including coverage, effectiveness of the intervention, safety, feasibility, drug resistance, and cost-effectiveness.
Methods
For this observational study, we collected data on the delivery, effectiveness, safety, influence on drug resistance, costs of delivery, impact on malaria incidence and mortality, and cost-effectiveness of SMC, during its administration for 4 months each year (2015 and 2016) to children younger than 5 years, in Burkina Faso, Chad, The Gambia, Guinea, Mali, Niger, and Nigeria. SMC was administered monthly by community health workers who visited door-to-door. Drug administration was monitored via tally sheets and via household cluster-sample coverage surveys. Pharmacovigilance was based on targeted spontaneous reporting and monitoring systems were strengthened. Molecular markers of resistance to sulfadoxine–pyrimethamine and amodiaquine in the general population before and 2 years after SMC introduction was assessed from community surveys. Effectiveness of monthly SMC treatments was measured in case-control studies that compared receipt of SMC between patients with confirmed malaria and neighbourhood-matched community controls eligible to receive SMC. Impact on incidence and mortality was assessed from confirmed outpatient cases, hospital admissions, and deaths associated with malaria, as reported in national health management information systems in Burkina Faso and The Gambia, and from data from selected outpatient facilities (all countries). Provider costs of SMC were estimated from financial costs, costs of health-care staff time, and volunteer opportunity costs, and cost-effectiveness ratios were calculated as the total cost of SMC in each country divided by the predicted number of cases averted.
Findings
12 467 933 monthly SMC treatments were administered in 2015 to a target population of 3 650 455 children, and 25 117 480 were administered in 2016 to a target population of 7 551 491. In 2015, among eligible children, mean coverage per month was 76·4% (95% CI 74·0–78·8), and 54·5% children (95% CI 50·4–58·7) received all four treatments. Similar coverage was achieved in 2016 (74·8% [72·2–77·3] treated per month and 53·0% [48·5–57·4] treated four times). In 779 individual case safety reports over 2015–16, 36 serious adverse drug reactions were reported (one child with rash, two with fever, 31 with gastrointestinal disorders, one with extrapyramidal syndrome, and one with Quincke's oedema). No cases of severe skin reactions (Stevens-Johnson or Lyell syndrome) were reported. SMC treatment was associated with a protective effectiveness of 88·2% (95% CI 78·7–93·4) over 28 days in case-control studies (2185 cases of confirmed malaria and 4370 controls). In Burkina Faso and The Gambia, implementation of SMC was associated with reductions in the number of malaria deaths in hospital during the high transmission period, of 42·4% (95% CI 5·9 to 64·7) in Burkina Faso and 56·6% (28·9 to 73·5) in The Gambia. Over 2015–16, the estimated reduction in confirmed malaria cases at outpatient clinics during the high transmission period in the seven countries ranged from 25·5% (95% CI 6·1 to 40·9) in Nigeria to 55·2% (42·0 to 65·3) in The Gambia. Molecular markers of resistance occurred at low frequencies. In individuals aged 10–30 years without SMC, the combined mutations associated with resistance to amodiaquine (pfcrt CVIET haplotype and pfmdr1 mutations [86Tyr and 184Tyr]) had a prevalence of 0·7% (95% CI 0·4–1·2) in 2016 and 0·4% (0·1–0·8) in 2018 (prevalence ratio 0·5 [95% CI 0·2–1·2]), and the quintuple mutation associated with resistance to sulfadoxine–pyrimethamine (triple mutation in pfdhfr and pfdhps mutations [437Gly and 540Glu]) had a prevalence of 0·2% (0·1–0·5) in 2016 and 1·0% (0·6–1·6) in 2018 (prevalence ratio 4·8 [1·7–13·7]). The weighted average economic cost of administering four monthly SMC treatments was US$3·63 per child.
Interpretation
SMC at scale was effective in preventing morbidity and mortality from malaria. Serious adverse reactions were rarely reported. Coverage varied, with some areas consistently achieving high levels via door-to-door campaigns. Markers of resistance to sulfadoxine–pyrimethamine and amodiaquine remained uncommon, but with some selection for resistance to sulfadoxine–pyrimethamine, and the situation needs to be carefully monitored. These findings should support efforts to ensure high levels of SMC coverage in west and central Africa.
Funding
Unitaid.
Introduction
In the sub-Sahel region, from southern Senegal and northern Guinea to Chad and northern Cameroon, most malaria morbidity and mortality occurs during and immediately after a short rainy season. Seasonal malaria chemoprevention (SMC), whereby antimalarial sulfadoxine–pyrimethamine plus amodiaquine (SP + AQ) are administered once a month to prevent malaria,1, 2, 3, 4, 5, 6, 7, 8, 9 has been welcomed as a new tool, offering a high degree of personal protection at moderate cost. Following endorsement of SMC by WHO in 2012,10, 11, 12 countries have been quick to include SMC in their strategic plans for malaria control. Small-scale pilot schemes showed preliminary evidence of effectiveness13, 14, 15 and by 2014, eight countries had SMC programmes, reaching about 2·5 million children (appendix p 2), but insufficient funding and poor supplies of quality-assured drugs for SMC hindered further scale-up. The Unitaid-funded project, Achieving Catalytic Expansion of SMC in the Sahel (ACCESS-SMC), sought to remove barriers to scale-up. ACCESS-SMC implemented SMC on a large scale in seven countries, to create demand and influence the market for SMC drugs in terms of manufacturing capacity and prices, and to evaluate effectiveness of the intervention to address concerns about safety, feasibility, and drug resistance. SMC was delivered to a target population of about 3·6 million children (aged 3–59 months) in Burkina Faso, Chad, The Gambia, Guinea, Mali, Niger, and Nigeria in 2015, and about 7·6 million children in 2016 (appendix pp 3–4). From 2017, SMC delivery was continued by national programmes in all seven countries, and ACCESS-SMC continued in parts of Burkina Faso, Chad, and Nigeria not covered by national programmes. The project aimed to evaluate the safety and effectiveness of SMC at scale, the costs of delivery, cost-effectiveness, and effects on drug resistance, during 2015 and 2016. The current paper summarises the results of this evaluation.
Methods
Study design
The present observational study collected data on the delivery, effectiveness and safety of monthly treatments, influence on drug resistance, costs of delivery, impact on malaria incidence and mortality, and cost-effectiveness of SMC, during its administration for 4 months each year to children younger than 5 years, in Burkina Faso, Chad, The Gambia, Guinea, Mali, Niger, and Nigeria in 2015 and 2016. Children eligible to receive SMC were those aged between 3 and 59 months at the time of the first monthly treatment of the year (eligible to receive four treatments), and in a particular month, those aged at least 3 months and younger than 5 years at the time of the first cycle. Additionally, eligible children were those who were not unwell, were not known to have allergies to SMC drugs, and had not taken amodiaquine, sulfadoxine–pyrimethamine, or sulfa-containing antibiotics, in the previous 4 weeks. SMC delivery was monitored by distribution teams and assessed independently by household surveys. The effectiveness of SMC treatments in preventing malaria was measured with case-control studies. The effect of SMC introduction on the number of confirmed malaria cases at health facilities was assessed from cases reported in national health management information systems (HMIS) databases, and individual patient data collected from selected clinics, before and during SMC introduction. National pharmacovigilance was strengthened in all countries. In addition, in part of the implementation area in Nigeria, a cohort of children was monitored for illness symptoms. Large-scale surveys were used to measure the frequency of molecular markers of resistance to SMC drugs before and after two years of SMC at scale. Costs of SMC delivery, and cost-effectiveness, were estimated in each country. The timing of the various substudies is shown in the appendix (p 2).
Research in context.
Evidence before this study
We searched for publications with the search terms “seasonal malaria chemoprevention” (published since Jan 1, 2012) or “IPTc” or “intermittent preventive treatment in children” (published before Jan 1, 2012). Clinical trials done between 2002 and 2009 showed that intermittent preventive treatment for malaria in children younger than 5 years during the transmission season substantially reduced the incidence of malaria. An effectiveness study in Senegal of monthly seasonal malaria chemoprevention (SMC) with sulfadoxine–pyrimethamine plus amodiaquine over three years (2008–11) showed that SMC reduced the incidence of malaria by 60%, and hospital admissions for malaria by 45% during the transmission season. Following WHO policy recommendations for SMC in 2012, pilot schemes by Médecins Sans Frontières provided early evidence of effectiveness, and surveys have shown reductions in prevalence of infection and anaemia following implementation of SMC. Cost-effectiveness has also been studied in Ghana. However, questions remained about the feasibility of implementing SMC effectively on a large scale, the risk of serious adverse drug reactions, the ability of SMC to prevent deaths from malaria, and the effects of widespread use on selection for resistance. There were also substantial barriers to access due to shortages of quality-assured drugs, increased cost of drugs, and the absence of child-friendly formulations. By 2014, although many countries had included SMC in their strategic plans for malaria control, SMC was available for only about 10% of eligible children.
Added value of this study
The Achieving Catalytic Expansion of SMC in the Sahel (ACCESS-SMC) project was undertaken to improve the availability of SMC drugs and evaluate the effectiveness of SMC at scale.This study showed that high, equitable coverage was achieved overall, but coverage varied, with some countries achieving better coverage than others. Each monthly treatment provided a high degree of protection for 4 weeks. Molecular monitoring showed that drug resistant infections are uncommon, but some selection for resistance to sulfadoxine–pyrimethamine occurred. Serious side-effects were rare. SMC cost US$3·63 per child per year, and was highly cost-effective. The number of malaria cases at outpatient clinics and the number of deaths from malaria in hospital were substantially reduced when SMC was introduced.
Implications of all the available evidence
Despite the complexity of delivery, SMC has proved highly effective. 13 countries now have SMC programmes, which reached about 22 million children in 2019. Drug resistance is a threat, and continued molecular monitoring is needed to provide early warning of loss of effectiveness, and pharmacovigilance needs to be strengthened. SMC does not provide complete protection and additional measures are still needed, but its impact could be increased by optimising delivery, and, in some areas, increasing the number of monthly cycles to ensure children are protected throughout the high-risk period.
The protocol covering all substudies was approved by the research ethics committee of London School of Hygiene & Tropical Medicine (London, UK) and by ethics committees in each participating country. Signed consent was obtained from caregivers for participation in the surveys and case-control studies after explaining aims and procedures with a standard script.
Drugs
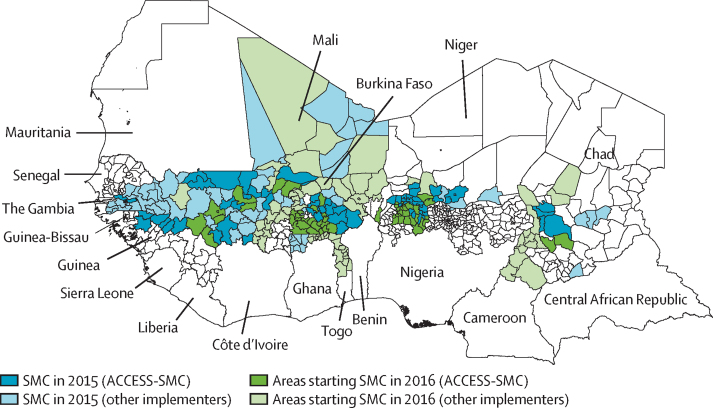
Infant and child co-blister packs of SP+AQ were manufactured by Guilin Pharmaceutical (Shanghai, China; 1 tablet of sulfadoxine–pyrimethamine 500 mg–25 mg and 3 tablets of amodiaquine 150 mg for children aged 12–59 months, and half-strength tablets for infants aged 3–11 months). In 2015, non-dispersible tablets were used. From Feb 7, 2016, dispersible tablets became available, and 80% of tablets used for SMC in 2016 were dispersible (unpublished data). Due to an international shortage of sulfadoxine in 2014, only half of the planned quantity of drugs could be procured for 2015, and implementation was therefore limited to half of the planned distribution areas in 2015, expanding to all planned areas in 2016 when manufacturing capacity had increased to meet demand. SMC implementation areas and estimates of populations reached are provided in figure 1.
Figure 1.
SMC implementation areas
Areas where SMC was implemented in 2015 are shown in blue, via the ACCESS-SMC project (dark blue) and other implementers (light blue). These areas continued SMC in 2016. Green shading shows the additional areas where SMC started in 2016, via the ACCESS SMC project (dark green) and other implementers (light green). In 2015, SMC programmes in nine countries reached about 7 million children, 3 million of them via ACCESS-SMC, and in 2016, programmes in 12 countries reached about 15 million children, 7 million via ACCESS-SMC (appendix p 3).16 SMC=seasonal malaria chemoprevention. ACCESS-SMC=Achieving Catalytic Expansion of SMC in the Sahel.
SMC delivery and coverage
A cascade model was used to train community drug distributors (volunteer distributors or community health workers [CHWs], referred to jointly as CHWs in this Article) and health facility staff to administer SMC drugs safely and to recognise and report adverse reactions. The job-aids used to help recognise adverse drug reactions and the training manual for SMC delivery (in English) are included in the appendix(pp 38, 43). Social mobilisation, via local announcements with criers, radio, and other local communication channels, explained the programme and notified communities, days in advance, of the dates of each monthly SMC campaign. SMC was delivered primarily door-to-door, supplemented by making treatment available at health facilities and at other fixed distribution points. CHWs administered sulfadoxine–pyrimethamine and the first dose of amodiaquine, leaving the blister pack with the caregiver to administer the remaining two doses of amodiaquine on each of the next 2 days. Children who were unwell were referred for assessment at the nearest health facility and could then receive SMC if they did not have malaria. In Mali, SMC was distributed by mobile teams from a central location in each community. These teams were equipped with malaria rapid diagnostic tests, artemisinin combination treatment, and other basic medicines in addition to SMC drugs so that they could do a rapid diagnostic test on any children who were unwell and treat them appropriately without the need for referral. Delivery exclusively at fixed points was used in urban areas in Niger in 2015, but this approach was replaced by door-to-door delivery in 2016. CHWs working in pairs for 4–5 days each month treated about 50 children per pair per day, recording treatments on record cards held by the caregiver, in a village register, and on a tally sheet held by the CHWs (or, in The Gambia only, a QR code on the child's SMC card was scanned with a smartphone and the data uploaded to a database via the Evaluate platform. At the end of each monthly campaign, tally sheets or database records were collated at health facilities and the number of treatments administered reported to the coordinating office of each country.
To assess SMC coverage, household cluster-sample surveys were done in each country at the end of each malaria transmission season (appendix p 4). Details of the survey sampling design are given in the appendix(p 7). Dates of treatments were noted from the SMCcard and caregivers were asked about monthly SMC treatments received, to determine the mean percentage of children who received SMC each month, and the percentages who received 0, 1, 2, 3, and 4 treatments, estimated with 95% CIs, using a survey-weighted ratio estimator in Stata (version 15). A questionnaire was used to ask caregivers about use of bednets, adherence to the daily SMC doses in the most recent month, awareness of SMC campaign dates in advance, knowledge about SMC, and ownership of household assets as a measure of wealth.
Treatment effectiveness
Effectiveness of SMC treatments (in terms of the percentage reduction in clinical malaria incidence in the 28 days and 29–42 days after administration of the first daily dose of SMC each month) was estimated in five countries during the 2015 (The Gambia and Mali) and 2016 (Burkina Faso, Chad, The Gambia, Mali, and Nigeria) transmission seasons with case-control studies. Cases were children aged 3–59 months presenting at health facilities with documented fever and microscopically confirmed asexual Plasmodium falciparum parasitaemia. The children were visited at home by fieldworkers where SMC record cards were inspected and caregivers asked about SMC treatments, adherence, and potential confounding factors including bednet use, caregiver education, and socio-economic status. The same information was collected for controls (two for each case), who were children from the same neighbourhood who were eligible to have received SMC. Further details and analysis methods are given in the appendix (pp 11–12).
Safety monitoring
Pharmacovigilance was based on targeted spontaneous reporting17 at health facilities, with a focus on known severe reactions to SMC drugs: severe skin reactions, liver disease, extrapyramidal syndrome, anaphylactic shock, and severe vomiting. Information on signs and symptoms was developed and distributed, with reporting forms, to health facilities (appendix pp 10–11). To assess the frequency of adverse reactions that did not lead to a health worker visit or health facility visit, CHWs administering SMC in three wards in Nigeria (two wards where there were four cycles of SMC and one ward where there was three cycles of SMC) to a cohort of about 10 000 children asked each caregiver about any illness symptoms in the child after administration of SP + AQ in the previous month using a symptom questionnaire. Individual case safety reports were entered into VigiFlow and submitted to VigiBase (WHO's individual case safety report database system). A committee was convened by WHO to review reports of serious adverse events associated with SMC, to provide advice about safety monitoring for SMC, and to report their findings to the WHO Advisory Committee on the Safety of Medicines and Medicinal Products. Individual case safety reports associated with SMC for the years 2015 and 2016, extracted from Vigibase on April 18, 2017, and additional case reports received by project teams which had not been submitted to Vigibase, were analysed.
Drug resistance
The prevalence of molecular markers of resistance to sulfadoxine–pyrimethamine and amodiaquine was measured in one second-level administrative area (district or equivalent area) per country, in representative household cluster-sample surveys of the district population in 2016 and 2018. Surveys were designed to have at least 90% power to detect an odds ratio for a change in marker prevalence over 2 years of 1·4. Districts were chosen that included a site used for routine monitoring efficacy of first-line antimalarials, and that were starting SMC in 2016, except for The Gambia where SMC had started in all eligible areas in 2014. The full survey design is provided in the appendix (pp 12–14). In 2016 and 2018, in each country per year, finger-prick blood samples on filter paper were taken from about 2000 children younger than 5 years and 2000 individuals between age 10 and 30 years, and shipped to the London School of Hygiene & Tropical Medicine (Department of Infection Biology) where samples positive for P falciparum by PCR were resistance-typed. DNA was extracted with a robotic platform, P falciparum chloroquine resistance transporter (pfcrt) genotyping was done via real-time PCR with hydrolysis probes, and direct sequencing was used for P falciparum multidrug resistance 1 (pfmdr1), dihydrofolate reductase (pfdhfr), and dihydropteroate synthetase (pfdhps) markers. For resistance mutations, prevalence each year was estimated with a ratio estimator, and the fold increase in prevalence and 95% CIs were estimated by survey Poisson regression in Stata software (version 15; appendix p 13). We primarily present mutation prevalence in the older age group (10–30 years), who had not received SMC and therefore reflected the trend in the circulating parasite population.
Impact on malaria rates
The reduction in the number of malaria outpatient cases, inpatient cases, and malaria deaths in hospital in children younger than 5 years associated with introduction of SMC, according to cases reported in HMIS databases and individual patient data, was estimated with a difference-in-differences approach (appendix pp 19–21). Individuals aged 5 years and older were the control age group, with data on the same age groups in areas that did not introduce SMC as additional controls. Poisson regression models were fitted to the data on numbers of cases before and during the intervention period in Stata. This approach corrected for changes in testing rates and use of insecticide-treated bednets, which increased in some countries during the study period but changed similarly in both age groups, and for the effect of removal of patient charges in Burkina Faso from 2016 (appendix pp 11–13). The Gambia and Burkina Faso had established District Health Information System 2 (DHIS2) databases before SMC scale-up and these national databases were used for analyses of the effect of SMC on the number of reported outpatient malaria cases, the number of reported severe (hospitalised) cases, and the number of deaths in district hospitals attributed to malaria. In the other five countries, data on confirmed outpatient cases were collected from outpatient clinics. In each country, facilities were selected (~30 per country) that had used parasitological confirmation of malaria cases for at least one year before introduction of SMC; had retained clinic registers; and were in areas where SMC was to be delivered via ACCESS-SMC starting in 2015 or 2016, or would not have implemented SMC by 2016. Data from facilities that had complete data for both age groups each month for at least one year before and one year after introduction of SMC (n=73 across all seven countries) were retained for analysis.
Costs and cost-effectiveness
Provider costs of SMC (in 2016 US$) in each country were estimated by use of an ingredients-based approach (appendix pp 17–18).18 Costed ingredients comprised financial costs (from accounting records of implementing non-governmental organisation partners and via interviews with their personnel), volunteer opportunity costs (calculated from the number of days spent on distribution and related activities during each monthly cycle, and the national average minimum daily wage), and costs of government staff time (based on time spent supporting the campaign according to the average monthly gross income). Per diem payments were considered financial costs. Start-up costs (such as those for the development of training materials and reporting tools) were excluded. The weighted average cost of four treatments per child was obtained by dividing the total recurrent cost by the total number of doses administered divided by 4. The case numbers of malaria, severe malaria, and malaria deaths that should have been averted by SMC were calculated from the number of SMC treatments administered, estimates of incidence rates without SMC derived from the malaria model of Imperial College London,19 and case-control estimates of the effectiveness of each monthly treatment (appendix p 34). Cost-effectiveness ratios were calculated by dividing the total cost of the SMC intervention in each country by the predicted number of cases averted. Potential cost savings from a provider perspective were calculated from the diagnostic and treatment costs for non-severe and severe malaria cases averted, and assumed 60% of malaria cases were diagnosed and treated. All cost analyses were done in Microsoft Excel.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Results
In 2015, a total of 12 467 933 treatments were administered over 4 monthly cycles to a target population of 3 650 455 children by 19 428 CHWs. In 2016, 25 117 480 treatments were administered to a target population of 7 551 491 children by 47 238 CHWs (table 1). Timings of the monthly cycles in 2015 are shown in the appendix (p 8). Four monthly cycles of treatment were administered each year, except in 2016 in Nigeria, where the first cycle was not implemented in some wards due to delays in registration of dispersible tablets, and in three districts in Chad, where the fourth cycle was not implemented in 2016 due to a shortage of drugs.
Table 1.
Monthly treatments of sulfadoxine–pyrimethamine plus amodiaquine administered in 2015 and 2016, and estimates of SMC coverage from cluster-sample surveys
| Burkina Faso | Chad | The Gambia | Guinea | Mali | Niger | Nigeria | Overall | |
|---|---|---|---|---|---|---|---|---|
| 2015 | ||||||||
| Target population | 707 317 | 268 956 | 88 748 | 253 252 | 875 330 | 596 355 | 860 497 | 3 650 455 |
| Total treatments administered | 2 721 731 | 1 061 417 | 308 830 | 805 131 | 2 752 912 | 1 668 015 | 3 149 897 | 12 467 933 |
| Number surveyed who were eligible for four treatments | 786 | 707 | 690 | 1258 | 740 | 4113 | 1082 | 9376 |
| Mean coverage per month | 92·2% (87·9–96·4) | 68·3% (63·5–73·1) | 81·8% (77·8–85·8) | 78·8% (74·6–83·0) | 68·3% (57·4–79·2) | 61·8% (58·1–65·4) | 83·0% (78·3–87·6) | 76·4% (74·0–78·8) |
| Percentage of children treated at least once | 95·8% (91·1–98·0) | 96·0% (91·6–98·2) | 93·7% (90·4–95·9) | 94·2% (90·1–96·7) | 87·2% (74·9–94·0) | 78·9% (75·4–81·9) | 76·8% (68·2–83·4) | 86·4% (83·4–89·3) |
| Percentage of children who received four treatments | 86·4% (78·8–91·5) | 24·0% (16·8–33·1) | 56·1% (46·4–65·4) | 56·8% (44·8–68·0) | 45·2% (33·3–57·7) | 43·0% (37·8–48·3) | 54·6% (45·3–63·6) | 54·5% (50·4–58·7) |
| Reported adherent percentage to 3-day regimen in the fourth cycle | 97·2% (93·7–98·8) | 96·0% (93·2–97·7) | 98·6% (96·2–99·5) | 94·3% (92·0–95·9) | 99·3% (95·7–99·9) | 99·4% (98·6–99·7) | 89·0% (83·2–93·0) | 95·9% (94·6–97·1) |
| 2016 | ||||||||
| Target population | 2 056 169 | 514 042 | 90 925 | 438 123 | 1 492 137 | 1 050 932 | 1 909 163 | 7 551 491 |
| Total treatments administered | 5 780 062 | 2 511 371 | 297 453 | 1 750 224 | 4 667 224 | 3 810 088 | 6 301 058 | 25 117 480 |
| Number surveyed who were eligible for four treatments | 874 | 1010 | 1138 | 1743 | 799 | 5646 | 1853 | 13 063 |
| Mean coverage per month | 96·4% (94·5–98·2) | 53·0% (47·1–58·8) | 67·4% (61·6–73·2) | 86·4% (84·0–88·9) | 77·9% (66·6–89·2) | 75·6% (70·7–80·5) | 52·1% (44·9–59·4) | 74·8% (72·2–77·3) |
| Percentage of children treated at least once | 99·3% (97·1–99·8) | 91·4% (85·0–95·3) | 83·5% (73·1–86·3) | 96·2% (94·7–97·3) | 90·1% (79·7–95·5) | 91·4% (88·2–93·8) | 82·7% (74·1–88·9) | 91·7% (89·3–94·2) |
| Percentage of children who received four treatments | 91·2% (86·6–94·4) | 12·4% (7·9–19·0) | 43·7% (36·6–51·2) | 73·0% (67·7–77·8) | 56·9% (37·9–74·1) | 50·2% (43·8–56·6) | 19·5% (13·1–28·2) | 53·0% (48·5–57·4) |
| Reported adherent percentage to 3-day regimen in the fourth cycle | 99·8% (99·0–99·9) | 95·6% (91·1–97·9) | 99·3% (97·9–99·8) | 98·1% (96·8–98·8) | 92·2% (48·5–99·3) | 99·6% (99·2–99·8) | 86·8% (81·7–90·7) | 94·6% (91·8–97·4) |
Target populations aged 3–59 months were estimated based on census projections.20 Mean coverage per month, the percentage of children who received SMC treatment at least once in the year, the percentage of children who received SMC four times, and reported adherence (percentage of those who received SMC in the month before the survey who received all three daily doses) were estimated for children who were eligible to receive four treatments of SMC. 95% CIs are shown in parentheses. Four monthly cycles of treatment were administered each year, except in 2016 in Nigeria, where the first cycle was not implemented in some wards due to delays in registration of dispersible tablets, and in three districts in Chad, where the fourth cycle was not implemented in 2016 due to a shortage of drugs. In addition to the number shown, a total of 1695 children aged 6–7 years were surveyed in 2015 and 2062 in 2016, of whom 53·0% (95% CI 48·7–57·3) in 2015 and 62·4% (55·7–69·1) in 2016 had received SMC at least once each year. SMC=seasonal malaria chemoprevention.
In 2015, 12 777 children were surveyed at the end of the transmission season; 9376 were eligible to receive four treatments, of whom 86·4% (95% CI 83·4–89·3) were treated at least once. The mean coverage per month was 76·4% (95% CI 74·0–78·8), and 54·5% children (95% CI 50·4–58·7) received all four treatments. In 2016, 15 366 children were surveyed; 13 063 were eligible to receive four treatments, of whom 91·7% (89·3–94·2) were treated at least once. The mean coverage per month was 74·8% (72·2–77·3), and, as in 2015, just more than half of eligible children received four treatments (53·0% [48·5–57·4]). Coverage varied among countries and was consistently highest in Burkina Faso (table 1). Reported adherence to the 3 day regimen in the most recent month ranged from 86·8% (95% CI 81·7–90·7; Nigeria in 2016) to 99·8% (99·0–99·9; Burkina Faso in 2016). Delivery was equitable with similar levels of coverage across wealth rankings in all countries in both years (appendix p 9). The surveys also showed that a high percentage of children aged 6–7 years received SMC (53·0% [95% CI 48·7–57·3] of children surveyed in 2015 [n=1695] and 62·4% [55·7–69·1] in 2016 [n=2062] were treated at least once). In 2015, 87·4% (85·7–89·1) of children eligible for SMC slept under a long-lasting insecticide-treated net the night before the survey, and 85·8% (83·3–88·2) did in 2016.
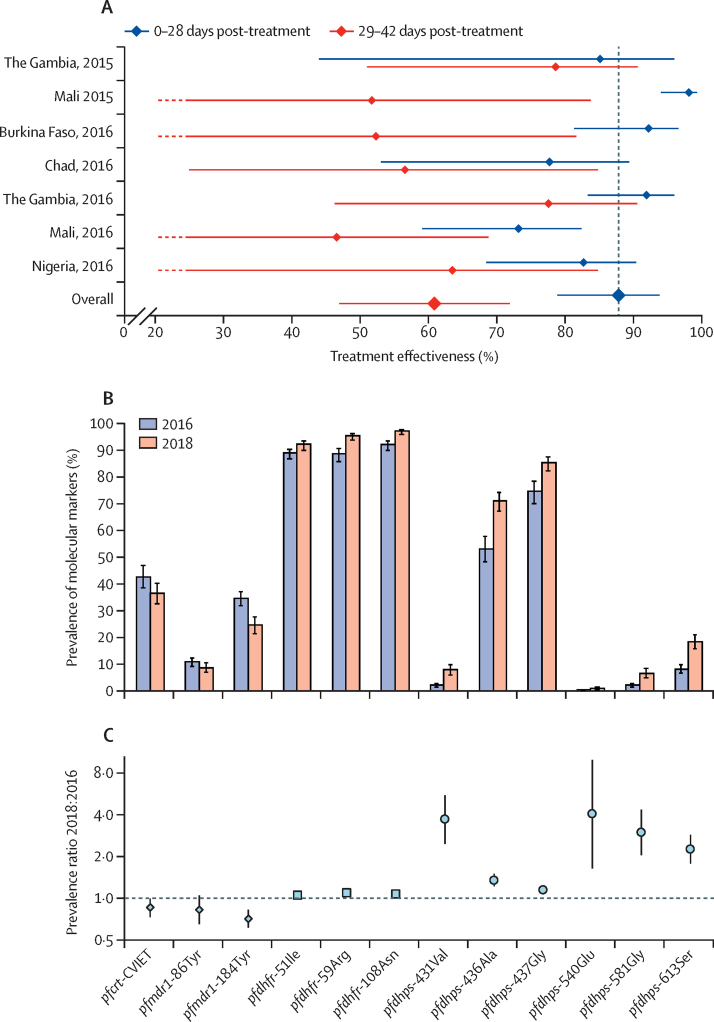
In our case-control studies, a total of 2185 cases with confirmed malaria, and 4370 controls, were enrolled during the 2015 (in The Gambia and Mali) and 2016 (Burkina Faso, Chad, The Gambia, Mali, and Nigeria) transmission seasons. The pooled estimate of the protective effectiveness of SMC in reducing incidence of clinical malaria within 28 days of administration was 88·2% (95% CI 78·7–93·4; figure 2A). Effectiveness from 29 to 42 days post-treatment was 61·4% (47·4–71·8).
Figure 2.
Effectiveness of SMC
(A) Case-control estimates of the effectiveness of SMC treatments. Datapoints are the percentage reduction in malaria incidence in 28 days since the start of treatment, and 29–42 days post-treatment, compared with the incidence in children who had not received SMC within the last 42 days (underlying incidence data reported previously;21 appendix pp 6, 36). Error bars show 95% CIs (lower confidence limits of <20% were truncated at 20% in the diagram, ending in a dashed line). The pooled estimates were obtained from a random effects meta-analysis (appendix pp 11–12). For comparison, the efficacy during 28 days from randomised trials was 86%.10 (B) The prevalence of molecular markers of resistance to sulfadoxine–pyrimethamine and amodiaquine in 2016 and 2018 in individuals not eligible to receive SMC (aged 10–30 years). Error bars show 95% CIs. (C) Prevalence ratios with 95% CIs representing the fold increase in each marker from 2016 to 2018, in the 10–30 years age group (95% CIs for the pfdhfr, pfdhps-436Ala, and pfdhps-4367Gly variants were too narrow to display). Results in children younger than 5 years are given in the appendix (p 15). SMC=seasonal malaria chemoprevention. pfcrt=Plasmodium falciparum chloroquine resistance transporter. pfmdr1=P falciparum multidrug resistance 1. pfdhfr=P falciparum dihydrofolate reductase. pfdhps=P falciparum dihydropteroate synthetase. pfcrt-CVIET=amino acid positions 72–76.
As of March 31, 2017, a total of 779 individual case safety reports related to SMC treatment were available for 2015 and 2016, of which 36 were graded serious: one child with rash, two with fever, 31 with gastrointestinal disorders, one with extrapyramidal syndrome, and one with Quincke's oedema. All children recovered from these serious adverse events. No cases of severe skin reactions (Stevens–Johnson syndrome or Lyell syndrome) were reported. A further serious adverse event was reported on Aug 16, 2017, a child who died due to suffocation from aspirating the SMC dissolved tablets after administration when not fully awake. In the cohort of children in Nigeria, the most commonly reported symptoms in 10 445 children seen after the first treatment cycle were fever (549 [5·3%] children), vomiting (333 [3·2%]), and diarrhoea (233 [2·2%]). In 6457 children seen after the third treatment cycle, again fever (178 [2·8%] children), vomiting (128 [2·0%]), and diarrhoea (70 [1·1%]) were the most common symptoms. In these children there were six spontaneous reports by facility staff of adverse drug-related reactions after presentation at health facilities with suspected adverse effects. These were four cases of rash, one of oedema, and one of vomiting, each in a separate child. None of these events were classed as serious.
In our 2016 survey of drug resistance markers, 29 274 samples from different individuals were analysed. 14 345 samples were from children younger than 5 years and 14 929 were from individuals aged 10–30 years, of which 2844 and 2286, respectively, were positive forP falciparum and were resistance-typed. In 2018,28 546 samples were analysed; 14 019 samples were from children younger than 5 years and 14 527 were from individuals aged 10–30 years, with 801 and 1375, respectively, that were positive and resistance-typed.
In children younger than 5 years, the combination of the pfcrt-CVIET (amino acid positions 72–76), pfmdr1-86Tyr, and pfmdr1-184Tyr variants, associated with resistance to amodiaquine, was found with a prevalence of 1·3% (95% CI 0·9–2·0) in 2016 and 0·5% (0·2–1·4) in 2018 (prevalence ratio 0·4 [0·1–1·1]). The prevalence of the quintuple mutation associated with resistance to sulfadoxine–pyrimethamine (triple mutation in pfdhfr with pfdhps-437Gly and pfdhps-540Glu) was 0·4% (0·2–0·8) in 2016 and 0·7% (0·3–1·5) in 2018 (prevalence ratio 1·8 [0·7–5·0). In the 10–30 age group, the corresponding estimates for the combination of pfcrt-CVIET, pfmdr1-86Tyr and pfmdr1-184Tyr were 0·7% (0·4–1·2) in 2016 and 0·4% (0·1–0·8) in 2018 (prevalence ratio 0·5 [0·2–1·2]), and for the quintuple mutation (triple mutation in pfdhfr with pfdhps-437Gly and pfdhps-540Glu), 0·2% (0·1–0·5) in 2016 and 1·0% (0·6–1·6) in 2018 (prevalence ratio 4·8 [1·7–13·7]). The prevalence of each mutation in 2016 and 2018 for the 10–30 age group is shown in figure 2B. The three variants associated with resistance to amodiaquine decreased in prevalence between the surveys, while mutations in the pfdhfr gene, associated with resistance to pyrimethamine, and in the pfdhps gene, associated with resistance to sulfadoxine, each increased in prevalence. The fold-rise for each variant is shown in figure 2C. The pfdhps-540Glu mutation, which, combined with pfdhps-436Ala or pfdhps-437Gly, confers resistance to sulfadoxine–pyrimethamine, was uncommon but its prevalence increased. Corresponding results in children younger than 5 years are shown in the appendix (p 15).
In 2016, only two samples (one in each age group) carried the quintuple mutation and pfcrt-CVIET, pfmrd1–86Tyr, and pfmdr1–184Tyr, the combination associated with resistance to sulfadoxine–pyrimethamine and amodiaquine (prevalence of 0·05% [0·01–0·18]). In 2018, no samples carried genotypes associated with resistance to both sulfadoxine–pyrimethamine and amodiaquine.
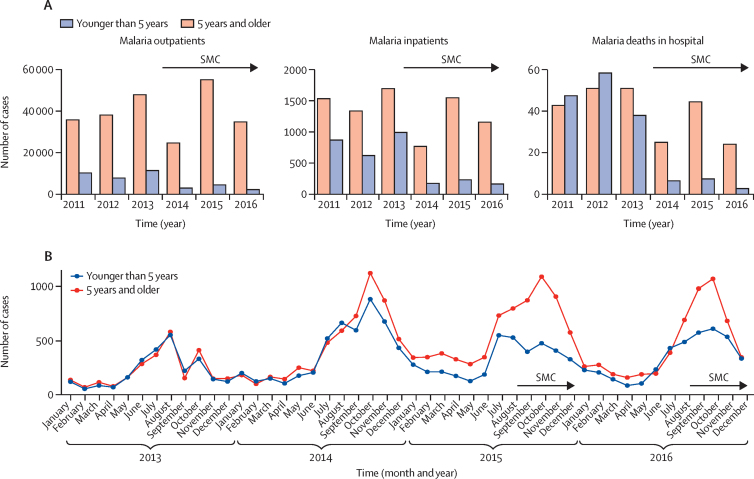
In The Gambia, SMC was introduced in the Upper River and Central River regions in 2014 (appendix p 2) and continued in the same areas via the ACCESS-SMC project in 2015 and 2016 (figure 1). Regarding the effect on malaria mortality, 155 malaria deaths were reported in hospitals (DHIS2 database) in children younger than 5 years during the transmission seasons in the 3 years before introduction of SMC (2011–13), compared with 18 deaths in the corresponding periods in the 3 years with SMC implementation (2014–16; figure 3). The overall reduction in malaria mortality associated with implementation of SMC (2014–16 vs 2011–13) estimated from Poisson regression was 56·6% (95% CI 28·9–73·5; table 2). In Burkina Faso, in areas where SMC was implemented from 2015, 612 malaria deaths were reported in district hospitals (DHIS2 database) in children younger than 5 years during the transmission seasons in the previous two years (2013–14), compared with 336 deaths in 2015 and 2016 when SMC was implemented. In the districts that introduced SMC from 2016, the number of malaria deaths in children younger than 5 years was 266 in 2013, 284 in 2014, and 281 in 2015, and 76 in 2016. The estimated reduction in deaths associated with SMC was 42·4% (5·9–64·7; table 2). The number of severe malaria cases reported in children younger than 5 years in the Upper River and Central River regions of The Gambia during the transmission season was 867 in 2011, 619 in 2012, and 989 in 2013 (figure 3). Following introduction of SMC, the number decreased to 175 in 2014, 233 in 2015, and 166 in 2016, representing a reduction of 54·8% (29·2–71·1; table 2). In Burkina Faso there was a 27·4% (20·5–33·7) reduction in 2015 (data for 2016 not available at time of analysis). Across all seven countries, estimated reductions in the number of confirmed outpatient malaria cases during the intervention periods (2015–16) ranged from 25·0% (5·4 to 40·5) in Nigeria in 2016 to 58·8% (43·0 to 70·3) in The Gambia in 2016 (table 2).
Figure 3.
Examples of the effect of SMC on malaria rates
(A) Numbers of confirmed cases of malaria in outpatient clinics, hospital inpatients admitted with a primary diagnosis of malaria, and deaths in hospital attributed to malaria, among children younger than 5 years and individuals aged 5 years and older, during transmission periods before and after SMC introduction for children younger than 5 years in the Upper River and Central River regions of The Gambia. (B) Numbers of confirmed cases of malaria among children younger than 5 years and individuals aged 5 years and older, in Kadiolo health centre, Sikasso region, Mali, each month before and after introduction of SMC for children younger than 5 years. SMC=seasonal malaria chemoprevention.
Table 2.
Reductions in malaria in children younger than 5 years when SMC was introduced
| Source* | 2015 | 2016 | Overall | |
|---|---|---|---|---|
| Malaria deaths in hospital | ||||
| Burkina Faso | DHIS2 | 47·2% (8·7 to 69·4) | 34·8% (−15·1 to 63·1) | 42·4% (5·9 to 64·7) |
| The Gambia† | DHIS2 | 48·4% (14·3 to 68·9) | 58·8% (−70·5 to 90·0) | 56·6% (28·9 to 73·5) |
| Malaria inpatients | ||||
| Burkina Faso‡ | DHIS2 | 27·4% (20·5 to 33·7) | .. | .. |
| The Gambia† | DHIS2 | 56·1% (33·3 to 71·1) | 41·7% (−65·2 to 79·4) | 54·8% (29·2 to 71·1) |
| Malaria outpatients | ||||
| Burkina Faso | DHIS2 | 40·6% (33·6 to 46·8) | 48·5% (39·0 to 56·5) | 45·0% (39·1 to 50·3) |
| The Gambia† | DHIS2 | 53·0% (37·5 to 64·7) | 58·8% (43·0 to 70·3) | 55·2% (42·0 to 65·3) |
| Chad | 11 clinics | 51·4% (−2·5 to 77·0) | 42·0% (15·6 to 60·2) | 43·6% (17·8 to 61·3) |
| Guinea | 15 clinics | 37·5% (7·6 to 57·7) | 49·2% (34·6 to 60·6) | 45·6% (31·0 to 57·1) |
| Mali | 26 clinics | 47·3% (27·6 to 61·6) | 39·0% (26·1 to 49·7) | 42·7% (28·7 to 53·9) |
| Niger | 13 clinics | 43·8% (14·3 to 63·1) | 29·2% (−14·1 to 56·0) | 35·3% (1·0 to 57·7) |
| Nigeria | 8 clinics | 26·0% (−0·7 to 45·7) | 25·0% (5·4 to 40·5) | 25·5% (6·1 to 40·9) |
The percentage reduction in the number of outpatient cases of malaria in children younger than 5 years at health facilities during the high transmission season, associated with the introduction of SMC, was estimated by fitting a Poisson regression model to the monthly number of confirmed cases treated at health facilities (appendix pp 25–29), with age group and calendar year as factors and the effect of SMC estimated with an indicator variable set to 1 for the age group during the months when SMC was implemented and set to 0 otherwise, with a robust standard error to calculate 95% CIs (appendix pp 19–24). Negative values indicate a relative increase. SMC=seasonal malaria chemoprevention. DHIS2=District Health Information System 2.
DHIS2 district-level data or data collected from selected outpatient clinics; 160 clinics were visited to inspect quality and completeness of data, and data were analysed from 73 clinics that had complete data on confirmed malaria cases for at least one year before and one year after SMC introduction.
For The Gambia, overall figures include data for 2014 (malaria deaths, 62·6% [16·9 to 83·2]; malaria inpatients, 61·5% [47·3 to 71·8]; malaria outpatients, 46·8% [42·0 to 51·2]).
Data on severe malaria not available for 2016 in the DHIS2 database at the time of data analysis.
The total recurrent economic cost of SMC in 2016 (for all ages) was US$22·8 million, comprising US$20·6 million in financial costs and US$2·2 million in volunteer opportunity costs. The weighted average economic cost of administering four monthly SMC cycles to a single child across the seven countries was US$3·63, ranging from US$2·71 in Niger to US$8·20 in The Gambia. The estimate of the average total economic cost per malaria case averted, based on modelled estimates of the incidence of malaria in the absence of SMC (appendix p 34), ranged from US$2·91 in Niger to US$30·73 in The Gambia. The average total cost per severe malaria case averted ranged from US$119·63 in Niger to US$506·00 in The Gambia, and the average cost per death averted ranged from US$533·56 in Niger to US$2256·92 in The Gambia. Potential cost savings were estimated to be US$66·0 million in total, ranging from US$291 966 in The Gambia to US$20·1 million in Nigeria. The net economic cost savings (deducting the costs of administering SMC) were US$43·2 million across the seven countries.
Discussion
The ACCESS-SMC project sought to show the effectiveness of SMC at scale and improve the market for SMC drugs, to overcome barriers to scale-up. Despite challenges of delivering SMC, almost 90% of children received at least one treatment, and more than 50% of children received all four treatments each year. Door-to-door distribution was successful in reaching the poorest in the community. Although adherence to unsupervised doses is difficult to verify, caregiver-reported adherence was high. The protective effectiveness of each monthly treatment was similar to the efficacy observed in randomised controlled trials.6, 7 In two countries with DHIS-2 databases established before SMC scale-up (The Gambia and Burkina Faso), estimated reductions of 57% and 42% in the number of malaria deaths in district hospitals were determined for the SMC intervention period, and reductions of 53% and 45% in the number of outpatient cases. Similar reductions were found in the number of outpatient malaria cases in other countries. These results represent the first large-scale evaluation of SMC implemented by national programmes, and provide the first evidence of an impact on malaria deaths. Earlier studies in Burkina Faso and Mali showed effects on prevalence22, 23 and preliminary data on cost-effectiveness was obtained in Ghana.24
ACCESS-SMC substantially increased the demand for SMC drugs, accounting for about 50% of all SMC treatments procured in 2016 (unpublished data). In 2015, a sweetened, dispersible product was submitted to the WHO Prequalification of Medicines Programme and to the Global Fund Expert Review Panel for Pharmaceutical Products. Panel approval was obtained in February, 2016, allowing ACCESS-SMC to use dispersible tablets for most treatments administered in 2016 (WHO prequalification was obtained on Aug 21, 2018). By 2017, all SP+AQ orders for SMC were for the dispersible formulation. National malaria control programmes have been able to transition to other sources of funding to sustain SMC in all ACCESS-SMC areas and to expand delivery, supported primarily by the Global Fund, national governments, the President's Malaria Initiative, UNICEF, and the World Bank. In addition, philanthropic funding supports Malaria Consortium programmes in Burkina Faso, Chad, and Nigeria.
Estimates of the effect on malaria rates could have been influenced by confounding, due to concurrent effects of other control measures, changes in access to health care, rates of parasitological testing of suspected malaria cases, and improvements in management of severe cases. However, as SMC is limited to children younger than 5 years, data could also be used from older age groups that did not receive SP + AQ, to at least partly control for temporal trends. Use of insecticide-treated bednets, which was assessed during SMC coverage surveys, did not increase differentially in eligible children during the period of SMC scale-up. Malaria testing rates increased, but this increase was similar in all age groups (appendix p 12).
No cases of severe skin reactions were reported in this study, although cases have occurred after SMC.25 It is possible that serious cases occurred and were not reported. A limitation in assessing case reports has been the absence of biochemical and haematological parameters, which might have led to underdetection of liver injury and we were not able to monitor cases of agranulocytosis. An independent review of the safety of SMC by the WHO Advisory Committee on Safe of Medicines and Medicinal Products, based on data from this project and reports from other countries implementing SMC,25, 26 endorsed the activities undertaken to promote safe administration of SMC and to strengthen safety monitoring, and concluded that the risk–benefit profile of SMC is positive,27, 28 but noted that further strengthening of pharmacoviligance is needed to ensure prompt investigation of suspected cases and improve completeness of reports. Assessment of a causal link to SMC dugs was often problematic. Assessing causality from the information in Vigibase was not possible, due to incompleteness of information and difficulty in eliminating other causes of adverse effects such as dysentery and malaria. CHWs were trained to exclude children who were unwell from receiving SMC, but symptoms might not be apparent in the early stages of an illness, and therefore children who were unwell shortly after SMC due to a pre-existing illness might have been included.
SMC will reduce natural acquisition of immunity,29 as is the case for effective malaria prevention by any method, but we have shown that SMC improves survival in children. Children older than 5 years who stop receiving SMC are expected to be at increased risk of malaria compared with when they received SMC, and this risk should be monitored. Steps should be taken to ensure this age group use long-lasting insecticide-treated bednets and other preventive measures, and seek treatment promptly if they have fever.
Molecular markers of resistance to SMC drugs occurred at low prevalence, consistent with the effectiveness of SMC observed in the case-control studies. However, there was evidence of selection for resistance to sulfadoxine–pyrimethamine in parasites sampled from the age group that did not receive SMC but lived in areas where SMC was deployed. The use of artemether–lumefantrine as a first-line therapy for malaria provides some protection against amodiaquine resistance, as it is effective against amodiaquine-resistant parasites, but it provides no corresponding protection against sulfadoxine–pyrimethamine resistance. Resistance to both sulfadoxine–pyrimethamine and amodiaquine should continue to be monitored via standardised methods, across all regions where SMC is used, to provide early warning of loss of effectiveness.
SMC is a relatively low-cost intervention, and contributed to substantial cost savings for national health systems, by substantially reducing malaria burden and costs for malaria diagnosis and treatment. However, the level of SMC coverage varied. High levels of SMC coverage were possible with door-to-door delivery, but were not achieved everywhere. Important factors in maintaining high levels of coverage month-to-month include effective communication to inform communities about dates of campaigns, effective systems to ensure prompt payment of drug distributors, and adequate quantification to avoid stock-outs.
Supported by results from this project (referenced in the appendix [pp 6, 36]), the use of SMC has been rapidly expanded since 2016, with programmes in 13 countries (Benin, Burkina Faso, Cameroon, Chad, The Gambia, Ghana, Guinea, Guinea Bissau, Mali, Niger, Nigeria, Senegal, and Togo) reaching about 22 million children in 2019 (appendix p 14; unpublished data). However, about 8 million children live in areas identified as suitable for SMC that did not have SMC programmes in 2019, and this gap needs to be closed. As the use of SMC is expanded, monitoring coverage and adapting delivery approaches will be important, to ensure all children can be reached each month. Additionally, cycles should be strictly 28 days apart in view of the rapid drop in protection after this time. In view of the success with which SMC has been implemented in recent years, wider use could be considered,30 with addition of a fifth monthly cycle in regions with highly seasonal transmission but where the main risk period is longer than 4 months. Where justified by the disease burden, inclusion of older children should also be considered, as has been done successfully in Senegal.25 The ACCESS-SMC project drove demand for SMC drugs, which encouraged manufacturers to increase capacity and develop child-friendly formulations. This evaluation showed that despite the challenges of delivering monthly treatments door to door, high coverage can be achieved, reducing morbidity and mortality caused by malaria. These results should support efforts to sustain and optimise the use of SMC to prevent malaria in children.
Correspondence to: Prof Paul Milligan, Faculty of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, London WC1E 7HT, UK paul.milligan@lshtm.ac.uk
Data sharing
Anonymised individual-level data from the surveys, case-control studies, drug resistance analyses, and the impact data, including data dictionaries, data collection tools, and protocol, will be made available through Data Compass. Requests for access will be reviewed by a data access committee.
Acknowledgments
Acknowledgments
The ACCESS-SMC project was funded by Unitaid. ACCESS-SMC was led by the Malaria Consortium in partnership with Catholic Relief Services, and in collaboration with the London School of Hygiene & Tropical Medicine, Management Sciences for Health, Medicines for Malaria Venture, and Speak Up Africa. ACCESS-SMC has supported national malaria control programmes to expand access to SMC to save children's lives across seven countries in the Sahel (Burkina Faso, Chad, Guinea, Mali, Niger, Nigeria, and The Gambia). The ACCESS-SMC project director was Diego Moroso and the deputy director was Lantorina Razafindralambo. The technical committee comprised Paul Milligan (chair), Diego Moroso, Harriet Kivumbi, Lantorina Razafindralambo, Suzanne Van Hulle, Eric Hubbard, Gladys Tetteh, David Collins, and Rhosyn Tuta. The safety committee comprised Rachida Soulaymani (chair), Nilima Kshirsagar, Houda Sefani, Ambrose Isah, and Alex Dodoo. Safety data from VigiBase, the WHO global database of individual case safety reports maintained by Uppsala Monitoring Centre, comes from a variety of sources, and the probability that the suspected adverse effect is drug-related is not the same in all cases. The safety information does not represent the opinion of UMC or WHO. Corinne Merle, Christine Halleux, Shanthi Pal, and Noha Iessa are staff members of WHO; these individuals alone are responsible for the views expressed in this publication, and the views expressed herein do not necessarily represent the decisions, policy, or views of WHO.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps.
Contributors
A-MT, CG, CM, CS, DC, DM, EB, FN, GJ, GT, HS, KBe, MCa, PM, RS, SVH, and YD designed the study. ADic, AK, A-MT, AS, CE, BA, BK, CG, CS, DAS, DC, DK, DM, EB, EH, EKL, EO, FJ, FN, GN, GT, HJac, HJah, HKe, HKi, IL, IO, IS, IZ, JBO, JM, JSJ, KBe, KBou, KML, LR, MCo, MCT, HAM, MJK, MK, MKa, MKe, MSS, NN, PC, PM, RA, SC, SJO, TE, TG, YD, and YS collected data. AR-F, CG, CM, CH, CS, DC, DM, EH, HS, KBe, MCa, PM, and RS planned the data analyses. Authors who verified the data and analyses and had full access to the data were: (safety data) HS, RS, CH, CM, IN, SP, and PM; (drug resistance data) KBe, CS, RM, and PM; (coverage data) SS, MCa, SL, and PM; (impact data) SL, PM, MK, IL, IZ, SC, KML, IS, HKe, YS, MK, and BK; (costs data) CG, DC, PM, and MCa; (delivery data): DM, HK, KM, AS, AR-F, JSJ, LR, EH, PC, HJah, EKL, RA, and PM; (effectiveness data) MCa, PM, SC, TE, SJO, IZ, JBO, IS, ADic, and HKe. ADic, ADia, ADj, A-MT, AR-F, CG, CH, CM, CS, DC, DD, DM, EB, EH, GJ, GT, HKe, HKi, HS, IL, IS, IZ, JA, JBO, JLN, JT, KBe, KBo, KM, KML, MCa, MCo, MKa, MM, NI, PH, PM, RG, RM, RS, RT, SC, SJO, SL, SP, SS, SVH, TE, and YF interpreted data. PM wrote the first draft of the paper and appendix and A-MT, AR-F, CG, CH, CM, CS, DC, DM, JT, KBe, MCa, MM, NI, SP, PM, SVH, SS and TE edited the paper. All authors approved the final version of the manuscript, decided to publish the manuscript, and agreed to be accountable for all aspects of the work.
ACCESS-SMC Partnership
Malaria Consortium: Uganda Diego Moroso, Harriet Kivumbi, Ebenezer Baba; UK Arantxa Roca-Feltrer, James Tibenderana, Prudence Hamade; USA Maddy Marasciulo; Burkina Faso Joanna Stenstrom Johansson; Chad Adama Sanogo; Nigeria Kolawole Maxwell. Catholic Relief Services: Senegal Lantorina Razafindralambo; Mali Eric Hubbard; USA Suzanne Van Hulle; Mali Patrice Coulibaly; Guinea Eugene Kaman Lama; Niger Rahila Abdoulaye; The Gambia Huja Jah. Collaborators: Burkina Faso Jean Bosco Ouedraogo, Issaka Zongo (Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso), Yacouba Savodogo, Alice Kiba (Programme National de Lutte contre le Paludisme, Ouagadougou), Emile Ouedraogo (Centre national des vigilances des produits de santé, Ouagadougou); Chad Hamit Kesseley, Daugla Doumagoum (Centre de Support en Santé Internationale, N'Djamena), Djiddi Ali Sougoudi, Kodbesse Boulotigam (Programme National de Lutte contre le Paludisme, N'Djamena), Nadine Ngarnaye (Direction de la Pharmacie, du Médicament et de la Pharmacopée, N'Djamena); Guinea Kovana Marcel Loua (L'Université Gamal Abdel Nasser de Conakry, Conakry), Timothee Guilavogui, Moussa Keita (Programme National de Lutte contre le Paludisme, Conakry), Mariama Sire Sano (la Direction Nationale de la Pharmacie et du Médicament, Conakry); The Gambia Serign Ceesay, Kalifa Bojang, Jane Achan (Medical Research Council Laboratories, Banjul), Balla Kandeh, Momodou Kalleh (National Malaria Control Programme, Banjul), Markieu Janneh Kaira, Fatou Jah (Medicines Control Agency, Banjul); Mali Alassane Dicko, Issaka Sagara, Abdoulaye Djimde (Malaria Research and Training Center, University of Bamako, Bamako), Diakalidia Kone (Programme National de Lutte contre le Paludisme, Bamako), Mamadou Chérif Traore (la Direction de la Pharmacie et du Médicament, Bamako); Niger Ibrahim Laminou (Centre de Recherche Médicale et Sanitaire, Niamey), Matt Coldiron, Rebecca Grais (Epicentre, Niamey), Hadiza Jackou, Ibrahim Ouba (Programme National de Lutte contre le Paludisme, Niamey), Halimatou Alassana Messan (Centre National de Pharmacovigilance, Niamey); Nigeria Musa Kana (Federal University Lafia, Nasarawa State), Sonny Johnbull Ogboi, Tony Eloike (Jedima International Health Consult, Lekki, Lagos), Godwin Ntadom, Bala Audu (National Malaria Elimination Programme, Abuja), Cassandra Elagbaje (The National Agency for Food and Drug Administration and Control, Abuja); Senegal Abdoulaye Diallo, Jean Louis Ndiaye (l'Université Cheikh Anta Diop, Dakar), Fara Ndiaye, Yacine Djibo (Speak Up Africa, Dakar); Ghana Gladys Tetteh (Management Sciences for Health, Accra); USA David Collins (Management Sciences for Health, Boston, MA), Colin Gilmartin (Management Sciences for Health, Arlington, VA); Switzerland André-Marie Tchouatieu, George Jagoe (Medicines for Malaria Venture, Geneva), Corinne Merle, Christine Halleux (Special Programme for Research and Training in Tropical Diseases, WHO, Geneva), Noha Iessa, Shanthi Pal (Regulation and Safety, Pharmacovigilance, WHO, Geneva); Morocco Rachida Souleymani, Houda Sefiani (Centre Anti-Poison de Maroc, Rabat); UK Colin Sutherland, Matt Cairns, Khalid Beshir, Julian Muwanguzi, Paul Snell, Rhosyn Tuta, Yolanda Fernandez, Susana Scott, Sham Lal, Raoul Mansukhani, Paul Milligan (London School of Hygiene & Tropical Medicine, London).
Declaration of interests
We declare no competing interests.
Contributor Information
ACCESS-SMC Partnership:
Ebenezer Baba, Prudence Hamade, Harriet Kivumbi, Maddy Marasciulo, Kolawole Maxwell, Diego Moroso, Arantxa Roca-Feltrer, Adama Sanogo, Joanna Stenstrom Johansson, James Tibenderana, Rahila Abdoulaye, Patrice Coulibaly, Eric Hubbard, Huja Jah, Eugene Kaman Lama, Lantorina Razafindralambo, Suzanne Van Hulle, George Jagoe, André-Marie Tchouatieu, David Collins, Colin Gilmartin, Gladys Tetteh, Yacine Djibo, Fara Ndiaye, Momodou Kalleh, Balla Kandeh, Bala Audu, Godwin Ntadom, Alice Kiba, Yacouba Savodogo, Kodbesse Boulotigam, Djiddi Ali Sougoudi, Timothee Guilavogui, Moussa Keita, Diakalidia Kone, Hadiza Jackou, Ibrahim Ouba, Emile Ouedraogo, Halimatou Alassana Messan, Fatou Jah, Markieu Janneh Kaira, Mariama Sire Sano, Mamadou Chérif Traore, Nadine Ngarnaye, Aishatu Yinusa Cassandra Elagbaje, Christine Halleux, Corinne Merle, Noha Iessa, Shanthi Pal, Houda Sefiani, Rachida Souleymani, Ibrahim Laminou, Daugla Doumagoum, Hamit Kesseley, Matt Coldiron, Rebecca Grais, Musa Kana, Jean Bosco Ouedraogo, Issaka Zongo, Tony Eloike, Sonny Johnbull Ogboi, Jane Achan, Kalifa Bojang, Serign Ceesay, Alassane Dicko, Abdoulaye Djimde, Issaka Sagara, Abdoulaye Diallo, Jean Louis NdDiaye, Kovana Marcel Loua, Khalid Beshir, Matt Cairns, Yolanda Fernandez, Sham Lal, Raoul Mansukhani, Julian Muwanguzi, Susana Scott, Paul Snell, Colin Sutherland, Rhosyn Tuta, and Paul Milligan
Supplementary Material
References
- 1.WHO . World Health Organization; Geneva: 2019. World malaria report 2018. [Google Scholar]
- 2.Wilson AL. A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc) PLoS One. 2011;6 doi: 10.1371/journal.pone.0016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meremikwu MM, Donegan S, Sinclair D, Esu E, Oringanje C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst Rev. 2012;2 doi: 10.1002/14651858.CD003756.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cissé B, Sokhna C, Boulanger D. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367:659–667. doi: 10.1016/S0140-6736(06)68264-0. [DOI] [PubMed] [Google Scholar]
- 5.Sokhna C, Cissé B, Ba EH. A trial of the efficacy, safety and impact on drug resistance of four drug regimens for seasonal intermittent preventive treatment in Senegalese children. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dicko A, Diallo AI, Tembine I. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konaté AT, Yaro JB, Ouédraogo AZ. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cissé B, Ba EH, Sokhna C. Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: a stepped-wedge cluster-randomised trial. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinclair D, Meremikwu MM, Garner P. Seasonal malaria chemoprevention for preventing malaria morbidity in children aged less than 5 years living in areas of marked seasonal transmission: GRADE tables to assist guideline development and recommendations. Oct 26, 2011. http://www.who.int/malaria/mpac/feb2012/smc_grade_tables.pdf
- 10.WHO Report of the technical consultation on seasonal malaria chemoprevention. 2012. http://www.who.int/malaria/publications/atoz/smc_report_teg_meetingmay2011/en/index.html
- 11.WHO WHO policy recommendation: seasonal malaria chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. March, 2012. http://www.who.int/malaria/publications/atoz/who_smc_policy_recommendation/en/index.html
- 12.WHO . World Health Organization; Geneva: 2012. Seasonal malaria chemoprevention with sulfadoxine-pyrimethamine plus amodiaquine in children. A field guide. [Google Scholar]
- 13.Lasry E. A novel prevention programme has dramatically reduced malaria cases in Mali and Chad. Sept 24, 2012. https://www.msf.org/mali-and-chad-novel-prevention-programme-has-dramatically-reduced-malaria-cases
- 14.Médecins Sans Frontières Malaria: MSF provides preventive treatment to children in the Sahel region. Nov 12, 2014. https://www.msf-me.org/article/malaria-msf-provides-preventive-treatment-to-children-in-the-sahel-region
- 15.Koscolacova A. Chemical prevention of seasonal malaria in Niger. Executive summary. February, 2015. https://evaluation.msf.org/sites/evaluation/files/attachments/execsummarycps_niger_final.pdf
- 16.WHO Global Malaria Programme Minutes of the Technical Expert Group on Drug Efficacy and Response, 1–2 June 2017. 5.1 Monitoring efficacy of seasonal malaria chemoprevention in the ACCESS-SMC project. October, 2017. https://www.who.int/malaria/mpac/mpac-oct2017-teg-drug-efficacy-response-session3.pdf?ua=1
- 17.Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;2013:75–81. doi: 10.1007/s40264-012-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilmartin C, Nonvignon J, Cairns M, et al. Seasonal malaria chemoprevention in the Sahel subregion of Africa: a cost-effectiveness and cost-savings analysis. Lancet Glob Health (in press). [DOI] [PubMed]
- 19.Winskill P, Slater HC, Griffin JT, Ghani AC, Walker PGT. The US President's malaria initiative, Plasmodium falciparum transmission and mortality: a modelling study. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott S, Cairns M, Lal S. London School of Hygiene & Tropical Medicine; London: 2018. Seasonal malaria chemoprevention coverage in seven West African countries, 2015–2016. Report for UNITAID. [Google Scholar]
- 21.Cairns M, Sagara I, Zongo I. London School of Hygiene & Tropical Medicine; London: 2018. Assessment of the protective efficacy of seasonal malaria chemoprevention with sulfadoxine-pyrimethamine plus amodiaquine in Burkina Faso, Chad, The Gambia, Mali and Nigeria, 2015–2016. Report for UNITAID. [Google Scholar]
- 22.Druetz T, Corneau-Tremblay N, Millogo T. Impact evaluation of seasonal malaria chemoprevention under routine program implementation: a quasi-experimental study in Burkina Faso. Am J Trop Med Hyg. 2018;98:524–533. doi: 10.4269/ajtmh.17-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diawara F, Steinhardt LC, Mahamar A. Measuring the impact of seasonal malaria chemoprevention as part of routine malaria control in Kita, Mali. Malar J. 2017;16:325. doi: 10.1186/s12936-017-1974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nonvignon J, Aryeetey GC, Issah S. Cost-effectiveness of seasonal malaria chemoprevention in upper west region of Ghana. Malar J. 2016;15:367. doi: 10.1186/s12936-016-1418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NDiaye JL, Diallo I, NDiaye Y. Evaluation of two strategies for community-based safety monitoring during seasonal malaria chemoprevention campaigns in Senegal, compared with the national spontaneous reporting system. Pharmaceut Med. 2018;32:189–200. doi: 10.1007/s40290-018-0232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NDiaye JL, Cissé B, Ba EH. Safety of seasonal malaria chemoprevention (SMC) with sulfadoxine–pyrimethamine plus amodiaquine when delivered to children under 10 years of age by district health services in Senegal: results from a stepped-wedge cluster randomized trial. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO . Integrating pharmacovigilance in seasonal malaria chemoprevention: the story so far. In: WHO, editor. WHO pharmaceuticals newsletter no. 4. World Health Organization; Geneva: 2017. pp. 33–36. [Google Scholar]
- 28.WHO . Fourteenth meeting of the WHO Advisory Committee on Safety of Medicinal Products (ACSoMP) In: WHO, editor. WHO pharmaceuticals newsletter no. 4. World Health Organization; Geneva: 2017. pp. 26–32. [Google Scholar]
- 29.Greenwood BM, David PH, Otoo-Forbes LN. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans R Soc Trop Med Hyg. 1995;89:629–633. doi: 10.1016/0035-9203(95)90419-0. [DOI] [PubMed] [Google Scholar]
- 30.NDiaye JLA, NDiaye Y, Ba MS. Seasonal malaria chemoprevention combined with community case management of malaria in children under 10 years of age, over 5 months, in southern Senegal: a cluster-randomized trial. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised individual-level data from the surveys, case-control studies, drug resistance analyses, and the impact data, including data dictionaries, data collection tools, and protocol, will be made available through Data Compass. Requests for access will be reviewed by a data access committee.