Key Points
Question
Among patients with recent myocardial infarction or hospitalization for heart failure, is high-dose trivalent inactivated influenza vaccine more effective than standard-dose quadrivalent inactivated influenza vaccine for reducing all-cause mortality or hospitalizations for cardiac or pulmonary causes?
Findings
In this randomized clinical trial that involved 5260 adults and was conducted over 3 influenza seasons, there was no significant difference in the time to first occurrence of all-cause death or cardiopulmonary hospitalization during each enrolling season for those in the high-dose group vs the standard-dose group (hazard ratio, 1.06).
Meaning
In patients with high-risk cardiovascular disease, high-dose trivalent influenza vaccine, compared with standard-dose quadrivalent vaccine, did not significantly reduce all-cause mortality or hospitalizations for cardiac or pulmonary causes; influenza vaccination remains strongly recommended in this population.
Abstract
Importance
Influenza is temporally associated with cardiopulmonary morbidity and mortality among those with cardiovascular disease who may mount a less vigorous immune response to vaccination. Higher influenza vaccine dose has been associated with reduced risk of influenza illness.
Objective
To evaluate whether high-dose trivalent influenza vaccine compared with standard-dose quadrivalent influenza vaccine would reduce all-cause death or cardiopulmonary hospitalization in high-risk patients with cardiovascular disease.
Design, Setting, and Participants
Pragmatic multicenter, double-blind, active comparator randomized clinical trial conducted in 5260 participants vaccinated for up to 3 influenza seasons in 157 sites in the US and Canada between September 21, 2016, and January 31, 2019. Patients with a recent acute myocardial infarction or heart failure hospitalization and at least 1 additional risk factor were eligible.
Interventions
Participants were randomly assigned to receive high-dose trivalent (n = 2630) or standard-dose quadrivalent (n = 2630) inactivated influenza vaccine and could be revaccinated for up to 3 seasons.
Main Outcomes and Measures
The primary outcome was the time to the composite of all-cause death or cardiopulmonary hospitalization during each enrolling season. The final date of follow-up was July 31, 2019. Vaccine-related adverse events were also assessed.
Results
Among 5260 randomized participants (mean [SD] age, 65.5 [12.6] years; 3787 [72%] men; 3289 [63%] with heart failure) over 3 influenza seasons, there were 7154 total vaccinations administered and 5226 (99.4%) participants completed the trial. In the high-dose trivalent vaccine group, there were 975 primary outcome events (883 hospitalizations for cardiovascular or pulmonary causes and 92 deaths from any cause) among 884 participants during 3577 participant-seasons (event rate, 45 per 100 patient-years), whereas in the standard-dose quadrivalent vaccine group, there were 924 primary outcome events (846 hospitalizations for cardiovascular or pulmonary causes and 78 deaths from any cause) among 837 participants during 3577 participant-seasons (event rate, 42 per 100 patient-years) (hazard ratio, 1.06 [95% CI, 0.97-1.17]; P = .21). In the high-dose vs standard-dose groups, vaccine-related adverse reactions occurred in 1449 (40.5%) vs 1229 (34.4%) participants and severe adverse reactions occurred in 55 (2.1%) vs 44 (1.7%) participants.
Conclusions and Relevance
In patients with high-risk cardiovascular disease, high-dose trivalent inactivated influenza vaccine, compared with standard-dose quadrivalent inactivated influenza vaccine, did not significantly reduce all-cause mortality or cardiopulmonary hospitalizations. Influenza vaccination remains strongly recommended in this population.
Trial Registration
ClinicalTrials.gov Identifier: NCT02787044
This randomized clinical trial compares the effect of high-dose vs standard-dose influenza vaccine on all-cause mortality and hospitalizations due to cardiovascular or pulmonary causes in patients with high-risk cardiovascular disease.
Introduction
Influenza leads to significant morbidity and mortality and increased health care burden.1 Individuals with underlying cardiovascular disease are more susceptible to influenza-related complications and adverse clinical outcomes.2 Observational studies have demonstrated a temporal association between influenza and acute cardiac events, including myocardial infarction and acute heart failure.3,4,5,6,7 In a meta-analysis of randomized clinical trials, influenza vaccination was associated with a lower risk of major adverse cardiovascular events compared with no vaccination.8
Influenza vaccine formulations vary in their preparation, the amount and number of viral antigens, and the presence of adjuvant. High-dose influenza vaccine contains 4 times the amount of hemagglutinin compared with standard-dose vaccine, and reduced laboratory-confirmed symptomatic influenza in a large randomized clinical trial compared with standard-dose influenza vaccine.9,10 High-dose vaccine is approved for adults 65 years and older, who may derive less protection from standard-dose vaccine due to reduced antibody-mediated responses.11,12 Compared with patients of similar age without cardiovascular disease, patients with cardiovascular disease have also been shown to mount a less robust humoral immune response to standard-dose influenza vaccine, suggesting that a higher-dose vaccine might confer greater protection in this population.13 In patients with heart failure, higher-dose influenza vaccine elicited higher antibody titers compared with standard-dose vaccine.14 The Centers for Disease Control and Prevention recommends annual influenza vaccination in those 6 months or older and professional cardiovascular societies emphasize annual influenza vaccination in patients with cardiovascular conditions,15 without specific guidance regarding the choice of vaccine formulation. This pragmatic randomized clinical trial sought to determine whether high-dose influenza vaccine would reduce all-cause mortality and hospitalizations due to cardiovascular or pulmonary causes compared with standard-dose vaccine in a high-risk cardiovascular population.
Methods
Ethics and Regulatory Issues
A National Heart, Lung, and Blood Institute–appointed protocol review committee and ethics committees at each enrolling site approved the protocol. All participants provided written informed consent in accordance with established guidelines.
Trial Design
The Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure (INVESTED) study was a pragmatic, randomized, double-blind, active comparator trial conducted at 157 participating centers in the US and Canada over 3 influenza seasons. The protocol and statistical analysis plan are presented in Supplement 1 and Supplement 2.
The study was overseen by a joint clinical coordinating center at Brigham and Women’s Hospital and the University of Minnesota and a data coordinating center at the University of Wisconsin-Madison. An independent data and safety monitoring board monitored trial conduct and patient safety.16 The study group members and investigators are listed in Supplement 3.
Participants
Eligible participants included those hospitalized for acute myocardial infarction in the past 12 months or for heart failure in the past 24 months who had at least 1 of the following additional risk factors: older than 65 years, current or prior left ventricular ejection fraction less than 40%, diabetes, body mass index greater than or equal to 30, history of chronic kidney disease (defined as estimated glomerular filtration rate ≤60 for at least 2 readings in the past year), ischemic stroke, peripheral artery disease, current tobacco use, or a myocardial infarction or heart failure hospitalization prior to the index hospitalization. Race and ethnicity were assessed per National Institutes of Health guidelines and were self-reported based on fixed categories.
Key exclusion criteria included known allergy, hypersensitivity (anaphylaxis), or Guillain-Barré syndrome related to influenza vaccine; severe allergy to egg protein; life expectancy less than 9 months; previous receipt of influenza vaccine during enrolling season; acute infection requiring use of antibiotics within 14 days of randomization; known fever within 7 days prior to randomization; pregnancy; or lactation.
Study Procedures
Patients were enrolled between mid-September until December 31 during the 2016-2017 season and until January 31 for the 2017-2018 and 2018-2019 seasons. Participants were randomized in a 1:1 ratio using permuted blocks of random block size ranging from 4 to 6 to receive double-blind treatment with high-dose trivalent inactivated influenza vaccine (containing 60 μg of hemagglutinin per strain) or standard-dose quadrivalent inactivated influenza vaccine (containing 15 μg of hemagglutinin per strain) administered intramuscularly. Randomization was centralized and performed electronically, balanced by site, without stratification using Stars software (Frontier Science Foundation), with site balancing using a minimization method.17,18 Participants received vaccination annually for up to 3 years according to their initial randomized group assignment. If a participant did not wish to continue receiving influenza vaccine as part of the trial, they were censored on July 31 of the last season in which they participated and were no longer followed up. Participants were contacted by phone approximately 1 week following vaccination for ascertainment of local injection site reactions and other adverse effects and again during the spring and summer following vaccination each year for ascertainment of hospitalization events and vital status.
Study Outcomes
The primary study outcome was time to a composite of all-cause death or hospitalization for cardiovascular or pulmonary causes during each enrolling influenza season, with censoring in the first 2 weeks after vaccination and after July 31 of the respective season. Secondary outcomes were total (first and recurrent) hospitalizations for cardiovascular or pulmonary causes or all-cause death across all enrolling influenza seasons, the time to first occurrence of death due to cardiovascular causes or cardiovascular hospitalization within each enrolling season, the time to first occurrence of all-cause death or hospitalization due to cardiovascular or pulmonary causes across all enrolling seasons, and the time to first occurrence of individual components of the primary efficacy end point during each enrolling season. An independent clinical events committee blinded to study treatment used prespecified criteria to adjudicate and categorize all deaths and hospitalizations based on source records or investigator narratives, including hospitalizations due to influenza or pneumonia (Supplement 4).19 Vaccine-related adverse reactions were assessed 1 week after vaccination (primary safety outcome); secondary safety outcomes included serious adverse events, including prespecified severe adverse events of special interest (Guillain-Barré syndrome, Bell palsy, encephalitis/myelitis, optic neuritis, Stevens-Johnson syndrome, and toxic epidermal necrolysis).
Statistical Considerations
The trial was originally designed to have at least 90% power to detect an 18% relative risk reduction (ie, a hazard ratio of 0.82 in the primary composite end point of all-cause mortality or cardiopulmonary hospitalization). The control event rate at 1 year was estimated to be 9%, with lower event rates in subsequent years. The effect size of 18% was based on a diluted estimate of a 27% risk reduction for the composite end point from data comparing 2 active vaccination treatments.8
The target enrollment was initially 9300 patients, aiming for a total of 1296 primary outcome events over 4 influenza seasons. A formal interim analysis for efficacy was planned at the end of the 2017-2018 and 2018-2019 influenza seasons with information fraction of 0.259 and 0.599 based on the O’Brien-Fleming group sequential method. The first formal interim analysis was performed on February 21, 2019.
The primary and select secondary efficacy analyses and the analysis of adverse events were based on an analysis in which only those receiving vaccinations in any given year were included and events of interest were accrued from 14 days after vaccination until July 31 of each enrolling season. For the primary analysis, participants without an event of interest were censored on July 31 of each season. Other secondary efficacy analyses were performed according to randomization group and included all randomized participants and events of interest accrued across multiple seasons from randomization until patients were censored due to decision not to participate the following year, withdrawal of consent, or time of loss to follow-up or until July 31, 2019 (the final date of follow-up).
The primary efficacy analysis was based on a log-rank test with robust variance estimate to account for within-participant correlation across multiple seasons, stratified by enrolling season; the corresponding hazard ratio and 95% CI were estimated using an unadjusted Cox proportional hazards model, stratified by enrolling season, with robust variance estimate.20 Proportionality was assessed using the Schoenfeld test.21 The subgroup × treatment interaction for 12 prespecified subgroups was assessed in a similar model to the primary analysis with the subgroup × treatment interaction term as an additional covariate in the model. Analysis of adverse events was based on a χ2 test, treating adverse events from the same participant over multiple seasons as independent events. As a secondary efficacy analysis, the composites of cardiovascular death or hospitalization were analyzed using standard methods for competing risk22 with a sandwich-type robust variance estimate.23 The composite of all-cause mortality or cardiopulmonary hospitalization across multiple seasons was analyzed using a log-rank test and Cox proportional hazards model, stratified by randomization season. The number of events of all-cause mortality and recurrent cardiopulmonary hospitalizations was analyzed using a proportional means model.24 In a prespecified sensitivity analysis, participants were censored at a time most consistent with influenza activity below epidemic threshold (May 15) for an in-season analysis. In another prespecified sensitivity analysis, the primary analysis was repeated using inverse probability weighting to account for differential survivorship bias and bias due to dropout after randomization. A post hoc comparison of prospectively collected and adjudicated influenza and pneumonia hospitalizations between groups was performed. The primary results were also analyzed post hoc in an analysis stratified by site. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Time-to-event data were censored at the last time of contact before loss to follow-up under the assumption of random loss. A 2-sided P value of less than .05 was considered statistically significant. The statistical analysis plan was finalized on July 31, 2020, before unblinding of the results. All analyses were performed using SAS, version 9.4, and R, version 3.6.1.
Early Trial Termination
On September 23, 2019, prior to initiation of the study’s fourth enrolling season, the data and safety monitoring board recommended early termination of the trial based on 5260 enrolled participants and 1770 events (159 deaths and 1611 cardiopulmonary hospitalizations), determining that the trial exceeded the number of events necessary to test the hypothesis and that determining superiority of high-dose vaccine to standard-dose vaccine would be futile. This decision was based solely on the data and safety monitoring board’s interpretation of the data and not on prespecified stopping rules or futility analysis; the National Heart, Lung, and Blood Institute accepted this recommendation.
Results
Trial Flow and Baseline Characteristics of Enrolled Participants
During 3 enrolling seasons from September 21, 2016, through January 31, 2019, a total of 5373 patients were randomly assigned to receive either high-dose trivalent or standard-dose quadrivalent influenza vaccine at 157 centers in the US and Canada (Figure 1). Over 3 seasons, 9% of patients were enrolled in the month of September, 48% in October, 26% in November, 10% in December, and 7% in January. A total of 113 patients were excluded from all efficacy analyses prior to database lock because they had been enrolled at a site that was closed for major violations of good clinical practice, leaving 5260 participants in the efficacy analysis. Baseline characteristics and concomitant therapies of patients enrolled were well balanced between groups (Table 1) and indicated a high-risk population with substantial comorbidity. A total of 3289 participants (63%) were enrolled in the heart failure hospitalization stratum and 1960 (37%) in the post–myocardial infarction stratum. Over the 3 seasons, 2630 participants were assigned to receive high-dose influenza vaccine and 2630 participants were assigned to receive standard-dose vaccine, with a total of 3577 vaccinations provided to the high-dose group and 3577 provided to the standard-dose group (Figure 1). A total of 979 patients in the high-dose group and 993 patients in the standard-dose group returned for more than 1 season (eTable 1 in Supplement 3). At the end of the study, vital status was known in all but 15 patients in the high-dose group and all but 19 patients in the standard-dose group.
Figure 1. Patient Selection, Randomization, and Flow in a Study of the Effect of High-Dose vs Standard-Dose Influenza Vaccine on Patients With High-risk Cardiovascular Disease.
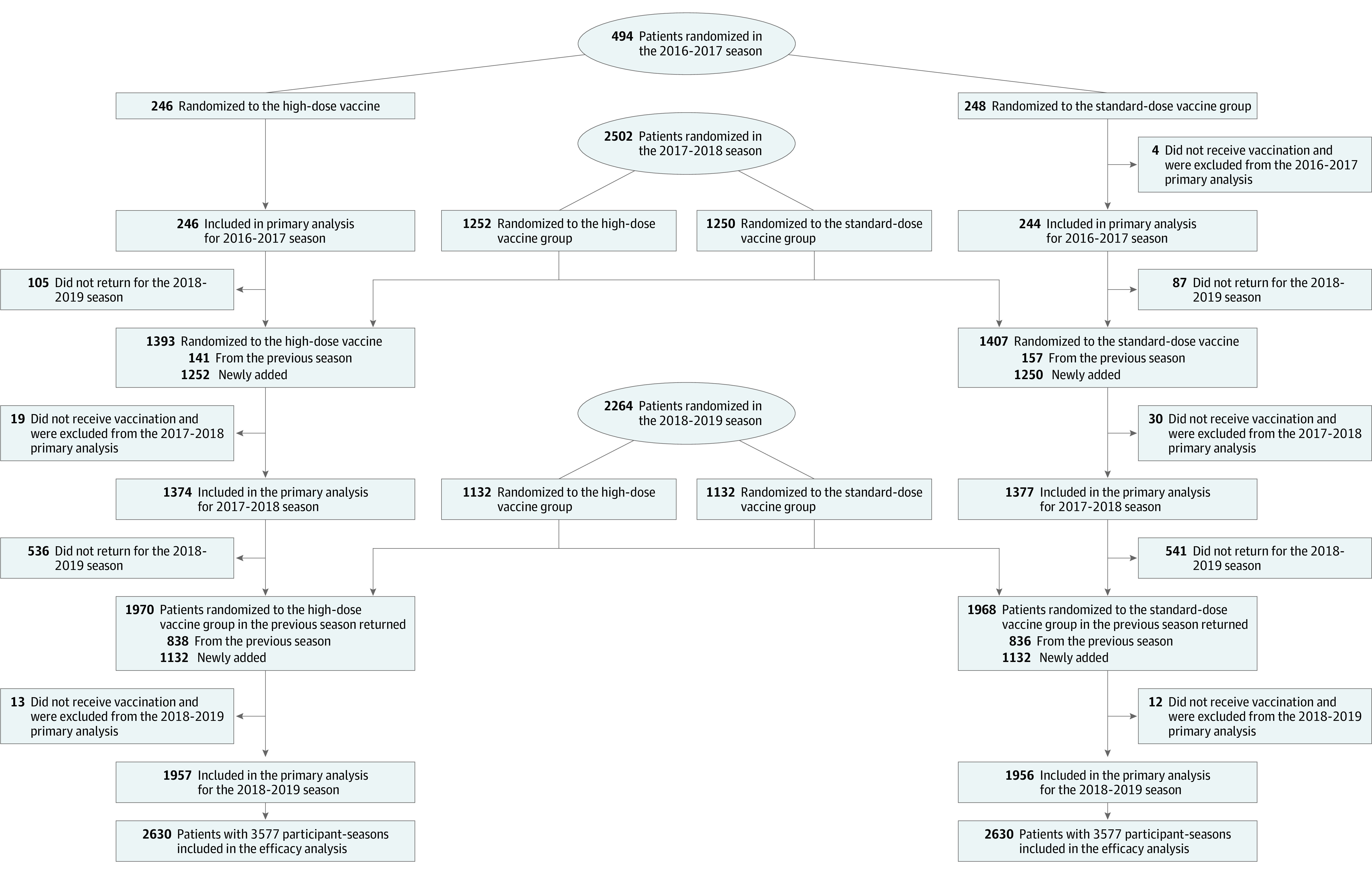
The study was designed so that sites were able to assess each potential participant for eligibility prior to enrollment and consent based on inclusion and exclusion criteria as defined in the Methods. Only eligible patients were consented. All patients who were consented are included in the figure.
Table 1. Baseline Characteristics of Participants in a Study of the Effect of High-Dose vs Standard-Dose Influenza Vaccine on Patients With High-risk Cardiovascular Disease.
| Baseline characteristic | No. (%) | |
|---|---|---|
| High dose (n = 2630) | Standard dose (n = 2630) | |
| Randomization year | ||
| 2016-2017 | 246 (9.4) | 248 (9.4) |
| Return in 2017-2018, No. | 141 | 157 |
| Return in 2018-2019, No. | 104 | 101 |
| 2017-2018 | 1252 (47.6) | 1250 (47.5) |
| Return in 2018-2019, No. | 734 | 735 |
| 2018-2019 | 1132 (43.0) | 1132 (43.0) |
| Region | ||
| US | 1792 (68.1) | 1792 (68.1) |
| Canada | 838 (31.9) | 838 (31.9) |
| Age, mean (SD), y | 65.5 (12.6) | 65.5 (12.5) |
| Median (IQR), y | 66 (58-74) | 67 (58-74) |
| Sex | ||
| Men | 1904 (72.6) | 1869 (71.2) |
| Women | 717 (27.3) | 756 (28.8) |
| Racea | ||
| White | 2042 (77.8) | 2061 (78.5) |
| Black | 407 (15.5) | 377 (14.4) |
| Asian | 81 (3.1) | 74 (2.8) |
| First Nations/American Indian | 17 (0.6) | 32 (1.2) |
| Otherb | 76 (2.9) | 82 (3.1) |
| Ethnicitya | ||
| Non-Hispanic/Latino | 2349 (89.6) | 2334 (88.9) |
| Hispanic/Latino | 250 (9.5) | 267 (10.2) |
| Otherc | 24 (0.9) | 25 (1.0) |
| Ejection fraction, mean (SD), % | 42.5 (16.1) [n = 2275] | 41.9 (16.2) [n = 2274] |
| BMI, mean (SD) | 30.7 (7.2) [n = 2275] | 31.0 (7.7) [n = 2274] |
| Qualifying event | ||
| Heart failure | 1641 (62.6) | 1648 (62.8) |
| MI | 982 (37.4) | 978 (37.2) |
| Eligibility risk factorsd | ||
| Age ≥65 y | 1467 (55.9) | 1520 (57.9) |
| Current BMI ≥30 | 1270 (48.4) | 1281 (48.8) |
| Current or past LVEF <40% | 1091 (41.6) | 1117 (42.5) |
| Type 1 or type 2 diabetes | 976 (37.2) | 974 (37.1) |
| History of chronic kidney disease | 792 (30.2) | 795 (30.3) |
| Current tobacco smoker | 483 (18.4) | 419 (16.0) |
| Prior heart failure hospitalization | 450 (17.2) | 453 (17.3) |
| Prior MI | 371 (14.1) | 374 (14.2) |
| History of ischemic stroke | 206 (7.9) | 227 (8.6) |
| History of peripheral artery disease | 119 (4.5) | 113 (4.3) |
| No. of eligibility risk factors | ||
| 1 | 537 (20.5) | 539 (20.5) |
| 2 | 737 (28.1) | 686 (26.1) |
| 3 | 616 (23.5) | 679 (25.9) |
| ≥4 | 733 (27.9) | 722 (27.5) |
| Other medical/surgical historyd,e | ||
| Hypertension | 1986 (75.7) | 2060 (78.4) |
| Dyslipidemia | 1793 (68.4) | 1823 (69.4) |
| Percutaneous coronary intervention | 1103 (42.1) | 1059 (40.3) |
| Atrial fibrillation | 854 (32.6) | 871 (33.2) |
| Coronary artery bypass graft | 503 (19.2) | 537 (20.4) |
| Chronic obstructive pulmonary disease | 486 (18.5) | 520 (19.8) |
| Implantable cardioverter-defibrillator | 463 (17.7) | 493 (18.8) |
| Asthma | 308 (11.7) | 294 (11.2) |
| Canadian Cardiovascular Society grading classf | (n = 982) | (n = 978) |
| I (no limitation) | 577 (58.8) | 539 (55.1) |
| II (slight limitation) | 238 (24.2) | 260 (26.6) |
| III (moderate limitation) | 74 (7.5) | 68 (7.0) |
| IV (severe limitation) | 31 (3.2) | 37 (3.8) |
| New York Heart Association functional classificationg | (n = 1641) | (n = 1648) |
| I (no limitation) | 279 (17.0) | 264 (16.0) |
| II (slight limitation) | 776 (47.3) | 808 (49.0) |
| III (moderate limitation) | 473 (28.8) | 458 (27.8) |
| IV (severe limitation) | 50 (3.0) | 54 (3.3) |
| Maintenance medications for those with MI as index eventd | (n = 982) | (n = 978) |
| Statins | 920 (93.7) | 915 (93.6) |
| Aspirin | 907 (92.4) | 878 (89.8) |
| β-Adrenergic blocker | 839 (85.4) | 840 (85.9) |
| Maintenance medications for those with heart failure as index eventd | (n = 1641) | (n = 1648) |
| β-Adrenergic blocker | 1381 (84.2) | 1398 (84.8) |
| Diuretic | 1294 (78.9) | 1313 (79.7) |
| ACE inhibitor, ARB, or ARN inhibitor | 1086 (66.2) | 1111 (67.4) |
| Mineralocorticoid receptor antagonist | 572 (34.9) | 546 (33.1) |
| Digoxin | 155 (9.4) | 159 (9.6) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ARN, angiontensin receptor neprilysin; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Race and ethnicity were collected via participant self-report.
Other race includes Native Hawaiian (n = 9), Pacific Islander (n = 16), more than 1 race (n = 26), participant does not want to report (n = 62), participant does not know (n = 40), and race not available or missing (n = 16).
Other ethnicity includes participant does not want to report (n = 32), participant does not know (n = 13), and ethnicity not available or missing (n = 15).
Total may be greater than 100% because of multiple risk factors per participant.
Medical and surgical history were collected via self-report and chart review.
The Canadian Cardiovascular Society grading scale is used for classification of angina severity, in which class I indicates angina only during strenuous or prolonged physical activity; class II, slight limitation with angina only during vigorous physical activity; class III, symptoms with everyday living activities (ie, moderate limitation); and class IV, inability to perform any activity without angina or angina at rest (ie, severe limitation).
The New York Heart Association class is a subjective categorization of heart failure by functional limitation. Class I indicates no limitation on physical activity; class II, comfort at rest with slight limitation on physical activity; class III, more significant limitation of physical activity; and class IV, symptoms at rest.
Outcomes
For the primary outcome, there were 975 primary events (883 hospitalizations for cardiovascular or pulmonary causes and 92 deaths from any cause) in 884 participants (event rate, 45 per 100 patient-years) among 3577 participant-seasons in the high-dose group compared with 924 primary events (846 hospitalizations for cardiovascular or pulmonary causes and 78 deaths from any cause) in 837 participants (event rate, 42 per 100 patient-years) among 3577 participant-seasons in the standard-dose group (hazard ratio, 1.06 [95% CI, 0.97-1.17]; P = .21) (Table 2 and Figure 2). No significant violations of proportional hazards were detected. Results were qualitatively similar for the components of the primary end point and within each enrolling season (Table 2). No significant differences between treatment groups were observed for the secondary outcomes (Table 2). The primary results were consistent across all prespecified subgroups (eFigure in Supplement 3). Results were qualitatively consistent with the main study findings in a prespecified secondary in-season analysis (eTable 2 in Supplement 3) and in a prespecified sensitivity analysis using inverse probability weighting to account for differential dropout (hazard ratio, 1.05 [95% CI, 0.96-1.13]; P = .45). The results were also similar when the primary analysis was stratified by site (hazard ratio, 1.08 [95% CI, 0.99-1.19]). In a post hoc analysis of prospectively collected and adjudicated hospitalizations, those ascribed primarily to influenza or pneumonia occurred infrequently in both treatment groups (influenza: 10 in the high-dose group and 8 in the standard-dose group [P = .63]; pneumonia: 47 in the high-dose group and 41 in the standard-dose group [P = .56]; eTable 3 in Supplement 3).
Table 2. Primary and Secondary Outcomes in a Study of the Effect of High-Dose vs Standard-Dose Influenza Vaccine on Patients With High-risk Cardiovascular Disease.
| Outcome | High dose (n = 3577 participant-seasons in 2630 participants) | Standard dose (n = 3577 participant-seasons in 2630 participants) | Absolute difference per 100 person-years (95% CI)a | Hazard ratio (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| Total | Rate per 100 person-years | Total | Rate per 100 person-years | ||||
| Primary | |||||||
| First cardiopulmonary hospitalization or all-cause death in each vaccination seasonb | 975 | 44.5 | 924 | 41.9 | 1.06 (0.97 to 1.17) | .21 | |
| 2016-2017 | 91 | 64.1 | 84 | 59.3 | 4.8 (–13.5 to 23.1) | 1.08 (0.80 to 1.45) | .61 |
| 2017-2018 | 413 | 50.0 | 377 | 44.5 | 5.6 (–1.0 to 12.1) | 1.12 (0.98 to 1.29) | .10 |
| 2018-2019 | 471 | 38.5 | 463 | 38.0 | 0.5 (–4.5 to 5.4) | 1.01 (0.89 to 1.15) | .86 |
| All-cause death as first event | 92 | 4.2 | 78 | 3.5 | |||
| Cardiopulmonary hospitalization as first event | 883 | 40.3 | 846 | 38.3 | |||
| Secondary | |||||||
| Cardiovascular death or hospitalization in each vaccination seasonb | 805 | 35.9 | 752 | 33.3 | 1.08 (0.97 to 1.20) | .16 | |
| All-cause deathc | 223 | 7.8 | 222 | 7.7 | 0.1 (–1.4 to 1.5) | 1.01 (0.84 to 1.21) | .96 |
| Total cardiopulmonary hospitalizations and all-cause death across all enrolling seasonsc | 1857 | 64.9 | 1784 | 62.2 | 2.7 (–4.0 to 9.3) | 1.04 (0.94 to 1.15)d | .44 |
| First cardiopulmonary hospitalization or all-cause death across all enrolling seasonsc | 955 | 42.3 | 918 | 40.0 | 2.3 (–1.4 to 6.0) | 1.05 (0.96 to 1.15) | .26 |
Absolute differences are calculated for results within single seasons; hazard ratios are presented for calculations accounting for correlation within patients over multiple seasons.
Based on the number of participant-seasons.
Based on the number of participants.
Rate ratio (95% CI).
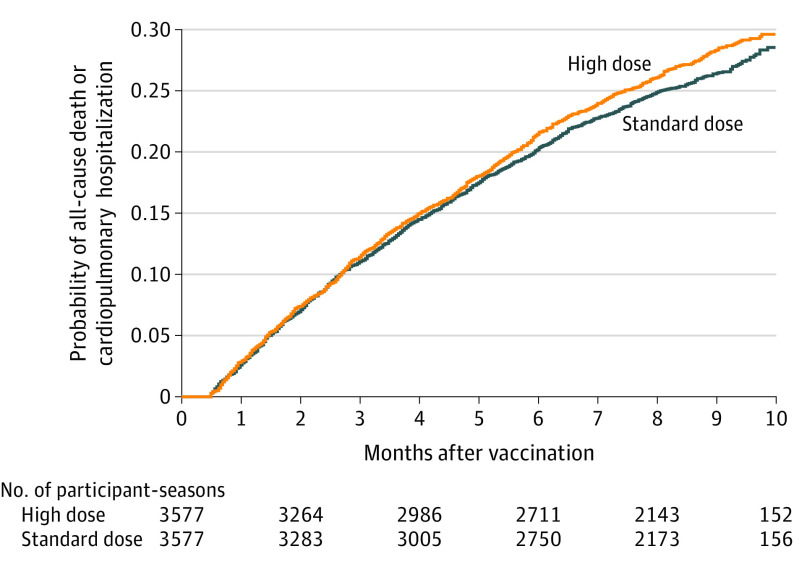
Figure 2. Survival Curves for All-Cause Mortality or Cardiopulmonary Hospitalizations in a Study of the Effect of High-Dose vs Standard-Dose Influenza Vaccine on Patients With High-risk Cardiovascular Disease .
The median (interquartile range) observation time was 8.5 (6.1-9.4) months in the high-dose group and 8.5 (6.2-9.4) months in the standard-dose group. All 3 influenza seasons are combined with each patient’s observation time beginning anew with each influenza season. Each patient can appear up to 3 times. Hazard ratio, 1.06 (95% CI, 0.97-1.17); P = .21.
Adverse Events
Overall, influenza vaccinations were well tolerated. The most frequent vaccine-related adverse events were injection site pain (26.5%), myalgia (17.7%), and swelling (6.1%), which were more common among patients in the high-dose group (Table 3 and eTable 4 in Supplement 3). A total of 55 patients (2.1%) in the high-dose group and 44 patients (1.7%) in the standard-dose group reported vaccine-related reactions that they considered severe. A total of 6 serious adverse events were reported (2 in the high-dose group and 4 in the standard-dose group; eTable 5 in Supplement 3).
Table 3. Postvaccination Adverse Events (Within 1 Week) in a Study of the Effect of High-Dose Trivalent vs Standard-Dose Quadrivalent Influenza Vaccine on Patients With High-risk Cardiovascular Disease.
| Vaccine-related adverse event | No. (%) | |
|---|---|---|
| High dose (3577 participant-seasons in 2606 participants) |
Standard dose (3577 participant-seasons in 2604 participants) |
|
| Pain | 932 (26.1) | 683 (19.1) |
| Myalgia | 500 (14.0) | 423 (11.8) |
| Overall discomfort | 310 (8.7) | 280 (7.8) |
| Headache | 279 (7.8) | 272 (7.6) |
| Swelling | 198 (5.5) | 119 (3.3) |
| Erythema | 157 (4.4) | 157 (4.4) |
| Fever | 102 (2.9) | 78 (2.2) |
| Any of above | 1449 (40.5) | 1229 (34.4) |
| Severity (by vaccination)a | ||
| None | 2070 (58) | 2288 (64) |
| Mild | 1108 (31) | 989 (28) |
| Moderate | 343 (9.6) | 255 (7.1) |
| Severe | 56 (1.6) | 45 (1.3) |
| Severity (by participant)a | (n = 2606 participants) | (n = 2604 participants) |
| None | 1368 (52) | 1513 (58) |
| Mild | 864 (33) | 810 (31) |
| Moderate | 319 (12) | 237 (9.1) |
| Severe | 55 (2.1) | 44 (1.7) |
| Participants with any vaccine-related adverse event | 1241 (47.6) | 1096 (42.1) |
| Participants with any severe vaccine-related adverse eventa | 55 (2.1) | 44 (1.7) |
Severity of postvaccination adverse events was determined by participant self-report from a 7-day symptom diary. Mild severity was defined as “symptom did not affect his/her daily activities”; moderate, “symptom bothered the participant to the point it somewhat interfered with his/her daily activities”; and severe, “symptom bothered the participant to the point she/he needed medical attention and major help with daily activities.” There were 6 serious adverse events (eTable 4 in Supplement 3). Vaccine-related adverse events were based on patient self-report. All potential serious adverse events were reviewed by study monitors.
Discussion
In this pragmatic, randomized, double-blind, active comparator trial of individuals at high cardiovascular risk enrolled over 3 influenza seasons, high-dose trivalent inactivated influenza vaccine did not significantly reduce the composite of all-cause death or hospitalizations for cardiac or pulmonary causes compared with standard-dose quadrivalent inactivated influenza vaccine. There was no significant difference between vaccine groups for any prespecified secondary end points, and results were consistent for analyses including all randomized participants, within each season, and in prespecified subgroups, including individuals younger than 65 years in whom high-dose vaccine is not currently indicated. Those who received the high-dose vaccine had a higher overall frequency of vaccine-related adverse effects, but severe adverse events were infrequent and occurred at similar rates in both groups.
This trial was predicated on the concept that reducing influenza in a high-risk population would lead to reduction in cardiovascular and pulmonary hospitalizations and deaths. High-dose trivalent influenza vaccine, compared with standard-dose trivalent vaccine, had previously reduced laboratory-confirmed influenza, hospitalizations, and serious cardiopulmonary events in medically stable older adults in a large randomized trial, in which only 17% had known coronary disease and 2.8% had heart failure.9,10 In a cluster randomized trial, nursing home residents who received a high-dose vaccine compared with standard-dose vaccine had fewer pulmonary hospitalizations based on Medicare claims, but no difference in mortality,25 which is consistent with observational analyses in claims-based data sets showing reduced influenza or pulmonary-based hospitalizations among those receiving high-dose trivalent influenza vaccine.26,27 However, in the current trial, the potential benefit for high-dose vaccine to prevent influenza infection did not translate to a reduction in all-cause mortality or hospitalizations with high-dose vaccine. Although it is possible that high-dose compared with standard-dose vaccine may have reduced influenza infection or illness, outcomes that were not specifically assessed in this trial, there were no significant differences in deaths, hospitalizations for cardiac or pulmonary causes, or influenza-related or pneumonia-related hospitalizations. Because event rates for death or hospitalization were markedly higher than in prior studies, the high-dose strategy did not appear to alter the clinical trajectory of very high-risk patients with cardiovascular disease in whom the incremental risk associated with influenza may have been lower than expected and in whom the high ambient event rate may have diluted any potential differential benefit of the high-dose formulation. Whether these results would have been different in a lower-risk population remains unclear.
There are several additional potential factors that may have contributed to these findings. One possible explanation is the difference in valence between the 2 formulations, because the quadrivalent standard-dose comparator contains an additional B/Yamagata strain with broader coverage for influenza B. Although the influenza A strain viruses (specifically influenza A/New York/55/2004 [H3N2] and novel influenza A [H1N1]) predominated during the enrolling seasons of this trial, the influenza B strain can contribute substantively to spring morbidity from influenza; thus, the additional influenza B strain in the quadrivalent formulation may have mitigated benefits associated with antigen dose alone. Previous comparisons of high-dose vs standard-dose influenza vaccine used trivalent vaccine formulations. Given the shifting standard of care in the US in favor of using quadrivalent influenza vaccine in most individuals, the trial steering committee felt that the quadrivalent vaccine as a comparator was essential to maintain equipoise, and high-dose quadrivalent vaccine was not available during the years of the trial. Nevertheless, although B/Yamagata, a strain absent in the high-dose vaccine formulation tested, accounted for 28% to 30% of influenza infections in 2016-2017 and 2017-2018, it only accounted for 1% of influenza infections in 2018-2019. Thus, even if the benefit of high-dose compared with standard-dose was attenuated due to the absence of the additional influenza B strain during the first 2 years of the trial, this should not have made a meaningful difference in the third year, which had the most events. Because the trial results did not vary by year, the additional influenza B strain in the standard-dose vaccine seems unlikely to account for these overall findings. Additionally, previous studies have shown that vaccine effectiveness does not vary significantly in adults during influenza B strain mismatched years.28 Contemporary studies have also demonstrated cross protection from the influenza B strain contained in the vaccine against the absent influenza B strain.29,30
Another potential explanation for these findings is that low vaccine effectiveness during the years that the trial enrolled participants (29%-40% overall), with lower vaccine effectiveness among those 65 years and older (12%-20%),31 may have attenuated any potential difference between high-dose and standard-dose vaccines. Additionally, the vaccines used in this study are prepared in chicken eggs, which are subject to adaptive variations in the hemagglutinin protein during vaccine preparation, leading to vaccine mismatch.32,33 Whether these results would have been different during other influenza seasons with higher vaccine effectiveness with a recombinant influenza vaccine that forgoes the egg-grown process or with adjuvant-based formulations is unknown.34,35
Although this comparative effectiveness trial did not find a significant difference between the 2 vaccine formulations for the end points studied in a high-risk population, the incidence of hospitalization due to influenza was low in both vaccinated groups, and the benefit of either vaccine may be far greater than the incremental benefit of high-dose over standard-dose vaccines for influenza outcomes. Although these data do not refute the greater efficacy of high-dose vs standard dose vaccines at reducing influenza among older adults, for the purposes of reducing death or cardiopulmonary hospitalizations in a high-risk cardiovascular population, the high-dose vaccine was not more effective than the standard-dose vaccine. Overall, both vaccines were well tolerated with low frequencies of severe vaccine-related adverse effects. Thus, these results do not detract from current Centers for Disease Control and Prevention recommendations to vaccinate all individuals 6 months or older or the strong guideline-based recommendations for vaccination of high-risk cardiovascular patients.
Limitations
This study has several limitations. First, influenza infection or illness was not specifically assessed, because this trial was testing the effect of this therapeutic strategy on clinical outcomes and it was impractical to capture laboratory-confirmed influenza in a pragmatic trial. Whether influenza infection or illness specifically were reduced in this population remains unknown. Second, although there were very few hospitalizations ascribed specifically to influenza during the trial, and these did not differ significantly different between vaccine groups, incomplete capture of influenza as a contributing factor to some hospitalizations or deaths is possible. Third, this trial did not include an unvaccinated control group because influenza vaccination is currently strongly recommended for high-risk individuals by current US guidelines15,36,37 and it was not considered ethical to deny influenza vaccine to patients at high risk for influenza-related complications. Nevertheless, several trials outside North America are testing whether influenza vaccine, compared with placebo, reduces cardiac events in patients with cardiovascular disease.38,39 Fourth, results may not be generalizable to other regions of the world where there are different vaccination patterns. Fifth, although this trial was stopped early because it had far exceeded the number of required end points, it had sufficient power to test the hypothesis, including in those younger than 65 years, a group for whom high-dose vaccine is not currently indicated and who have not previously been studied rigorously.
Conclusions
In patients with high-risk cardiovascular disease, high-dose trivalent influenza vaccine, compared with standard-dose quadrivalent vaccine, did not significantly reduce all-cause mortality or hospitalizations for cardiac or pulmonary causes. Influenza vaccination remains strongly recommended in this population.
Trial protocol
Statistical analysis plan
INVESTED Committees
Investigators
eTables and eFigures
Clinical endpoints committee operations and definitions
Data sharing statement
References
- 1.Rolfes MA, Foppa IM, Garg S, et al. . Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-137. doi: 10.1111/irv.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estabragh ZR, Mamas MA. The cardiovascular manifestations of influenza: a systematic review. Int J Cardiol. 2013;167(6):2397-2403. doi: 10.1016/j.ijcard.2013.01.274 [DOI] [PubMed] [Google Scholar]
- 3.Kwong JC, Schwartz KL, Campitelli MA, et al. . Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345-353. doi: 10.1056/NEJMoa1702090 [DOI] [PubMed] [Google Scholar]
- 4.Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51(3):51. doi: 10.1183/13993003.01794-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow EJ, Rolfes MA, O’Halloran A, et al. . Acute cardiovascular events associated with influenza in hospitalized adults : a cross-sectional study. Ann Intern Med. 2020;173(8):605-613. doi: 10.7326/M20-1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panhwar MS, Kalra A, Gupta T, et al. . Effect of influenza on outcomes in patients with heart failure. JACC Heart Fail. 2019;7(2):112-117. doi: 10.1016/j.jchf.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Tripathi B, Kumar V, Kalra A, et al. . Influence of influenza infection on in-hospital acute myocardial infarction outcomes. Am J Cardiol. 2020;130:7-14. doi: 10.1016/j.amjcard.2020.05.045 [DOI] [PubMed] [Google Scholar]
- 8.Udell JA, Zawi R, Bhatt DL, et al. . Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711-1720. doi: 10.1001/jama.2013.279206 [DOI] [PubMed] [Google Scholar]
- 9.DiazGranados CA, Dunning AJ, Kimmel M, et al. . Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635-645. doi: 10.1056/NEJMoa1315727 [DOI] [PubMed] [Google Scholar]
- 10.DiazGranados CA, Robertson CA, Talbot HK, Landolfi V, Dunning AJ, Greenberg DP. Prevention of serious events in adults 65 years of age or older: a comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015;33(38):4988-4993. doi: 10.1016/j.vaccine.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 11.Fulop T, Larbi A, Dupuis G, et al. . Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8:1960. doi: 10.3389/fimmu.2017.01960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons: a meta-analysis and review of the literature. Ann Intern Med. 1995;123(7):518-527. doi: 10.7326/0003-4819-123-7-199510010-00008 [DOI] [PubMed] [Google Scholar]
- 13.Vardeny O, Sweitzer NK, Detry MA, Moran JM, Johnson MR, Hayney MS. Decreased immune responses to influenza vaccination in patients with heart failure. J Card Fail. 2009;15(4):368-373. doi: 10.1016/j.cardfail.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Ermen A, Hermanson MP, Moran JM, Sweitzer NK, Johnson MR, Vardeny O. Double dose vs. standard dose influenza vaccination in patients with heart failure: a pilot study. Eur J Heart Fail. 2013;15(5):560-564. doi: 10.1093/eurjhf/hfs207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MM, Taubert K, Benin AL, et al. ; American Heart Association; American College of Cardiology; American Association of Cardiovascular and Pulmonary Rehabilitation; American Association of Critical Care Nurses; American Association of Heart Failure Nurses; American Diabetes Association; Association of Black Cardiologists, Inc; Heart Failure Society of America; Preventive Cardiovascular Nurses Association; American Academy of Nurse Practitioners; Centers for Disease Control and Prevention and the Advisory Committee on Immunization . Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. J Am Coll Cardiol. 2006;48(7):1498-1502. doi: 10.1016/j.jacc.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 16.Vardeny O, Udell JA, Joseph J, et al. . High-dose influenza vaccine to reduce clinical outcomes in high-risk cardiovascular patients: rationale and design of the INVESTED trial. Am Heart J. 2018;202:97-103. doi: 10.1016/j.ahj.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7-8):365-375. doi: 10.1016/0021-9681(74)90015-0 [DOI] [PubMed] [Google Scholar]
- 18.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443-453. doi: 10.1002/cpt1974155443 [DOI] [PubMed] [Google Scholar]
- 19.Hicks KA, Mahaffey KW, Mehran R, et al. ; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI) . 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137(9):961-972. doi: 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 20.Wei LJ, Lin DY. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc 1989;84:1065-73. doi: 10.1080/01621459.1989.10478873 [DOI] [Google Scholar]
- 21.Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. 1980;67(1):145-153. doi: 10.1093/biomet/67.1.145 [DOI] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 23.Zhou B, Fine J, Latouche A, Labopin M. Competing risks regression for clustered data. Biostatistics. 2012;13(3):371-383. doi: 10.1093/biostatistics/kxr032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao L, Lin DY. Semiparametric regression for the weighted composite endpoint of recurrent and terminal events. Biostatistics. 2016;17(2):390-403. doi: 10.1093/biostatistics/kxv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gravenstein S, Davidson HE, Taljaard M, et al. . Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med. 2017;5(9):738-746. doi: 10.1016/S2213-2600(17)30235-7 [DOI] [PubMed] [Google Scholar]
- 26.Izurieta HS, Thadani N, Shay DK, et al. . Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis. 2015;15(3):293-300. doi: 10.1016/S1473-3099(14)71087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young-Xu Y, Van Aalst R, Mahmud SM, et al. . Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis. 2018;217(11):1718-1727. doi: 10.1093/infdis/jiy088 [DOI] [PubMed] [Google Scholar]
- 28.Flannery B, Chung JR, Monto AS, et al. ; US Flu VE Investigators . Influenza vaccine effectiveness in the United States during the 2016-2017 season. Clin Infect Dis. 2019;68(11):1798-1806. doi: 10.1093/cid/ciy775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean HQ, Thompson MG, Sundaram ME, et al. . Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type. J Infect Dis. 2015;211(10):1529-1540. doi: 10.1093/infdis/jiu647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyer WEP, Palache AM, Boulfich M, Osterhaus ADME. Rationale for two influenza B lineages in seasonal vaccines: a meta-regression study on immunogenicity and controlled field trials. Vaccine. 2017;35(33):4167-4176. doi: 10.1016/j.vaccine.2017.06.038 [DOI] [PubMed] [Google Scholar]
- 31.CDC seasonal flu vaccine effectiveness studies. Centers for Disease Control and Prevention. Accessed September 26, 2020. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm
- 32.Chen Z, Zhou H, Jin H. The impact of key amino acid substitutions in the hemagglutinin of influenza A (H3N2) viruses on vaccine production and antibody response. Vaccine. 2010;28(24):4079-4085. doi: 10.1016/j.vaccine.2010.03.078 [DOI] [PubMed] [Google Scholar]
- 33.Zost SJ, Parkhouse K, Gumina ME, et al. . Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114(47):12578-12583. doi: 10.1073/pnas.1712377114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunkle LM, Izikson R, Patriarca P, et al. ; PSC12 Study Team . Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med. 2017;376(25):2427-2436. doi: 10.1056/NEJMoa1608862 [DOI] [PubMed] [Google Scholar]
- 35.Behrouzi B, Araujo Campoverde MV, Liang K, et al. . Influenza vaccination to reduce cardiovascular morbidity and mortality in patients with COVID-19: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(15):1777-1794. doi: 10.1016/j.jacc.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yancy CW, Jessup M, Bozkurt B, et al. . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128(16):e240-e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 37.Piepoli MF, Hoes AW, Agewall S, et al. ; ESC Scientific Document Group . 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fröbert O, Götberg M, Angerås O, et al. . Design and rationale for the influenza vaccination after myocardial infarction (IAMI) trial: a registry-based randomized clinical trial. Am Heart J. 2017;189:94-102. doi: 10.1016/j.ahj.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 39.Loeb M, Dokainish H, Dans A, et al. ; IVVE investigators . Randomized controlled trial of influenza vaccine in patients with heart failure to reduce adverse vascular events (IVVE): rationale and design. Am Heart J. 2019;212:36-44. doi: 10.1016/j.ahj.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
INVESTED Committees
Investigators
eTables and eFigures
Clinical endpoints committee operations and definitions
Data sharing statement