Abstract
Introduction.
Improved methods are needed to detect and quantify age-related muscle change. Here we assessed the electrical properties of muscle impacted by acquired mitochondrial DNA mutations via the PolG mouse, which exhibits typical age-associated features, and the impact of a potential therapy, nicotinamide mononucleotide (NMN).
Methods.
The gastrocnemii of 24 PolG and 30 wild-type (WT) mice (8 PolG and 17 WT treated with NMN) were studied in an electrical impedance-measuring cell. Conductivity and relative permittivity were determined from the impedance data. Myofiber cross-sectional area (CSA) was quantified histologically.
Results.
Untreated PolG mice demonstrated alterations in several impedance features, including 50 kHz relative permittivity and center frequency. A potential effect of NMN was also observed in these parameters in PolG but not in WT animals. Impedance values correlated to myofiber CSA.
Discussion.
Electrical impedance is sensitive to myofiber features considered characteristic of aging and to the impact of a potential therapy.
Introduction
Convenient tools to evaluate the development, progression, and therapy of aging-related muscle change and ultimately sarcopenia remain poorly developed. Current clinical approaches for quantifying these alterations have mostly have relied upon relatively nonspecific clinical measures such as hand grip strength and the short physical performance battery.1 Certain diagnostic tools have also been used to some extent for this purpose, including dual x-ray absorptiometry,2 computerized tomography and magnetic resonance imaging,3 and ultrasound;4 however, all have limitations, including inconvenience, high cost, and limited repeatability.
One approach that has offered potential benefit for this purpose is electrical impedance myography (EIM). In EIM, a low-amplitude, high-frequency electrical current is passed through a muscle of interest and the consequent voltage is measured.5 Alterations in muscle condition, including myofiber atrophy, and the deposition of fat and connective tissue, impact the resulting voltages. Thus, EIM is potentially sensitive to histological changes in muscle that are thought to be closely related to the development of sarcopenia. Major advantages of EIM over the other technologies listed above include potentially low cost, convenience, and high reproducibility. Several previous studies have evaluated the technology for this purpose, including two studies in older adults6,7 and one study in aged mice.8 These studies have shown alterations in EIM parameters change over time consistent with aging-associated deterioration, and in the case of the mouse study, correlation to functional measures and muscle mass. Nevertheless, additional measurements to support the value of EIM in this application are needed, especially in helping to explain the intrinsic alterations in EIM data and how they relate to myofiber health as well as whether they are responsive to a therapeutic intervention.
The mitochondrial DNA (mtDNA) mutator mouse, a model of mtDNA mutation accumulation with a well-characterized phenotype of premature aging9–11 is an appropriate model to examine the efficacy of EIM. These transgenic mice carry a substitution mutation in the PolgA gene that encodes mtDNA polymerase-γ. This PolgAD257A mutation alters the proofreading function of the polymerase, resulting in the age-associated accumulation of a high number of point mutations in the mitochondrial genome.9 This leads to systemic mitochondrial dysfunction and a progressive phenotype that becomes apparent at 7–9 months. Many features of this phenotype are associated with aging, such as weight loss, reduced subcutaneous fat, greying and loss of fur, pronounced kyphosis, osteoporosis, anemia, reduced fertility, enlargement of the heart, reduced lifespan, and muscle deterioration.9,10,12
Recent work has emphasized the importance of nicotinamide adenine dinucleotide (NAD+) decline in aging and muscle loss.13–15 The NAD+ precursor nicotinamide mononucledotide (NMN) effectively augments levels of NAD+ in skeletal muscle cells16,17 and NMN supplementation reverses the signs of aging in muscles of older wild type mice.14,16 The present study was designed to assess the ability of EIM and muscle histological analysis to detect differences in wild-type versus mtDNA mutator mice of the same age and whether any potential impact of NMN treatment could also be identified. We were also particularly interested in one impedance parameter, the fc, obtained from the multifrequency data set, that is highly related to myofiber cross-sectional area.18
Materials and Methods
Animals and Drug Therapy
All animal procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center (BIDMC). Homozygous knock-in mtDNA mutator mice (PolgAD257A/D257A) and wild type (WT) littermates (PolgA+/+) were bred and maintained at BIDMC Animal Facility as previously published.19,20 The presence of the PolG knock-in mutation was confirmed in this line using standard methods.9,19,20 PolG and WT mice (male and female) were treated with either normal drinking water or drinking water containing NMN (obtained from David Sinclair, PhD, Harvard Medical School, Boston, MA) at 300 mg/kg/day for 20 weeks starting at 40 weeks of age. A total of 30 WT (water n = 13, NMN n= 17) and 24 mtDNA mutator gastrocnemii (water n= 16, NMN n= 8) were available and used in this study. Mice were euthanized at ~ 60 weeks of age. Body mass was recorded immediately prior to euthanization.
The PolG mice used for these experiments were also used for a simultaneously conducted study of central nervous system alterations, to be published separately. Due to the constraints imposed by those other investigations, only ex vivo EIM experiments could be completed.
Gastrocnemius muscle extraction
Mice were euthanized by overdose of ketamine and xylazine via intraperitoneal injection. The entire gastrocnemius muscle excised from the limb at its proximal extent just below the knee and distally by cutting the gastrocnemius tendon at its insertions, after removing the more superficial bicep femoris muscle. The wet mass of the entire muscle was determined using a standard analytical balance. The gastrocnemius muscle was cut to approximately 5 × 5 mm2 (with variable height) so as to fit into the dielectric cell used for impedance measurements.
Dielectric cell
We used a Plexiglass dielectric measuring cell developed for the measurement of muscle dielectric properties as previously described.21 The dielectric cell consists of two flat plate stainless steel current electrodes contacting the entire side of the slab of muscle. Two disposable monopolar electromyography needle electrodes (Natus 902-DMG50-TP) were used as voltage drop measuring electrodes that projected through the holes of the tight-fitting cover. These voltage electrodes were inserted through the holes of the plastic cover so that they just contacted the surface of the muscle. For measurements of the gastrocnemius, a cell W = 5 mm x L = 5 mm base and H = 5 mm high was used. The exact height of each GA was measured using calipers; typical muscle height was ~ 3 mm. Voltage electrodes being placed at a single distance of d = 3 mm apart. The gastrocnemius muscle was first placed in the cell with the fibers oriented perpendicularly to the metal plates (for longitudinal muscle measurements), then removed and placed with the fibers placed parallel to the plates (for transverse muscle measurements).
Impedance measurements
Electrical impedance measurements were made with the EIM mView impedance spectroscopy system (Myolex Inc., Boston, MA) using a frequency sweep spectroscopy technique. In total, 41 logarithmically-spaced frequencies (10 frequencies per decade) were measured from 1 kHz to 10 MHz.
Data analysis, calibration, and parameterization
Resistance (R) and reactance (X) impedance values were collected across the frequency range. Next, in order to calibrate the entire impedance measuring setup for potential measurement errors due to, for example, electrode polarization and stray capacitances, identical measurements were made with normal (0.9%) saline solution placed into the dielectric cell. From the calibrated impedance values, we then calculated the muscle conductivity (σ), in S/m, and the relative permittivity (εr), dimensionless, at each individual frequency measured, as previously described.21
Obtained conductivity and relative permittivity data were fitted in MATLAB (The Mathworks, Natick, MA, USA) to the complex resistivity version of the Cole–Cole dielectric model,22 with the weighted complex nonlinear least square method,23 to yield the parameters resistivity at 0 and infinite frequency, ρo and ρinf, respectively; center frequency fc, and α. These parameters are associated with cell density (ρo and ρinf), myofiber cross-sectional area (fc) and cell size distribution (α). If the data was not of sufficient quality for modeling (e.g., if the frequency spectrum was contaminated by contact artifact or other noise), the data was not used in this analysis.
Muscle histology
After the impedance measurements, the gastrocnemius muscle tissue was placed in 10% buffered formalin and fixed for at least 48 hr. Samples were then embedded in paraffin blocks, sectioned into 10 μm slices and stained with anti-collagen VI antibody (Abcam ab6588) to identify the cell membranes and 4’, 6-diamidino-2-phenylindole to stain nuclei. Sections were subsequently imaged at 20x with a Zeiss AxioImager M1 epifluorescence microscope and myofiber area was measured using muscle morphometry plug-in (developed by Anthony Sinadinos using Eclipse IDE) in FIJI, the open source image processing software (ImageJ Version 2.0.0-rc-68/1.52d) (NIH Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997–2016.). On average, approximately 225 fibers were counted per WT muscle and 450 myofibers per PolG muscle.
Statistical analysis
Statistical analysis on the extracted physiological data, histological data, and the Cole-Cole parameters was performed using GraphPad Prism V8 (GraphPad Software, Inc. La Jolla, CA). Unless otherwise noted, all data are reported as mean ± SEM. Multiple group comparisons were performed by one-way ANOVA with post-hoc t-tests using Tukey’s multiple comparisons test. For correlation analyses, the Pearson correlation coefficient was calculated. Data was considered significant with p < 0.05.
Results
See Table 1 for a complete summary of the results.
Table 1.
Summary of data
| Female | Male | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| WT H2O | WT NMN | PolG H2O | PolG NMN | WT H2O | WT NMN | PolG H2O | PolG NMN | ||
| Body mass (g) | 26.58±1.07 | 31.28±0.71 | 18.88±0.37 | 20.45±0.53 | 33.89±0.69 | 40.30±201 | 23.34±1.20 | 22.68±0.92 | |
| Gastrocnemius mass (g) | 0.156±0.005 | 0.146±0.004 | 0.083±0.005 | 0.094±0.014 | 0.183±0.004 | 0.175±0.005 | 0.138±0.004 | 0.121±0.009 | |
| Myofiber CSA (μm2) | 2245±94 | 2100±94 | 1066±72 | 983±63 | 2552±154 | 2238±141 | 1368±75 | 1298±131 | |
| 50 kHz Conductivity longitudinal (S/m) | 0.342±0.033 | 0.327±0.023 | 0.300±0.033 | 0.251±0.047 | 0.324±0.029 | 0.358±0.009 | 0.407±0.042 | 0.271±0.047 | |
| 50 kHz Relative Permittivity longitudinal (dimensionless) | 36550±4353 | 39878±2602 | 24067±2128 | 24333±4274 | 41711±3181 | 44477±2634 | 35311±1794 | 44554±5907 | |
| 50 kHz Conductivity transverse (S/m) | 0.182±0.027 | 0.164±0.016 | 0.144±0.016 | 0.068±0.008 | 0.160±0.019 | 0.160±0.014 | 0.249±0.039 | 0.222±0.036 | |
| 50 kHz Relative Permittivity transverse (dimensionless) | 33706±3047 | 35210±929 | 19758±1034 | 16714±660 | 36204±2156 | 34008±1495 | 32768±2039 | 38401±3288 | |
| ρo, longitudinal (Ω m) | 6.191±1.372 | 7.264±0.965 | 5.790±0.982 | 7.496±2.01 | 7.717±0.787 | 6.365±0.229 | 3.831±0.404 | 5.718±0.685 | |
| ρinf longitudinal (Ω m) | 1.200±0.085 | 1.253±0.049 | 1.551±0.091 | 1.716±0.077 | 1.270±0.071 | 1.274±0.068 | 1.068±0.087 | 1.367±0.156 | |
| fc longitudinal (Hz) | 15059±8036 | 9545±1810 | 59478±14074 | 19866±3242 | 11366±2753 | 8143±1474 | 56997±14635 | 24384±3765 | |
| α longitudinal (dimensionless) | 0.522±0.033 | 0.581±0.020 | 0.628±0.064 | 0.552±0.049 | 0.575±0.027 | 0.546±0.022 | 0.558±0.037 | 0.677±0.030 | |
| ρo transverse (Ω m) | 10.720±1.930 | 11.370±0.915 | 11.010±1.812 | 24.530±4.379 | 12.400±1.079 | 11.840±1.243 | 6.600±1.296 | 7.937±1.597 | |
| ρinf transverse (Ω m) | 1.416±0.148 | 1.577±0.081 | 2.078±0.119 | 2.314±0.290 | 1.507±0.075 | 1.679±0.067 | 1.294±0.101 | 1.191±0.074 | |
| fc transverse (Hz) | 28921±6360 | 23047±2380 | 62192±10926 | 31086±5137 | 19399±1512 | 22713±3911 | 60291±14823 | 34082±3334 | |
| α transverse (dimensionless) | 0.690±0.015 | 0.756±0.022 | 0.774±0.022 | 0.784±0.017 | 0.736±0.029 | 0.744±0.023 | 0.775±0.038 | 0.697±0.016 | |
All values given as mean ± standard error of mean.
WT, wild type; NMN, nicotinamide mononucledotide, CSA, cross-sectional area; H2O, water; ρo, resistivity at 0 frequency; ρinf, resistivity at infinite frequency; fc, center frequency; α alpha parameter.
Animal and muscle mass.
Figure 1 compares body mass between the PolG and WT animals, treated with either H2O or NMN, both for individual (Figure 1A) and combined sexes (Figure 1B), demonstrating the considerably smaller body mass for the PolG animals (35% lower than WT; range 29–44%). Figures 1C and 1D provide the mass of the gastrocnemius muscle for individual and combined sexes, respectively, again revealing the considerably lower muscle mass in the PolG mice (35% lower than WT; range 26–47%). These observations were unchanged when gastrocnemius muscle mass was normalized to body mass (data not shown). A small but significant increase in body mass was also observed in WT mice treated with NMN.
Figure 1.
Body mass for WT and PolG animals at time of euthanasia, separated by treatment group and sex (A) and treatment group alone (B). Parallel comparisons for gastrocnemius muscle mass (C, D). Note anticipated reductions in body and muscle mass in PolG versus WT animals, as well as in the females compared to males, but no clear effect of NMN therapy in the PolG animals, and only a slight increase in the body mass in the WT animals. All values given as mean ± standard error of mean. One-way ANOVA with Tukey’s test; * p < 0.05, *** p < 0.001, **** p<0.0001; WT H20, N=13; WT NMN, N=17; PolG H20, N=16, PolG NMN=8.
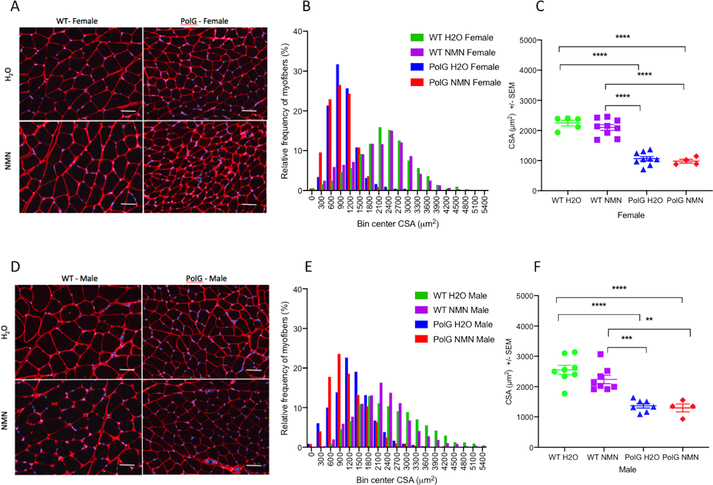
Histological analysis.
Figure 2A,D shows representative histological data from PolG and WT animals, female and male respectively, revealing a marked difference in fiber size between the WT and PolG groups, far outweighing the anticipated sex-associated differences (with male animals having slightly larger average myofiber CSA than female). Figure 2B,E provides histograms depicting the frequency distribution of the overall individual myofiber CSA data across all the groups, again, quantifying the marked reduction in fiber size in the PolG mice (treated and untreated) as compared to their WT counterparts. Figure 2C,F provides scatter plots showing the mean CSA for each animal. As with muscle and body mass values, there is no apparent effect of NMN on myofiber size in either PolG or WT animals of either sex (Table 1).
Figure 2.
Representative examples of gastrocnemius (GA) muscle histology (stained with Collagen VI (red, cell membranes and DAPI (blue, nuclei) in both untreated and NMN-treated WT and PolG female (A) and male (D) mice. The corresponding frequency distributions of the GA cross-sectional area are shown in histograms for female (B) and male (E) animals. Note the major shift to smaller size fibers in the both NMN-treated and untreated PolG animals in both sexes. Individual animal data showing consistent reductions in myofiber size in both female (C) and male (D) PolG animals, regardless of treatment status. Values given as mean ± standard error of mean. Scale bar = 50 μm. One-way ANOVA with Tukey’s test; ** p < 0.01, *** p < 0.001, **** p<0.0001; WT H20, N=13; WT NMN, N=17; PolG H20, N=16, PolG NMN=8.
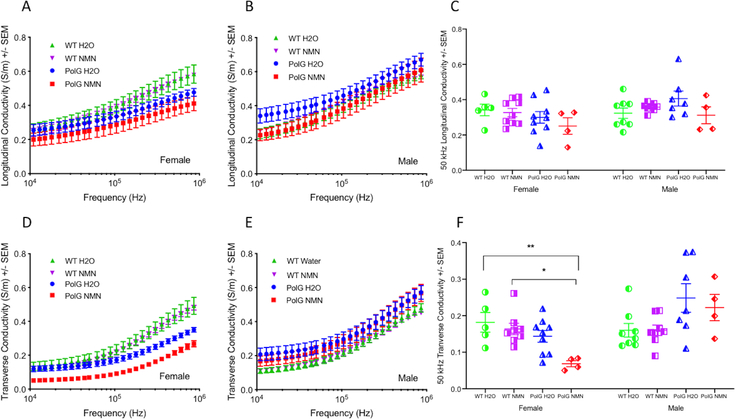
Electrical properties.
The multifrequency conductivity and relative permittivity data are shown in Figures 3A,B and 4D,E, respectively, in both the longitudinal and transverse directions. Reductions in both conductivity and relative permittivity multifrequency data can be seen in the female PolG mice as compared to female WT, which is most obvious in the transverse direction. In contrast, we observed no differences in either multifrequency conductivity or relative permittivity in the male PolG compared to male WT animals. Single frequency 50 kHz analyses (Figure 3C,F and Figure 4C,F) show significant reductions in the transverse conductivity and in both the longitudinal and transverse relative permittivity values in female but not in male PolG animals at 50 kHz. No treatment effect of NMN was observed in either PolG or WT animals.
Figure 3.
Conductivity from 10 kHz to 1 MHz in both longitudinal and transverse directions of NMN-treated as well as untreated female (A, B) and male (D, E) animals. Note the reduction in the transverse conductivity values for both NMN- treated and untreated PolG female mice across the entire frequency range. Comparisons of the effect of treatment and sex in both longitudinal and transverse conductivity at 50 kHz are shown (C) and (D), respectively. Values given as mean ± standard error of mean. One-way ANOVA with Tukey’s test; * p < 0.05, ** p < 0.01; WT H20, N=13; WT NMN, N=17; PolG H20, N=16, PolG NMN=8.
Figure 4.
Relative permittivity from 10 kHz to 1 MHz in both longitudinal and transverse directions of NMN-treated as well as untreated female (A, B) and male (D, E) animals. Note the clear reduction in longitudinal as well as transverse relative permittivity values for both the untreated and NMN-treated PolG female mice particularly at lower frequencies. Comparisons of the effect of treatment and sex in both longitudinal and transverse relative permittivity at 50 kHz are shown (C) and (D), respectively. Note the significant reduction in both longitudinal and transverse relative permittivity for the untreated as well as NMN-treated female PolG mice at this frequency. Values given as mean ± standard error of mean. One-way ANOVA with Tukey’s test; * p < 0.05, ** p < 0.01, **** p<0.0001; WT H20, N=13; WT NMN, N=17; PolG H20, N=16, PolG NMN=8.
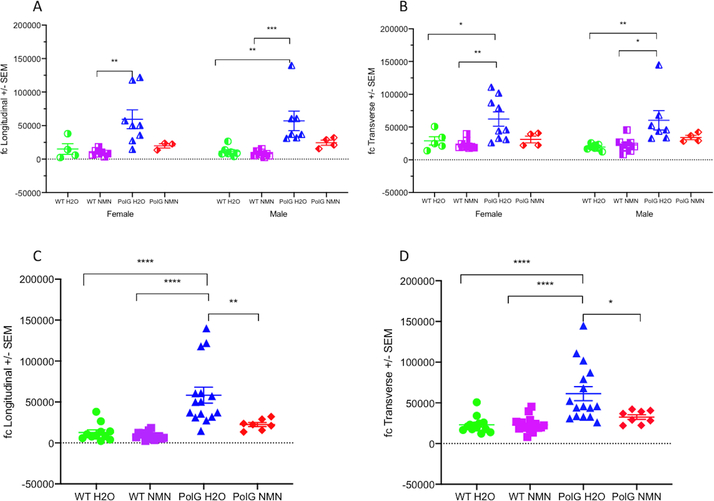
Modeled impedance parameters.
In contrast to conductivity and relative permittivity, fc showed a consistent increase in both female and male PolG animals (Figure 5A,B), as well as when the sexes were combined in both the longitudinal and transverse directions (Figure 5 C,D); none of the other modelled parameters were different. In addition, when the sexes were combined, the NMN-treated PolG animals had significantly lower fc values than water-treated PolG animals, reaching values similar to those obtained in the WT animals. No therapy effects were observed in the WT mice. Of note, for the longitudinal analysis only, the data from 6 of the 54 animals were excluded since our Cole model program could not fit the data set and provide a value. This usually occurs when the shape of the multifrequency data is atypical in some fashion, likely due to contact artifact or noise.
Figure 5.
Scatter fc plots in the four groups of animals (i.e., WT vs PolG, NMN-treated vs untreated) for female (A), male (B), or combined sex (C,D) animals in both longitudinal (A,C) and transverse (B,D) directions. Note the significant increase in fc values in the untreated PolG mice irrespective of sex, and the trend in PolG animals treated with NMN towards fc values of WT animals. Values given as mean ± standard error of mean. One-way ANOVA with Tukey’s test; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p<0.0001; Longitudinal: WT H20, N=11; WT NMN, N=15; PolG H20, N=15, PolG NMN=7. Transverse: WT H20, N=13; WT NMN, N=17; PolG H20, N=16, PolG NMN=8.
Correlation between fc and histological data.
Figure 6 provides bivariate correlation analysis comparing the fc values in both the longitudinal (Figure 6A) and transverse (Figure 6B) directions with the measured myofiber CSA across all the groups. A significant correlation was identified for both orientations.
Figure 6.
Scatter plots showing correlation between mean myofiber size per animal and measured fc values in both longitudinal (A) and transverse (B) directions. Pearson correlation; Longitudinal: WT H20, N=11; WT NMN, N=15; PolG H20, N=15, PolG NMN=7; Transverse: WT H20, N=13; WT NMN, N=17; PolG H20, N=16, PolG NMN=8.
Discussion
This study confirms the ability of EIM to detect alterations in myofiber CSA, with the PolG animals most notably showing differences in the fc parameter, a value strongly related to cell size.24,25 Previous studies11,26, not using EIM, pooled male and female PolG mice to increase the statistical power based on a prior report indicating that there were no sex differences in mitochondrial oxygen consumption in the heart, skeletal muscle and liver, ATP content, mitochondrial H2O2 generation, oxidative stress and markers of apoptosis in WT mice of the same background.27 However, here we found statistically significant differences in both conductivity and relative permittivity in female PolG compared to female WT mice but not in male PolG compared to WT male mice. Combining all the group data, we also observed a correlation between fc values and the average myofiber CSA.
Neither the analysis of muscle mass nor myofiber CSA demonstrated a significant difference with NMN supplementation. Still, the NMN-treated PolG mice (combined sex) showed significant reductions in the fc value in both the longitudinal and transverse directions as compared to the water-treated animals. There are several potential hypotheses to explain this observation. First, it is possible that there was a population of myofibers on the periphery of the muscle block in which CSA had increased with NMN treatment but were not included in the histological analysis. Second, fc values could also have changed due to alterations in either the intracellular conductivity of the myofibers, the myofiber density, and/or the myofiber membrane capacitance per unit of area, none of which were evaluated in this study.
In agreement with a previous study that evaluated EIM in a group of live 3-month-old and 25-month-old C57/B6 mice and found an increase in both longitudinal and transverse fc in the aged mice,8 here, we found a consistent increase in the fc value in the untreated PolG mice, that correlates with the typical premature aging features associated with this model. Moreover, in the present study, fc also correlates with the differences in myofiber CSA observed in the PolG animals, as opposed to the findings reported in the previous work, which showed no significant differences in myofiber CSA between the 3-month-old and 25-month-old groups.
The present study has a number of limitations. First, since tissue was only measured shortly after death, post-mortem ischemia could have altered the impedance data as compared to living muscle.28,29 However, our previous work in both humans and mice support that these impedance alterations are readily detectable using surface EIM methods.6–8 Second, our total sample numbers were small. Third, errors positioning the excised muscle in the impedance-measuring cell could have compromised the accuracy of the conductivity and relative permittivity properties measured. Nonetheless, the range of values reported here are well within the range of data available in the literature.30 Additionally, we did not evaluate mitochondrial content or the presence of fat and connective tissue in the muscle histologically, the latter known to be altered in the mtDNA mutator mice.9 Finally, we did not assess oxidative damage in the muscle tissue because the presence of oxidative damage in this model is controversial, and likely at a low level that affects pro-apoptotic and pro-inflammatory pathways without causing actual damage to proteins. In fact, early publications describing this model suggested the absence of oxidative stress;9,11only later in later publications, using specially developed techniques, were increased mitochondrial reactive oxygen species in this model found and beneficial effects of antioxidant treatment demosntrated31,32 Moreover, EIM will be sensitive only to secondary histological change and not to increased oxidation itself.
Despite these limitations, these data provide new insights into the relationship between EIM’s fc value and tissue histology alterations observed in the PolG mouse model of premature aging. Further investigation into EIM for the assessment of muscle aging and potential therapies should be considered.
Acknowledgements:
This work was funded by the National Institutes of Health grant R01 NS091159 (SBR), R21NS094840 (DKS and JC-M) and a BIDMC Neurology Pilot grant (DKS and JC-M).
Abbreviations:
- CSA
cross-sectional area
- EIM
electrical impedance myography
- mtDNA
mitochondrial DNA
- NAD+
nicotinamide adenine dinucleotide
- NMN
nicotinamide mononucleotide
- WT
wild type
Units:
- Ω m
Ohms meter
- kHz
kiloHertz
- MHz
megaHertz
- S/m
Siemens/meter
Footnotes
Disclosure of Conflicts of Interest:
Dr. Rutkove has equity in, and serves a consultant and scientific advisor to, Myolex, Inc. a company that designs impedance devices for clinical and research use; he is also a member of the company’s Board of Directors. The company also has an option to license patented impedance technology of which Dr. Rutkove is named as an inventor. Dr. Sanchez also serves as a consultant to Myolex, Inc. as well as to Impedimed, Inc. another company that develops impedance technology for clinical use.
Ethical Publication Statement:
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Beaudart C, Reginster JY, Slomian J, Buckinx F, Dardenne N, Quabron A, et al. Estimation of sarcopenia prevalence using various assessment tools. Experimental Gerontology 2015; 61:31–37. [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clinical and Experimental Research 2016; 28:1047–1060. [DOI] [PubMed] [Google Scholar]
- 3.Sergi G, Trevisan C, Veronese N, Lucato P, Manzato E. Imaging of sarcopenia. European Journal of Radiology 2016; 85:1519–1524. [DOI] [PubMed] [Google Scholar]
- 4.Minetto MA, Caresio C, Menapace T, Hajdarevic A, Marchini A, Molinari F, et al. Ultrasound-Based Detection of Low Muscle Mass for Diagnosis of Sarcopenia in Older Adults. PM and R 2016; 8:453–462. [DOI] [PubMed] [Google Scholar]
- 5.Rutkove SB, Sanchez B. Electrical Impedance Methods in Neuromuscular Assessment: An Overview. Cold Spring Harbor Perspectives in Medicine 2018:a034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiological measurement 2006; 27:953–959. [DOI] [PubMed] [Google Scholar]
- 7.Kortman HG, Wilder SC, Geisbush TR, Narayanaswami P, Rutkove SB. Age- and gender-associated differences in electrical impedance values of skeletal muscle. Physiological measurement 2013; 34:1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold WD, Taylor RS, Li J, Nagy JA, Sanchez B, Rutkove SB. Electrical impedance myography detects age-related muscle change in mice. PLoS ONE 2017; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujoth CC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Medicine: Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005; 309:481–484. [DOI] [PubMed] [Google Scholar]
- 10.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004; 429:417–423. [DOI] [PubMed] [Google Scholar]
- 11.Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, et al. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS ONE 2010; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgar D, Trifunovic A. The mtDNA mutator mouse: Dissecting mitochondrial involvement in aging. Aging 2009; 1:1028–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshino J, Mills KF, Yoon MJ, Imai SI. Nicotinamide mononucleotide, a key NAD + intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metabolism 2011; 14:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013; 155:1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederick DW, Loro E, Liu L, Davila A, Chellappa K, Silverman IM, et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metabolism 2016; 24:269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metabolism 2016; 24:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher RS, Ratajczak J, Doig CL, Oakey LA, Callingham R, Da Silva Xavier G, et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Molecular Metabolism 2017; 6:819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy JA, Kapur K, Taylor RS, Sanchez B, Rutkove SB. Electrical impedance myography as a biomarker of myostatin inhibition with ActRIIB-mFc: a study in wild-type mice. Future Science OA 2018; 4:FSO308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai Y, Kiselak T, Clark J, Clore E, Zheng K, Cheng A, et al. Behavioral and metabolic characterization of heterozygous and homozygous POLG mutator mice. Mitochondrion 2013; 13:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai Y, Clark J, Zheng K, Kujoth GC, Prolla TA, Simon DK. Somatic mitochondrial DNA mutations do not increase neuronal vulnerability to MPTP in young POLG mutator mice. Neurotoxicology and Teratology 2014; 46:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Jafarpoor M, Bouxsein M, Rutkove SB. Distinguishing neuromuscular disorders based on the passive electrical material properties of muscle. Muscle Nerve 2015; 51:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole KS, Cole RH. Dispersion and absorption in dielectrics. I. Alternating current characteristics. J Chem Phys 1941; 9:341–51. [Google Scholar]
- 23.Sanchez B, Bandarenka AS, Vandersteen G, Schoukens J, Bragos R. Novel approach of processing electrical bioimpedance data using differential impedance analysis. Medical engineering & physics 2013; 35:1349–1357. [DOI] [PubMed] [Google Scholar]
- 24.Grimnes S, Martinsen OG. Bioimpedance and Bioelectricity Basics. 3rd ed. Academic Press, 2014. [Google Scholar]
- 25.Kapur K, Taylor RS, Qi K, Nagy JA, Li J, Sanchez B, et al. Predicting myofiber size with electrical impedance myography: A study in immature mice. Muscle Nerve 2018; 58:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph AM, Adhihetty PJ, Wawrzyniak NR, Wohlgemuth SE, Picca A, Kujoth GC, et al. Dysregulation of Mitochondrial Quality Control Processes Contribute to Sarcopenia in a Mouse Model of Premature Aging. PLoS ONE 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz A, Hiona A, Kujoth GC, Seo AY, Hofer T, Kouwenhoven E, et al. Evaluation of sex differences on mitochondrial bioenergetics and apoptosis in mice. Experimental Gerontology 2007; 42:173–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gersing E Impedance spectroscopy on living tissue for determination of the state of organs. Bioelectrochemistry and Bioenergetics 1998; 45:145–149. [Google Scholar]
- 29.D’Entremont MI, Paulson AT, Marble AE. Impedance spectroscopy: An accurate method of differentiating between viable and ischaemic or infarcted muscle tissue. Medical and Biological Engineering and Computing 2002; 40:380–387. [DOI] [PubMed] [Google Scholar]
- 30.Nagy JA, DiDonato CJ, Rutkove SB, Sanchez B. Permittivity of ex vivo healthy and diseased murine skeletal muscle from 10 kHz to 1 MHz. Scientific Data 2019; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong YXG, van Bergen N, Trounce IA, Bui BV., Chrysostomou V, Waugh H, et al. Increase in mitochondrial DNA mutations impairs retinal function and renders the retina vulnerable to injury. Aging Cell 2011; 10:572–583. [DOI] [PubMed] [Google Scholar]
- 32.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 2010; 9:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]