Abstract
Zymogen granule protein 16B (ZG16B) has been identified in various cancers, while so far the association between ZG16B and breast cancer hasn’t been explored. Our aim is to confirm whether it can serve as a prognostic biomarker in breast cancer. In this study, Oncomine, Cancer Cell Line Encyclopedia (CCLE), Ualcan, and STRING database analyses were conducted to detect the expression level of ZG16B in breast cancer with different types. Kaplan–Meier plotter was used to analyze the prognosis of patients with high or low expression of ZG16B. We found that ZG16B was significantly upregulated in breast cancer. Moreover, ZG16B was closely associated with foregone biomarkers and crucial factors in breast cancer. In the survival analysis, high expression of ZG16B represents a favorable prognosis in patients. Our work demonstrates the latent capacity of ZG16B to be a biomarker for prognosis of breast cancer.
Keywords: ZG16B, breast cancer, database, biomarker, prognosis
1. Introduction
Breast cancer is the most commonly diagnosed cancer in women, which is one major cause of cancer death especially in young women, second only to lung cancer [1,2,3,4]. Based on the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), breast cancer is divided into Luminal A, Luminal B, Basal-like, and HER2-positive subtypes [5]. Many therapies have been developed and used to detect and treat breast cancer [6,7,8]. However, due to the complex interactions between the environment and hereditary factors, it’s still difficult to diagnose or prevent breast cancer at initial stage [9]. It has been reported that the abnormal increase of biomarkers in tumorigenesis can be detected in blood, urine, and tissue and then help predict tumor’s grade malignancy, behaviors, and prognosis [10]. In previous studies, multiple kinds of conventional biomarkers related to early diagnosis and prognosis for breast cancer have been developed, such as uPA [11], Rs/DJ-1 [12], and PAI-1 [13]. And recently, circulating miRNAs [14], serum uPAR [15], KiSS1 [16], CD24 [17] etc. also have been recognized as strongly associated with breast cancer development. In order to improve the early detection, diagnosis, and prognosis or even discover therapeutic targets of breast cancer, more specific biomarkers need to be identified.
Zymogen granule protein 16A (ZG16A), also known as ZG16 or ZG16p, is a soluble lectin expressed in pancreatic acinar cells and digestive tract, which mediates the condensation of pancreatic enzymes to the zymogen granule membrane [18,19]. Zymogen granule protein 16B(ZG16B), also identified as Pancreatic adenocarcinoma upregulated factor, is a paralog of ZG16A which has a 65.5% of similarity and 36% identity, first found to be overexpressed in pancreatic ductal adenocarcinoma [19,20,21]. Both of these two zymogen granule proteins exist in human alimentary system and have the same structures such as β-prism fold and glycosaminoglycan-binding site, suggesting their potential functional similarity [19,21].
ZG16A has been recognized as a mucus ingredient in colon fluid which blocks bacteria and upregulates in colorectal cancer as a biomarker [22,23]. ZG16B was first discovered to act as a growth factor overexpressed in pancreatic cancer, which enhances tumor proliferation and helps escape from innate immune system by intriguing TLR-mediated ERK signaling, inhibiting TLR-mediated NF-kappa B signaling and keeping β-catenin stable through phosphorylation [21,24,25]. Furthermore, ZG16B enhances angiogenesis and vascular permeability and then promotes tumor progression and metastasis of pancreatic cancer by stimulating CXCR4 expression and FAK activation [26,27,28]. Additionally, ZG16B helps pancreatic cancer cells to resist oncolytic parvovirus H-1 infection via IFNAR-mediated signaling [29]. As a special factor in pancreatic cancer, ZG16B promotes activation and maturation of DCs through TLR4 signaling pathway to mediate immune system activation; meanwhile, it could also increase and activate MDSCs to benefit tumor formation, showing a double-sided effect on the immunotherapy [30,31]. And in chemotherapy, ZG16B enhances the effect of gemcitabine and 5-FU in pancreatic cancer [32,33].
In addition, ZG16B has been identified as a biomarker highly expressed in colorectal cancer and enhancing the migration and invasion, leading to a poor prognosis [20,34,35,36]. ZG16B is also confirmed to exist in HeLa cells and upregulates in cervical cancer [37,38]. Moreover, ZG16B can also have effect and be a biomarker for early diagnosis and prognosis of prostate cancer [39], oral squamous cell carcinoma [40], and especially ovarian cancer [41,42,43]. Besides, ZG16B is correlated with the prognosis of atherosclerosis and acute coronary syndrome [44], and it is demonstrated to be abundant in the reflex tears as the key point in the ocular surface protection, maintaining the tear film stability [45,46]. ZG16B has been detected as a biomarker in various malignant tumors; however, the association between ZG16B and breast cancer has not been noticed yet.
In this report, we found that ZG16B expressed highly in breast cancer, and depth analysis was conducted further to clarify the possible effect of ZG16B in breast cancer through public medical databases. Expression levels in different conditions, possible mechanisms of action and the effect of prognosis of ZG16B in breast cancer were presented, demonstrating its potential value to be a biomarker for breast cancer in clinical practice.
2. Materials and methods
2.1. Ethics statement
Our work has been approved by the Ethics Committee and Institutional Review Board of Qingdao University, China. Informed consent for publication was not required, as all patient data used in the study were obtained from publicly available databases.
2.2. Oncomine database analysis
Oncomine database (https://www.oncomine.org), which provides 715 datasets and 86,733 samples, was referred to analyze the expression pattern of ZG16B mRNA in different types of cancers. Pooled meta-analysis in different subtypes of breast cancer and gene co-expression analysis of ZG16B were conducted by Oncomine. The threshold of p-value was fixed to 1 × 10−4. The threshold of fold change was fixed to 2.
2.3. CCLE analysis
The expression level of ZG16B in different cell lines was analyzed by Cancer Cell Line Encyclopedia (CCLE, https://portals.broadinstitute.org/ccle), a database offering the expression level sorting of 84,434 genes in 1,457 cancer cell lines.
2.4. Ualcan analysis
Ualcan (http://ualcan.path.uab.edu/analysis.html) is a user-friendly cancer database based on TCGA database. Data from TCGA database were obtained through Ualcan to analyze the expression discrepancy of ZG16B in diverse molecular subtypes of breast cancer, as well as gender, age, cancer stages, and node metastasis status. The promoter methylation status of ZG16B was also analyzed using Ualcan.
2.5. STRING analysis
STRING (https://string-db.org) is a database that collects known and predicted protein–protein physical and functional interaction information, the data of which originate from computer prediction, knowledge sharing between organizations, and other databases. A protein network was drawn by STRING to find the interaction between ZG16B and other proteins.
2.6. Survival analysis
The Kaplan–Meier plotter (http://kmplot.com/analysis) is an online graph plotter including the survival data of patients with multiple types of cancers to draw survival curves. The effect of ZG16B on prognosis in breast cancer was analyzed by Kaplan–Meier plotter on the scale of relapse-free survival (RFS). ZG16B Affy ID: 228058_at. The Probe set option is the user selected probe set. The cut-off value is determined by the median.
2.7. Statistical analysis
Students t-tests were performed to detect the statistical difference of ZG16B mRNA expression between breast cancer and normal tissues, as well as the mRNA expression level and promotor methylation level in different clinical indicators. Log-rank test and hazard ratio (HR) analyses were conducted to examine the statistical difference of survival curves in different subtypes of breast cancer with low or high expression of ZG16B. Data were presented as the mean ± standard error of mean, and P < 0.05 was considered to be statistically significant.
3. Results
3.1. ZG16B expression in breast cancer
Two kinds of zymogen granule proteins have been recognized in human body cells, including ZG16A and ZG16B. However, no report has announced their function and correlation in breast cancer yet. Oncomine database was used to analyze the different expression levels of ZG16A and ZG16B between multiple types of cancer and the corresponding normal tissues. Among all kinds of cancers, it was significant that the expression of ZG16B was upregulated in 7 analyses of 3 datasets from Curtis’ [47], Finak’s [48], and TCGA database which met the threshold. And yet no dataset showed high expression of ZG16A in breast cancer (Figure 1a). We analyzed the datasets uploaded by Ma [49] and Curtis [47]. No significant difference of ZG16A expression was observed between breast cancer tissue and normal tissue (p = 0.088) (Figure 1b), while the fold change of ZG16B expression was 3.898 (p = 1.03 × 10−29) (Figure 1c), which showed high significance.
Figure 1.
Analysis of ZG16A and ZG16B mRNA expression in different types of cancer (Oncomine). (a) The mRNA expression pattern of ZG16A and ZG16B in various cancers. The number in the grid represented the number of datasets that meet the fixed threshold (p < 1 × 10−4, fold change >2). The color of the grid represented if the gene expressed higher (red) or lower (blue) (cancer vs. normal). The color depth of the grid depended on the best gene rank percentiles in all genes detected in each analysis. Box plots derived from gene expression data in Oncomine comparing the mRNA expression of ZG16A (b) and ZG16B (c) in normal and breast cancer tissues.
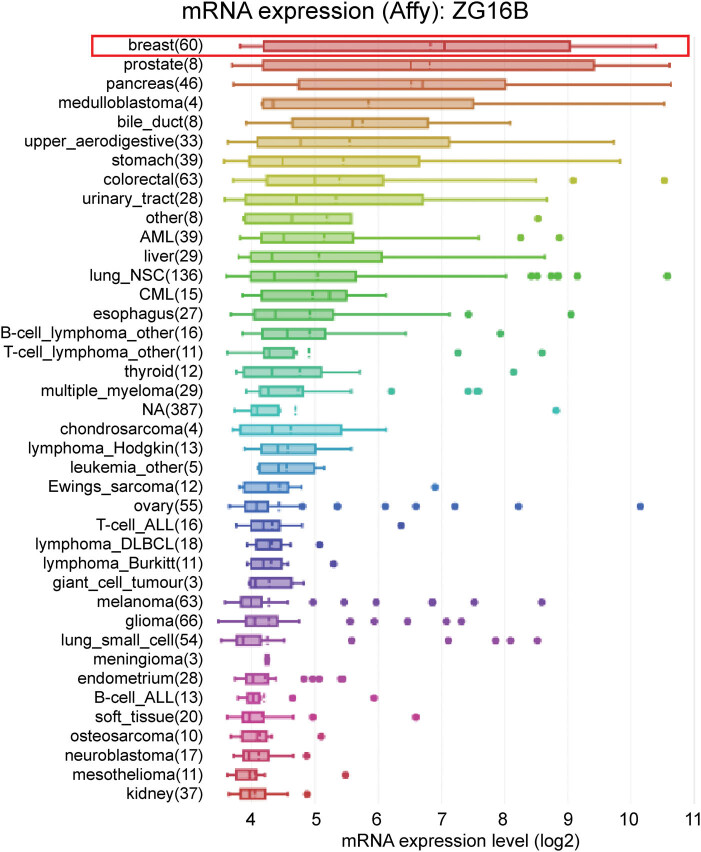
Furthermore, in order to verify the high-level expression signal of ZG16B in breast cancer, we used CCLE analysis to detect the transcription level of ZG16B in multiple cancer cell lines. The results demonstrated that the transcription level of ZG16B was the highest among all types of cancer cell lines (Figure 2). These results indicated the special role of ZG16B in breast cancer.
Figure 2.
Analysis of ZG16B mRNA expression in cancer cells. ZG16B mRNA expression levels in 40 types of cancer cell lines were measured by Affymetrix gene chips and arranged from the highest to the lowest (CCLE analysis). ZG16B mRNA expression level was the first highest in breast cancer cells among all cancers.
In consideration that the evidences described above indicate ZG16B expresses highly in breast cancer tissues and cell lines, we have done a further pooled meta-analysis including 2,780 samples from all the 18 researches of breast carcinoma in Curtis’ [47], Finak’s [48], and TCGA databases to confirm the high expression in general situation of breast cancer. The pooled meta-analysis confirmed that the mRNA upregulation of ZG16B was extremely significant in breast cancer (p = 5.97 × 10−4) (Figure 3). Persuasive testament was presented explaining the connection between the overexpression of ZG16B and breast cancer.
Figure 3.
The pooled meta-analysis of gene expression profiling for ZG16B gene across 18 analysis. The color of the grid represented if the gene expressed higher (red) or lower (blue). The color depth of the grid depended on the median gene rank percentiles. The rank of ZG16B was the median rank across each of the analyses. The p-value of ZG16B was for the median-ranked analysis.
3.2. Analysis of ZG16B expression in different clinical features of breast cancer
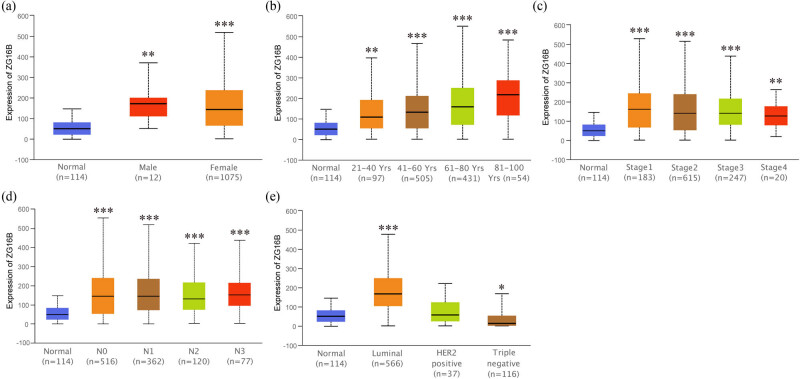
Since it showed that ZG16B indeed upregulated in breast cancer, in order to expound the expression pattern of ZG16B in breast cancer, Ualcan analysis was conducted to compare the expression level of ZG16B in different clinical indicators. The expression level of ZG16B increased in both female and rare male patients as shown in Figure 4a. Although ZG16B expression level is higher in all patient groups with different ages compared with normal groups, no significance could be found between groups (Figure 4b); and the situations could be classified in similar way by individual cancer stages and nodal metastasis status as shown in Figure 4c and d. Intriguingly, ZG16B expression level is significantly higher in luminal-like subtype (p = 1.624 × 10−12) and triple-negative subtype (p = 4.632 × 10−2) than in normal tissues, whereas no significance was shown in HER2-positive subtype (Figure 4e).
Figure 4.
Analysis of ZG16B expression patterns in breast cancer patients with different clinical-pathologic features. Ualcan analysis showed the mRNA expression level of ZG16B in breast cancer patients with distinct gender (a), age (b), cancer stages (c), node metastasis status (d), and molecular subtypes (e). Asterisks were marked to show the significance of each breast cancer group compared with normal group (*p < 0.05, **p < 0.01, ***p < 0.001).
3.3. Hypomethylation of ZG16B promoter in breast cancer
It has been reported that abnormal promoter hypomethylation induces irregular gene upregulation and then affects tumor progression [50,51,52]. Unsurprisingly, by the way of Ualcan, we found that the promoter methylation level of ZG16B significantly downregulated in primary breast tumor compared with that in normal tissues (Figure 5a). Patient groups with different gender, age, and nodal metastasis status showed similar results, when compared with the normal group; however, no significance was confirmed for within group comparison (Figure 5b–d). These results suggested that the overexpression regulatory mechanism of ZG16B in breast cancer might be the consequence of promoter demethylation.
Figure 5.
Analysis of ZG16B promoter methylation status in breast cancer patients with different clinical-pathologic features. Ualcan analysis showed the methylation level of ZG16B promoter in primary tumor of breast cancer (a) and in breast cancer patients with distinct gender (b), age (c), and cancer stages (d). Asterisks were marked to show the significance of each group compared with normal group (*p < 0.05, **p < 0.01, ***p < 0.001).
3.4. Protein interactions and gene co-expression analysis
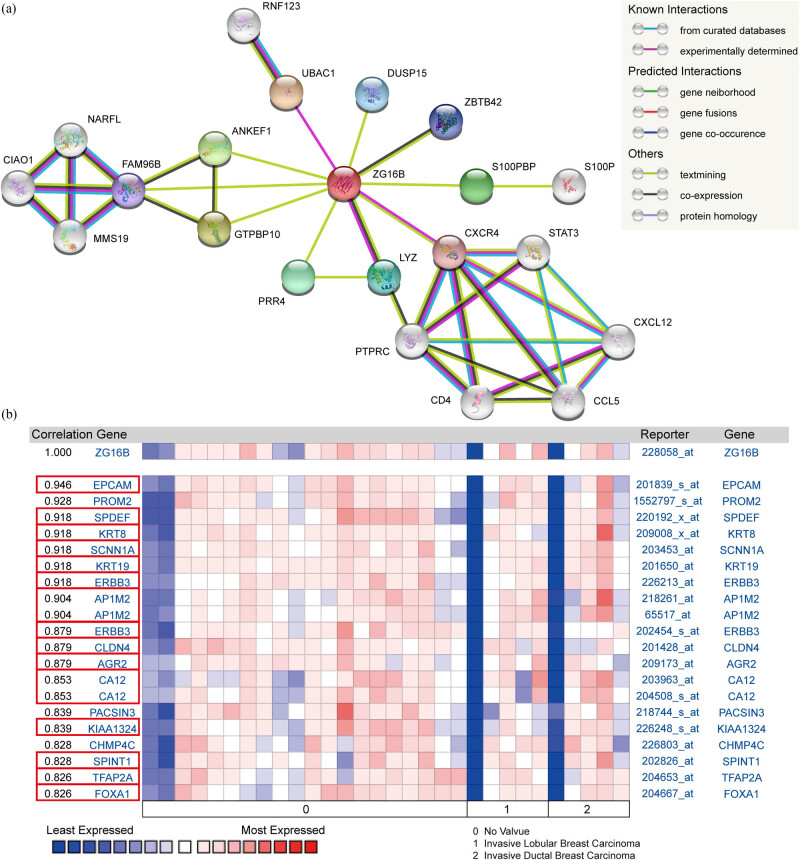
STRING analysis was performed to extrapolate the possible mechanism of ZG16B action in breast cancer and find the interaction between ZG16B and other proteins. The protein network showed that UBAC1, LYZ, and CXCR4 are experimentally determined to have interactions with ZG16B, while ZBTB42 and LYZ co-expressed with it. In addition, STRING computationally predicted ZG16B might have physical or functional relation with DUSP15, ZBTB42, S100PBP, PRR4, GTPBP10, FAM96B, and ANKEF1 (Figure 6a).
Figure 6.
Protein interactions and gene co-expression analysis of ZG16B. (a) The protein interaction network of ZG16B was drawn by STRING analysis. The colored nodes in the network represented ZG16B and its first stage of interactors, and the white nodes represented the second stage of interactors. The physical or functional relations between these proteins are interpreted in the conventional signs. (b) The gene co-expression analysis of ZG16B in Turashvili’s work was conducted by Oncomine. The co-expression genes of ZG16B in breast cancer and their coefficients were marked by red rectangles.
In order to obtain more detailed information at genetic level, we have detected the co-expression genes of ZG16B in breast cancer through Oncomine database. As reported in Turashvili’s research [53], the expression of ZG16B is highly related to EPCAM (r = 0.946), SPDEF (r = 0.918), KRT8 (r = 0.918), SCNN1A (r = 0.918), KRT19 (r = 0.918), ERBB3 (r = 0.918), AP1M2 (r = 0.904), CLDN4 (r = 0.879), AGR2 (r = 0.879), CA12 (r = 0.853), KIAA1324 (r = 0.839), SPINT1 (r = 0.828), TFAP2A (r = 0.826), and FOXA1 (r = 0.826) as shown in Figure 6b. These demonstrate that ZG16B has strong interactions with various proteins involved in breast cancer formation, indicating the special role of ZG16B in breast cancer from another point of view.
3.5. Overexpressed ZG16B represents favorable prognosis of breast cancer patients
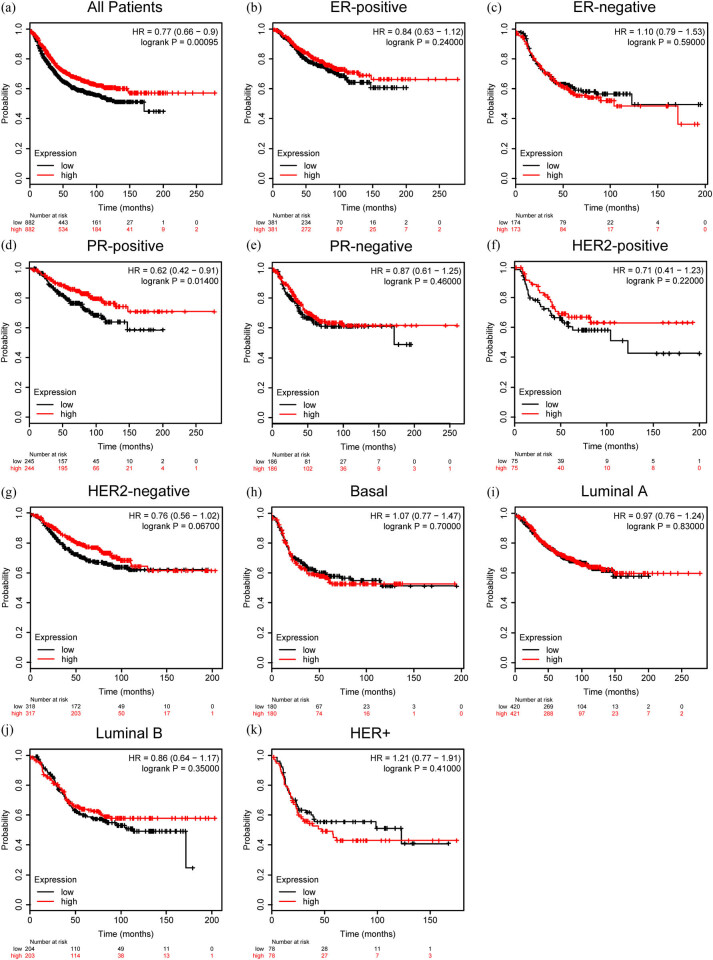
We further examined the impact of ZG16B on the prognosis of distinct molecular types of breast cancer by Kaplan–Meier plotter using the RFS as an indicator. Interestingly, higher expression of ZG16B represented a longer RFS for all breast cancer patients (HR = 0.77, p = 0.00095) (Figure 7a). The RFS of PR-positive patients also had a longer RFS as ZG16B expressed higher (HR = 0.62, p = 0.014) (Figure 7d), but there is no significance in other types of breast cancer (Figure 7b, c and e–k). These data indicated that ZG16B might be a general factor to mark a relatively favorable prognosis in breast cancer.
Figure 7.
Prognostic value of ZG16B expression in different subtypes of breast cancer. The RFS curves were drawn by Kaplan–Meier plotter in all breast cancer patients (a) and different subtypes of breast cancer, ER-positive (b), ER-negative (c), PR-positive (d), PR-negative (e), HER2-positive (f), HER2-negative (g), Basal (h), Luminal A (i), Luminal (j), and HER2+ (k).
4. Discussion
Currently, breast cancer threatens the health of women around the world [3,54]. Various traditional early detection methods including X-ray, CT, MRI, and ultrasound have been used to diagnose breast cancer [55,56]. In recent years, biomarkers in breast cancer are found and used not only to predict the invasiveness [57], recurrence, distant metastases, and prognosis [58,59], but also to predict the response to chemotherapy [60], monitor the use of medicine [61], and being the target of therapy [62] to earn time and quality of life for patients. Fortunately, consensus has been reached by biomedical researchers to establish bioinformatics database such as Oncomine [63], TCGA database [64], CCLE database [65], and so on to discover and predict potential biomarkers and therapeutic targets which could be verified by experiments furthermore.
ZG16B as a biomarker of pancreatic cancer, ovarian cancer, etc. has no research correlated to breast cancer yet. In our work, we specially noticed in Oncomine database that ZG16B was also upregulated in several reports. In order to further verify the observation, CCLE analysis and pooled meta-analysis have been explored and confirmed that ZG16B indeed has high expression in tissues and cell lines of breast cancer, as shown in Figures 1–3. To figure out the expression pattern of ZG16B in breast cancer, Ualcan database analysis confirms that ZG16B upregulates in different clinical classifications, including gender, age, and nodal metastasis status; it also demonstrates that ZG16B has significantly high expression in luminal and triple-negative molecular subtypes of breast cancer, while this high expression is not found in HER2-positive subtype (Figure 4). In addition, the mechanism of demethylation epigenetic factor has been represented by Ualcan to explain the upregulation of ZG16B in breast cancer (Figure 5).
In order to explore the possible role of ZG16B in breast cancer, STRING analysis and Oncomine co-expression analysis have been performed successively and we found that ZG16B had close relationships with UBCA1, DUSP15, ZBTB42, S100PBP, PRR4, CXCR4, LYZ, GTPBP10, FAM96B, ANKEF1, EPCAM, SPDEF, KRT8, SCNN1A, KRT19, ERBB3, AP1M2, CLDN4, AGR2, CA12, KIAA1324, SPINT1, TFAP2A, and FOXA1 (Figure 6). Among these proteins related to ZG16B, LYZ [66] and PRR4 [67] have protective function in tears and various body fluids, which are corresponding to Perumal’s research [45,46]. S100PBP [68], PRR4 [69], ANKEF1 [39,70], EPCAM [71], SPDEF [72], KRT8, KRT19 [73], KIAA1324 [74,75], CXCR4 [76,77], AGR2 [78,79,80], SCNN1A [81], AP1M2 [82], CLDN4 [83], and ERBB3 [84,85] have been reported to have correlations with breast cancer or have been identified as biomarkers for diagnosis, metastasis, and prognosis of breast cancer and even as therapeutic targets. Interestingly, FAM96B is reported to inhibit VEGF receptor 2 promoter to restrain endothelium activity through the control of E2-2 expression [86]. SPINT1, which is one of the Kunitz-type serine protease inhibitors and also known as HAI-1, can inhibit hepatocyte growth factor function via regulation of HGFA, matriptase, and hepsin to inhibit the migration, proliferation, and invasion of breast cancer, indicating a good prognosis [87,88,89]. TFAP2A, also known as AP-2-α, is a transcription factor regulating the differentiation and proliferation of breast, the upregulation of which inhibits cell cycle, promotes apoptosis, and suppresses invasion in breast cancer via the regulation of various miRNAs [90,91,92]. FOXA1 high expression connects with good prognosis in breast cancer by relieving the epithelial-to-mesenchymal transition process and inhibiting migration, invasion, and metastasis [93,94]. These four genes co-expressed with ZG16B are corresponding to our exploration, the interaction of which may interpret that ZG16B high expression represents a favorable prognosis in breast cancer. In addition, DUSP15 is recognized as a special regulator gene for oligodendrocytes differentiation [95]. GTPBP10 is a mitochondrial protein as a ribosome biogenesis factor [96,97] correlated with multicentric glioblastoma [98]. The two genes co-expressed with ZG16B may suggest a potential function of ZG16B in nervous system.
Finally, to confirm whether ZG16B had clinical significance in breast cancer patients and investigate its effect on prognosis, we have done Kaplan–Meier plotter analysis to access the RFS survival curves in different molecular subtypes of breast cancer shown in Figure 7, and it has been observed that although most molecular subtypes except PR-positive subtype ZG16B seemingly don’t show apparent effect, ZG16B does represent a long RFS and good prognosis for all kinds of patients. Patients with PR-positive breast cancer had the most favorable prognosis among breast cancer subtypes with ZG16B high expression, suggesting its special role in PR-positive breast cancer. All the data discussed above confirm that ZG16B might be a potential biomarker of breast cancer which represents a favorable prognosis.
In conclusion, ZG16B upregulates in breast cancer and represents a favorable prognosis in patients. Furthermore, ZG16B has correlations with various biomarkers and factors of breast cancer, some of which have precisely inhibitory effect on breast cancer. More work and experiments are needed in order to further reveal more fundamental mechanism for its role in breast cancer.
Acknowledgments
This study is supported by National Key R&D Program of China (2016YFC1000808), the National Natural Science Foundation of China (81470926, 81702785), Natural Science Foundation of Shandong Province (ZR2017PH013), Key R&D Program of Shandong Province (2019GSF107037), the Science and Technology Project of Qingdao, China (18-6-1-88-nsh), and Qingdao Postdoctoral Application Research Funded Project (2016067). In addition, we specially thank Dr. Rongtao Lu for comments on the manuscript.
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest in this work.
Contributor Information
Ling Li, Email: liling743@126.com.
Ying Liu, Email: liuying_hero@163.com.
References
- [1].DeSantis CE, Ma J, Sauer AG, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. Ca-a Cancer J Clinic. 2017;67(6):439–48. [DOI] [PubMed]; DeSantis CE, Ma J, Sauer AG, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. Ca-a Cancer J Clinic. 2017;67(6):439–48. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. Ca-a Cancer J Clinic. 2016;66(2):115–32. [DOI] [PubMed]; Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer Statistics in China, 2015. Ca-a Cancer J Clinic. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2016. Ca-a Cancer J Clinic. 2016;66(1):7–30. [DOI] [PubMed]; Siegel RL, Miller KD, Jemal A.. Cancer Statistics, 2016. Ca-a Cancer J Clinic. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Ao X, Jia Z, Bai XY, Xu Z, Hu G, et al. FOXK2 transcription factor suppresses ERalpha-positive breast cancer cell growth through down-regulating the stability of ERalpha via mechanism involving BRCA1/BARD1. Sci Rep. 2015;5:8796. [DOI] [PMC free article] [PubMed]; Liu Y, Ao X, Jia Z, Bai XY, Xu Z, Hu G. et al. FOXK2 transcription factor suppresses ERalpha-positive breast cancer cell growth through down-regulating the stability of ERalpha via mechanism involving BRCA1/BARD1. Sci Rep. 2015;5:8796. doi: 10.1038/srep08796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. [DOI] [PubMed]; Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA. et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- [6].Van de Wiel M, Dockx Y, Van den Wyngaert T, Stroobants S, Tjalma WAA, Huizing MT. Neoadjuvant systemic therapy in breast cancer: challenges and uncertainties. Eur J Obstet Gynecol Reprod Biol. 2017;210:144–56. [DOI] [PubMed]; Van de Wiel M, Dockx Y, Van den Wyngaert T, Stroobants S, Tjalma WAA, Huizing MT.. Neoadjuvant systemic therapy in breast cancer: challenges and uncertainties. Eur J Obstet Gynecol Reprod Biol. 2017;210:144–56. doi: 10.1016/j.ejogrb.2016.12.014. [DOI] [PubMed] [Google Scholar]
- [7].Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. [DOI] [PubMed]; Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- [8].Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017;389(10087):2430–42. [DOI] [PubMed]; Denkert C, Liedtke C, Tutt A, von Minckwitz G.. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet. 2017;389(10087):2430–42. doi: 10.1016/S0140-6736(16)32454-0. [DOI] [PubMed] [Google Scholar]
- [9].Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13(11):1387–97. [DOI] [PMC free article] [PubMed]; Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY. et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13(11):1387–97. doi: 10.7150/ijbs.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li G, Hu J, Hu G. Biomarker studies in early detection and prognosis of breast cancer. Adv Exp Med Biol. 2017;1026:27–39. [DOI] [PubMed]; Li G, Hu J, Hu G.. Biomarker studies in early detection and prognosis of breast cancer. Adv Exp Med Biol. 2017;1026:27–39. doi: 10.1007/978-981-10-6020-5_2. [DOI] [PubMed] [Google Scholar]
- [11].Janicke F, Schmitt M, Pache L, Ulm K, Harbeck N, Hofler H, et al. Urokinase (UPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node-negative breast-cancer. Breast Cancer Res Treat. 1993;24(3):195–208. [DOI] [PubMed]; Janicke F, Schmitt M, Pache L, Ulm K, Harbeck N, Hofler H. et al. Urokinase (UPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node-negative breast-cancer. Breast Cancer Res Treat. 1993;24(3):195–208. doi: 10.1007/BF01833260. [DOI] [PubMed] [Google Scholar]
- [12].Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S, et al. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7(11):3328–35. [PubMed]; Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S. et al. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7(11):3328–35. [PubMed] [Google Scholar]
- [13].Look MP, van Putten WLJ, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, et al. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 8377 breast cancer patients. J Natl Cancer Inst. 2002;94(2):116–28. [DOI] [PubMed]; Look MP, van Putten WLJ, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C. et al. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 8377 breast cancer patients. J Natl Cancer Inst. 2002;94(2):116–28. doi: 10.1093/jnci/94.2.116. [DOI] [PubMed] [Google Scholar]
- [14].Zhao H, Shen J, Medico L, Wang D, Ambrosone CB, Liu S. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLoS One. 2010;5(10):e13735. [DOI] [PMC free article] [PubMed]; Zhao H, Shen J, Medico L, Wang D, Ambrosone CB, Liu S.. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLoS One. 2010;5(10):e13735. doi: 10.1371/journal.pone.0013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hao W, Friedman A. Serum uPAR as biomarker in breast cancer recurrence: a mathematical model. PLoS One. 2016;11(4):e0153508. [DOI] [PMC free article] [PubMed]; Hao W, Friedman A.. Serum uPAR as biomarker in breast cancer recurrence: a mathematical model. PLoS One. 2016;11(4):e0153508. doi: 10.1371/journal.pone.0153508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singh R, Bhatt ML, Singh SP, Kumar V, Goel MM, Mishra DP, et al. Evaluation of KiSS1 as a prognostic biomarker in North Indian breast cancer cases. Asian Pac J Cancer Prev. 2016;17(4):1789–95. [DOI] [PubMed]; Singh R, Bhatt ML, Singh SP, Kumar V, Goel MM, Mishra DP. et al. Evaluation of KiSS1 as a prognostic biomarker in North Indian breast cancer cases. Asian Pac J Cancer Prev. 2016;17(4):1789–95. doi: 10.7314/apjcp.2016.17.4.1789. [DOI] [PubMed] [Google Scholar]
- [17].Jing X, Cui X, Liang H, Hao C, Yang Z, Li X, et al. CD24 is a potential biomarker for prognosis in human breast carcinoma. Cell Physiol Biochem. 2018;48(1):111–9. [DOI] [PubMed]; Jing X, Cui X, Liang H, Hao C, Yang Z, Li X. et al. CD24 is a potential biomarker for prognosis in human breast carcinoma. Cell Physiol Biochem. 2018;48(1):111–9. doi: 10.1159/000491667. [DOI] [PubMed] [Google Scholar]
- [18].Mito A, Nakano Y, Saitoh T, Gouraud SSS, Yamaguchi Y, Sato T, et al. Lectin ZG16p inhibits proliferation of human colorectal cancer cells via its carbohydrate-binding sites. Glycobiology. 2018;28(1):21–31. [DOI] [PubMed]; Mito A, Nakano Y, Saitoh T, Gouraud SSS, Yamaguchi Y, Sato T. et al. Lectin ZG16p inhibits proliferation of human colorectal cancer cells via its carbohydrate-binding sites. Glycobiology. 2018;28(1):21–31. doi: 10.1093/glycob/cwx088. [DOI] [PubMed] [Google Scholar]
- [19].Kanagawa M, Satoh T, Ikeda A, Nakano Y, Yagi H, Kato K, et al. Crystal structures of human secretory proteins ZG16p and ZG16b reveal a Jacalin-related beta-prism fold. Biochem Biophys Res Commun. 2011;404(1):201–5. [DOI] [PubMed]; Kanagawa M, Satoh T, Ikeda A, Nakano Y, Yagi H, Kato K. et al. Crystal structures of human secretory proteins ZG16p and ZG16b reveal a Jacalin-related beta-prism fold. Biochem Biophys Res Commun. 2011;404(1):201–5. doi: 10.1016/j.bbrc.2010.11.093. [DOI] [PubMed] [Google Scholar]
- [20].Escudero-Paniagua B, Bartolome RA, Rodriguez S, de Los Rios V, Pintado L, Jaen M, et al. PAUF/ZG16B promotes colorectal cancer progression through alterations of the mitotic functions and the Wnt/beta-catenin pathway. Carcinogenesis. 2020;41(2):203–13. [DOI] [PubMed]; Escudero-Paniagua B, Bartolome RA, Rodriguez S, de Los Rios V, Pintado L, Jaen M. et al. PAUF/ZG16B promotes colorectal cancer progression through alterations of the mitotic functions and the Wnt/beta-catenin pathway. Carcinogenesis. 2020;41(2):203–13. doi: 10.1093/carcin/bgz093. [DOI] [PubMed] [Google Scholar]
- [21].Kim SA, Lee Y, Jung DE, Park KH, Park JY, Gang J, et al. Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma. Cancer Sci. 2009;100(5):828–36. [DOI] [PMC free article] [PubMed]; Kim SA, Lee Y, Jung DE, Park KH, Park JY, Gang J. et al. Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma. Cancer Sci. 2009;100(5):828–36. doi: 10.1111/j.1349-7006.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bergstrom JH, Birchenough GM, Katona G, Schroeder BO, Schutte A, Ermund A, et al. Gram-positive bacteria are held at a distance in the colon mucus by the lectin-like protein ZG16. Proc Natl Acad Sci U S A. 2016;113(48):13833–8. [DOI] [PMC free article] [PubMed]; Bergstrom JH, Birchenough GM, Katona G, Schroeder BO, Schutte A, Ermund A. et al. Gram-positive bacteria are held at a distance in the colon mucus by the lectin-like protein ZG16. Proc Natl Acad Sci U S A. 2016;113(48):13833–8. doi: 10.1073/pnas.1611400113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meng H, Li W, Boardman LA, Wang L. Loss of ZG16 is associated with molecular and clinicopathological phenotypes of colorectal cancer. BMC Cancer. 2018;18(1):433. [DOI] [PMC free article] [PubMed]; Meng H, Li W, Boardman LA, Wang L.. Loss of ZG16 is associated with molecular and clinicopathological phenotypes of colorectal cancer. BMC Cancer. 2018;18(1):433. doi: 10.1186/s12885-018-4337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park HD, Lee Y, Oh YK, Jung JG, Park YW, Myung K, et al. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene. 2011;30(2):201–11. [DOI] [PMC free article] [PubMed]; Park HD, Lee Y, Oh YK, Jung JG, Park YW, Myung K. et al. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene. 2011;30(2):201–11. doi: 10.1038/onc.2010.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cho IR, Koh SS, Min HJ, Kim SJ, Lee Y, Park EH, et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the expression of beta-catenin, leading to a rapid proliferation of pancreatic cells. Exp Mol Med. 2011;43(2):82–90. [DOI] [PMC free article] [PubMed]; Cho IR, Koh SS, Min HJ, Kim SJ, Lee Y, Park EH. et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the expression of beta-catenin, leading to a rapid proliferation of pancreatic cells. Exp Mol Med. 2011;43(2):82–90. doi: 10.3858/emm.2011.43.2.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim SJ, Lee Y, Kim NY, Hwang Y, Hwang B, Min JK, et al. Pancreatic adenocarcinoma upregulated factor, a novel endothelial activator, promotes angiogenesis and vascular permeability. Oncogene. 2013;32(31):3638–47. [DOI] [PubMed]; Kim SJ, Lee Y, Kim NY, Hwang Y, Hwang B, Min JK. et al. Pancreatic adenocarcinoma upregulated factor, a novel endothelial activator, promotes angiogenesis and vascular permeability. Oncogene. 2013;32(31):3638–47. doi: 10.1038/onc.2012.366. [DOI] [PubMed] [Google Scholar]
- [27].Lee Y, Kim SJ, Park HD, Park EH, Huang SM, Jeon SB, et al. PAUF functions in the metastasis of human pancreatic cancer cells and upregulates CXCR4 expression. Oncogene 2010;29(1):56–67. [DOI] [PubMed]; Lee Y, Kim SJ, Park HD, Park EH, Huang SM, Jeon SB. et al. PAUF functions in the metastasis of human pancreatic cancer cells and upregulates CXCR4 expression. Oncogene. 2010;29(1):56–67. doi: 10.1038/onc.2009.298. [DOI] [PubMed] [Google Scholar]
- [28].Lee YS, Kim SJ, Min HJ, Jo JY, Park EH, Koh SS. PAUF promotes adhesiveness of pancreatic cancer cells by modulating focal adhesion kinase. Exp Mol Med. 2011;43(5):291–7. [DOI] [PMC free article] [PubMed]; Lee YS, Kim SJ, Min HJ, Jo JY, Park EH, Koh SS.. PAUF promotes adhesiveness of pancreatic cancer cells by modulating focal adhesion kinase. Exp Mol Med. 2011;43(5):291–7. doi: 10.3858/emm.2011.43.5.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kaowinn S, Cho IR, Moon J, Jun SW, Kim CS, Kang HY, et al. Pancreatic adenocarcinoma upregulated factor (PAUF) confers resistance to pancreatic cancer cells against oncolytic parvovirus H-1 infection through IFNA receptor-mediated signaling. Biochem Biophys Res Commun. 2015;459(2):313–8. [DOI] [PubMed]; Kaowinn S, Cho IR, Moon J, Jun SW, Kim CS, Kang HY. et al. Pancreatic adenocarcinoma upregulated factor (PAUF) confers resistance to pancreatic cancer cells against oncolytic parvovirus H-1 infection through IFNA receptor-mediated signaling. Biochem Biophys Res Commun. 2015;459(2):313–8. doi: 10.1016/j.bbrc.2015.02.107. [DOI] [PubMed] [Google Scholar]
- [30].Kang TH, Kim YS, Kim S, Yang B, Lee JJ, Lee HJ, et al. Pancreatic adenocarcinoma upregulated factor serves as adjuvant by activating dendritic cells through stimulation of TLR4. Oncotarget. 2015;6(29):27751–62. [DOI] [PMC free article] [PubMed]; Kang TH, Kim YS, Kim S, Yang B, Lee JJ, Lee HJ. et al. Pancreatic adenocarcinoma upregulated factor serves as adjuvant by activating dendritic cells through stimulation of TLR4. Oncotarget. 2015;6(29):27751–62. doi: 10.18632/oncotarget.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Song J, Lee J, Kim J, Jo S, Kim YJ, Baek JE, et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the accumulation and functional activity of myeloid-derived suppressor cells (MDSCs) in pancreatic cancer. Oncotarget. 2016;7(32):51840–53. [DOI] [PMC free article] [PubMed]; Song J, Lee J, Kim J, Jo S, Kim YJ, Baek JE. et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the accumulation and functional activity of myeloid-derived suppressor cells (MDSCs) in pancreatic cancer. Oncotarget. 2016;7(32):51840–53. doi: 10.18632/oncotarget.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gao CC, Xu XL, Li F, Gong BG, Liu S, Cui YQ, et al. Silencing pancreatic adenocarcinoma upregulated factor (PAUF) increases the sensitivity of pancreatic cancer cells to gemcitabine. Tumour Biol. 2016;37(6):7555–64. [DOI] [PubMed]; Gao CC, Xu XL, Li F, Gong BG, Liu S, Cui YQ. et al. Silencing pancreatic adenocarcinoma upregulated factor (PAUF) increases the sensitivity of pancreatic cancer cells to gemcitabine. Tumour Biol. 2016;37(6):7555–64. doi: 10.1007/s13277-015-4641-2. [DOI] [PubMed] [Google Scholar]
- [33].Cho JH, Kim SA, Park SB, Kim HM, Song SY. Suppression of pancreatic adenocarcinoma upregulated factor (PAUF) increases the sensitivity of pancreatic cancer to gemcitabine and 5FU, and inhibits the formation of pancreatic cancer stem like cells. Oncotarget. 2017;8(44):76398–407. [DOI] [PMC free article] [PubMed]; Cho JH, Kim SA, Park SB, Kim HM, Song SY.. Suppression of pancreatic adenocarcinoma upregulated factor (PAUF) increases the sensitivity of pancreatic cancer to gemcitabine and 5FU, and inhibits the formation of pancreatic cancer stem like cells. Oncotarget. 2017;8(44):76398–407. doi: 10.18632/oncotarget.19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Barderas R, Mendes M, Torres S, Bartolome RA, Lopez-Lucendo M, Villar-Vazquez R, et al. In-depth characterization of the secretome of colorectal cancer metastatic cells identifies key proteins in cell adhesion, migration, and invasion. Mol Cell Proteom. 2013;12(6):1602–20. [DOI] [PMC free article] [PubMed]; Barderas R, Mendes M, Torres S, Bartolome RA, Lopez-Lucendo M, Villar-Vazquez R. et al. In-depth characterization of the secretome of colorectal cancer metastatic cells identifies key proteins in cell adhesion, migration, and invasion. Mol Cell Proteom. 2013;12(6):1602–20. doi: 10.1074/mcp.M112.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu PF, Wu YY, Hu Y, Wang L, He SB, Zhu YB, et al. Silencing of pancreatic adenocarcinoma upregulated factor by RNA interference inhibits the malignant phenotypes of human colorectal cancer cells. Oncol Rep. 2013;30(1):213–20. [DOI] [PubMed]; Liu PF, Wu YY, Hu Y, Wang L, He SB, Zhu YB. et al. Silencing of pancreatic adenocarcinoma upregulated factor by RNA interference inhibits the malignant phenotypes of human colorectal cancer cells. Oncol Rep. 2013;30(1):213–20. doi: 10.3892/or.2013.2478. [DOI] [PubMed] [Google Scholar]
- [36].Kim JG, Chae YS, Lee SJ, Kang BW, Park JY, Lee EJ, et al. Genetic variation in microRNA-binding site and prognosis of patients with colorectal cancer. J Cancer Res Clin Oncol. 2015;141(1):35–41. [DOI] [PubMed]; Kim JG, Chae YS, Lee SJ, Kang BW, Park JY, Lee EJ. et al. Genetic variation in microRNA-binding site and prognosis of patients with colorectal cancer. J Cancer Res Clin Oncol. 2015;141(1):35–41. doi: 10.1007/s00432-014-1780-6. [DOI] [PubMed] [Google Scholar]
- [37].Wakana Y, van Galen J, Meissner F, Scarpa M, Polishchuk RS, Mann M, et al. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. Embo J. 2012;31(20):3976–90. [DOI] [PMC free article] [PubMed]; Wakana Y, van Galen J, Meissner F, Scarpa M, Polishchuk RS, Mann M. et al. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. Embo J. 2012;31(20):3976–90. doi: 10.1038/emboj.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Choi CH, Chung JY, Park HS, Jun M, Lee YY, Kim BG, et al. Pancreatic adenocarcinoma up-regulated factor expression is associated with disease-specific survival in cervical cancer patients. Hum Pathol. 2015;46(6):884–93. [DOI] [PMC free article] [PubMed]; Choi CH, Chung JY, Park HS, Jun M, Lee YY, Kim BG. et al. Pancreatic adenocarcinoma up-regulated factor expression is associated with disease-specific survival in cervical cancer patients. Hum Pathol. 2015;46(6):884–93. doi: 10.1016/j.humpath.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jin HJ, Jung S, DebRoy AR, Davuluri RV. Identification and validation of regulatory SNPs that modulate transcription factor chromatin binding and gene expression in prostate cancer. Oncotarget. 2016;7(34):54616–26. [DOI] [PMC free article] [PubMed]; Jin HJ, Jung S, DebRoy AR, Davuluri RV.. Identification and validation of regulatory SNPs that modulate transcription factor chromatin binding and gene expression in prostate cancer. Oncotarget. 2016;7(34):54616–26. doi: 10.18632/oncotarget.10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sasahira T, Kurihara M, Nishiguchi Y, Nakashima C, Kirita T, Kuniyasu H. Pancreatic adenocarcinoma up-regulated factor has oncogenic functions in oral squamous cell carcinoma. Histopathology. 2017;70(4):539–48. [DOI] [PubMed]; Sasahira T, Kurihara M, Nishiguchi Y, Nakashima C, Kirita T, Kuniyasu H.. Pancreatic adenocarcinoma up-regulated factor has oncogenic functions in oral squamous cell carcinoma. Histopathology. 2017;70(4):539–48. doi: 10.1111/his.13097. [DOI] [PubMed] [Google Scholar]
- [41].Kim SK, Song SY, Kim S, Cho NH, Yim GW, Kim SW, et al. Association of pancreatic adenocarcinoma up-regulated factor expression in ovarian mucinous adenocarcinoma with poor prognosis. Int J Clin Exp Pathol. 2014;7(8):5103–10. [PMC free article] [PubMed]; Kim SK, Song SY, Kim S, Cho NH, Yim GW, Kim SW. et al. Association of pancreatic adenocarcinoma up-regulated factor expression in ovarian mucinous adenocarcinoma with poor prognosis. Int J Clin Exp Pathol. 2014;7(8):5103–10. [PMC free article] [PubMed] [Google Scholar]
- [42].Choi CH, Kang TH, Song JS, Kim YS, Chung EJ, Ylaya K, et al. Elevated expression of pancreatic adenocarcinoma upregulated factor (PAUF) is associated with poor prognosis and chemoresistance in epithelial ovarian cancer. Sci Rep. 2018;8(1):12161. [DOI] [PMC free article] [PubMed]; Choi CH, Kang TH, Song JS, Kim YS, Chung EJ, Ylaya K. et al. Elevated expression of pancreatic adenocarcinoma upregulated factor (PAUF) is associated with poor prognosis and chemoresistance in epithelial ovarian cancer. Sci Rep. 2018;8(1):12161. doi: 10.1038/s41598-018-30582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang GH, Chen MM, Kai JY, Ma Q, Zhong AL, Xie SH, et al. Molecular profiling of mucinous epithelial ovarian cancer by weighted gene co-expression network analysis. Gene. 2019;709:56–64. [DOI] [PubMed]; Zhang GH, Chen MM, Kai JY, Ma Q, Zhong AL, Xie SH. et al. Molecular profiling of mucinous epithelial ovarian cancer by weighted gene co-expression network analysis. Gene. 2019;709:56–64. doi: 10.1016/j.gene.2019.05.034. [DOI] [PubMed] [Google Scholar]
- [44].Martin-Lorenzo M, Zubiri I, Maroto AS, Gonzalez-Calero L, Posada-Ayala M, de la Cuesta F, et al. KLK1 and ZG16B proteins and arginine-proline metabolism identified as novel targets to monitor atherosclerosis, acute coronary syndrome and recovery. Metabolomics. 2015;11(5):1056–67. [DOI] [PMC free article] [PubMed]; Martin-Lorenzo M, Zubiri I, Maroto AS, Gonzalez-Calero L, Posada-Ayala M, de la Cuesta F. et al. KLK1 and ZG16B proteins and arginine-proline metabolism identified as novel targets to monitor atherosclerosis, acute coronary syndrome and recovery. Metabolomics. 2015;11(5):1056–67. doi: 10.1007/s11306-014-0761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Perumal N, Funke S, Wolters D, Pfeiffer N, Grus FH. Characterization of human reflex tear proteome reveals high expression of lacrimal proline-rich protein 4 (PRR4). Proteomics. 2015;15(19):3370–81. [DOI] [PubMed]; Perumal N, Funke S, Wolters D, Pfeiffer N, Grus FH.. Characterization of human reflex tear proteome reveals high expression of lacrimal proline-rich protein 4 (PRR4) Proteomics. 2015;15(19):3370–81. doi: 10.1002/pmic.201400239. [DOI] [PubMed] [Google Scholar]
- [46].Perumal N, Funke S, Pfeiffer N, Grus FH. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci Rep. 2016;6:29629. [DOI] [PMC free article] [PubMed]; Perumal N, Funke S, Pfeiffer N, Grus FH.. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci Rep. 2016;6:29629. doi: 10.1038/srep29629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52. [DOI] [PMC free article] [PubMed]; Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–27. [DOI] [PubMed]; Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H. et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–27. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- [49].Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11(1):R7. [DOI] [PMC free article] [PubMed]; Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC.. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11(1):R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. [DOI] [PubMed]; Jones PA, Baylin SB.. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- [51].Van Tongelen A, Loriot A, De Smet C. Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Lett. 2017;396:130–7. [DOI] [PubMed]; Van Tongelen A, Loriot A, De, Smet C.. Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Lett. 2017;396:130–7. doi: 10.1016/j.canlet.2017.03.029. [DOI] [PubMed] [Google Scholar]
- [52].Lin IH, Chen DT, Chang YF, Lee YL, Su CH, Cheng C, et al. Hierarchical clustering of breast cancer methylomes revealed differentially methylated and expressed breast cancer genes. PLoS One. 2015;10(2):e0118453. [DOI] [PMC free article] [PubMed]; Lin IH, Chen DT, Chang YF, Lee YL, Su CH, Cheng C. et al. Hierarchical clustering of breast cancer methylomes revealed differentially methylated and expressed breast cancer genes. PLoS One. 2015;10(2):e0118453. doi: 10.1371/journal.pone.0118453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. [DOI] [PMC free article] [PubMed]; Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J. et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. Ca-a Cancer J Clinic. 2015;65(2):87–108. [DOI] [PubMed]; Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A.. Global Cancer Statistics, 2012. Ca-a Cancer J Clinic. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- [55].Roganovic D, Djilas D, Vujnovic S, Pavic D, Stojanov D. Breast MRI, digital mammography and breast tomosynthesis: comparison of three methods for early detection of breast cancer. Bosn J Basic Med Sci. 2015;15(4):64–8. [DOI] [PMC free article] [PubMed]; Roganovic D, Djilas D, Vujnovic S, Pavic D, Stojanov D. Breast MRI, digital mammography and breast tomosynthesis: comparison of three methods for early detection of breast cancer. Bosn J Basic Med Sci. 2015;15(4):64–8. doi: 10.17305/bjbms.2015.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang L. Early diagnosis of breast cancer. Sensors. 2017;17(7):1572. [DOI] [PMC free article] [PubMed]; Wang L.. Early diagnosis of breast cancer. Sensors. 2017;17(7):1572. doi: 10.3390/s17071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lim S, Janzer A, Becker A, Zimmer A, Schule R, Buettner R, et al. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31(3):512–20. [DOI] [PubMed]; Lim S, Janzer A, Becker A, Zimmer A, Schule R, Buettner R. et al. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31(3):512–20. doi: 10.1093/carcin/bgp324. [DOI] [PubMed] [Google Scholar]
- [58].Millar EKA, Graham PH, O’Toole SA, McNeil CM, Browne L, Morey AL, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27(28):4701–8. [DOI] [PubMed]; Millar EKA, Graham PH, O’Toole SA, McNeil CM, Browne L, Morey AL. et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27(28):4701–8. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- [59].Ao X, Ding W, Ge H, Zhang Y, Ding D, Liu Y. PBX1 is a valuable prognostic biomarker for patients with breast cancer. Exp Ther Med. 2020;20(1):385–94. [DOI] [PMC free article] [PubMed]; Ao X, Ding W, Ge H, Zhang Y, Ding D, Liu Y.. PBX1 is a valuable prognostic biomarker for patients with breast cancer. Exp Ther Med. 2020;20(1):385–94. doi: 10.3892/etm.2020.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Esserman LJ, Berry DA, Cheang MCU, Yau C, Perou CM, Carey L, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2012;132(3):1049–62. [DOI] [PMC free article] [PubMed]; Esserman LJ, Berry DA, Cheang MCU, Yau C, Perou CM, Carey L. et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2012;132(3):1049–62. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer – a study from the IMPACT trialists. J Clin Oncol. 2005;23(11):2477–92. [DOI] [PubMed]; Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I. et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer – a study from the IMPACT trialists. J Clin Oncol. 2005;23(11):2477–92. doi: 10.1200/JCO.2005.07.559. [DOI] [PubMed] [Google Scholar]
- [62].Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, et al. The HER-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8(4):307–25. [DOI] [PubMed]; Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M. et al. The HER-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8(4):307–25. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- [63].Rhodes DR, Yu JJ, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. [DOI] [PMC free article] [PubMed]; Rhodes DR, Yu JJ, Shanker K, Deshpande N, Varambally R, Ghosh D. et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chin L, Meyerson M, Aldape K, Bigner D, Mikkelsen T, VandenBerg S, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. [DOI] [PMC free article] [PubMed]; Chin L, Meyerson M, Aldape K, Bigner D, Mikkelsen T, VandenBerg S. et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7. [DOI] [PMC free article] [PubMed]; Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S. et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McCanna DJ, Oh S, Seo J, Coles-Brennan C, Fadli Z, Subbaraman LN, et al. The effect of denatured lysozyme on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2018;59(5):2006–14. [DOI] [PubMed]; McCanna DJ, Oh S, Seo J, Coles-Brennan C, Fadli Z, Subbaraman LN. et al. The effect of denatured lysozyme on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2018;59(5):2006–14. doi: 10.1167/iovs.17-22260. [DOI] [PubMed] [Google Scholar]
- [67].Ekizoglu S, Ulutin T, Guliyev J, Buyru N. PRR4: a novel downregulated gene in laryngeal cancer. Oncol Lett. 2018;15(4):4669–75. [DOI] [PMC free article] [PubMed]; Ekizoglu S, Ulutin T, Guliyev J, Buyru N.. PRR4: a novel downregulated gene in laryngeal cancer. Oncol Lett. 2018;15(4):4669–75. doi: 10.3892/ol.2018.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Savci-Heijink CD, Halfwerk H, Koster J, Horlings HM, van de Vijver MJ. A specific gene expression signature for visceral organ metastasis in breast cancer. BMC Cancer. 2019;19(1):333. [DOI] [PMC free article] [PubMed]; Savci-Heijink CD, Halfwerk H, Koster J, Horlings HM, van de Vijver MJ.. A specific gene expression signature for visceral organ metastasis in breast cancer. BMC Cancer. 2019;19(1):333. doi: 10.1186/s12885-019-5554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rabet MM, Cabaud O, Josselin E, Finetti P, Castellano R, Farina A, et al. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer Ann Oncol. 2017;28(4):769–76. [DOI] [PubMed]; Rabet MM, Cabaud O, Josselin E, Finetti P, Castellano R, Farina A. et al. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann Oncol. 2017;28(4):769–76. doi: 10.1093/annonc/mdw678. [DOI] [PubMed] [Google Scholar]
- [70].Nguyen NM, Andrade FD, Jin L, Zhang XY, Macon M, Cruz MI, et al. Maternal intake of high n-6 polyunsaturated fatty acid diet during pregnancy causes transgenerational increase in mammary cancer risk in mice. Breast Cancer Res. 2017;19:77. [DOI] [PMC free article] [PubMed]; Nguyen NM, Andrade FD, Jin L, Zhang XY, Macon M, Cruz MI. et al. Maternal intake of high n-6 polyunsaturated fatty acid diet during pregnancy causes transgenerational increase in mammary cancer risk in mice. Breast Cancer Res. 2017;19:77. doi: 10.1186/s13058-017-0866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gao S, Sun Y, Liu X, Zhang D, Yang X. EpCAM and COX2 expression are positively correlated in human breast cancer. Mol Med Rep. 2017;15(6):3755–60. [DOI] [PubMed]; Gao S, Sun Y, Liu X, Zhang D, Yang X.. EpCAM and COX2 expression are positively correlated in human breast cancer. Mol Med Rep. 2017;15(6):3755–60. doi: 10.3892/mmr.2017.6447. [DOI] [PubMed] [Google Scholar]
- [72].Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. [DOI] [PubMed]; Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C. et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- [73].Qiu J, Du Z, Wang Y, Zhou Y, Zhang Y, Xie Y, et al. Weighted gene co-expression network analysis reveals modules and hub genes associated with the development of breast cancer. Medicine. 2019;98(6):e14345. [DOI] [PMC free article] [PubMed]; Qiu J, Du Z, Wang Y, Zhou Y, Zhang Y, Xie Y. et al. Weighted gene co-expression network analysis reveals modules and hub genes associated with the development of breast cancer. Medicine. 2019;98(6):e14345. doi: 10.1097/MD.0000000000014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bolton KA, Holliday EG, Attia J, Bowden NA, Avery-Kiejda KA, Scott RJ. A novel polymorphic repeat in the upstream regulatory region of the estrogen-induced gene EIG121 is not associated with the risk of developing breast or endometrial cancer. BMC Res Notes. 2016;9:287. [DOI] [PMC free article] [PubMed]; Bolton KA, Holliday EG, Attia J, Bowden NA, Avery-Kiejda KA, Scott RJ.. A novel polymorphic repeat in the upstream regulatory region of the estrogen-induced gene EIG121 is not associated with the risk of developing breast or endometrial cancer. BMC Res Notes. 2016;9:287. doi: 10.1186/s13104-016-2086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schlumbrecht MP, Xie SS, Shipley GL, Urbauer DL, Broaddus RR. Molecular clustering based on ERalpha and EIG121 predicts survival in high-grade serous carcinoma of the ovary/peritoneum. Mod Pathol. 2011;24(3):453–62. [DOI] [PMC free article] [PubMed]; Schlumbrecht MP, Xie SS, Shipley GL, Urbauer DL, Broaddus RR.. Molecular clustering based on ERalpha and EIG121 predicts survival in high-grade serous carcinoma of the ovary/peritoneum. Mod Pathol. 2011;24(3):453–62. doi: 10.1038/modpathol.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998;393(6685):595–9. [DOI] [PubMed]; Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR.. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- [77].Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. [DOI] [PubMed]; Mantovani A, Allavena P, Sica A, Balkwill F.. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- [78].Jung SY, Yun J, Kim SJ, Kang S, Kim DY, Kim YJ, et al. Basic helix-loop-helix transcription factor Twist1 is a novel regulator of anterior gradient protein 2 homolog (AGR2) in breast cancer. Biochem Biophys Res Commun. 2019;516(1):149–56. [DOI] [PubMed]; Jung SY, Yun J, Kim SJ, Kang S, Kim DY, Kim YJ. et al. Basic helix-loop-helix transcription factor Twist1 is a novel regulator of anterior gradient protein 2 homolog (AGR2) in breast cancer. Biochem Biophys Res Commun. 2019;516(1):149–56. doi: 10.1016/j.bbrc.2019.05.191. [DOI] [PubMed] [Google Scholar]
- [79].Ondrouskova E, Sommerova L, Nenutil R, Coufal O, Bouchal P, Vojtesek B, et al. AGR2 associates with HER2 expression predicting poor outcome in subset of estrogen receptor negative breast cancer patients. Exp Mol Pathol. 2017;102(2):280–3. [DOI] [PubMed]; Ondrouskova E, Sommerova L, Nenutil R, Coufal O, Bouchal P, Vojtesek B. et al. AGR2 associates with HER2 expression predicting poor outcome in subset of estrogen receptor negative breast cancer patients. Exp Mol Pathol. 2017;102(2):280–3. doi: 10.1016/j.yexmp.2017.02.016. [DOI] [PubMed] [Google Scholar]
- [80].Salmans ML, Zhao F, Andersen B. The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: a potential drug target and biomarker. Breast Cancer Res. 2013;15(2):204. [DOI] [PMC free article] [PubMed]; Salmans ML, Zhao F, Andersen B.. The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: a potential drug target and biomarker. Breast Cancer Res. 2013;15(2):204. doi: 10.1186/bcr3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Varley KE, Gertz J, Roberts BS, Davis NS, Bowling KM, Kirby MK, et al. Recurrent read-through fusion transcripts in breast cancer. Breast Cancer Res Treat. 2014;146(2):287–97. [DOI] [PMC free article] [PubMed]; Varley KE, Gertz J, Roberts BS, Davis NS, Bowling KM, Kirby MK. et al. Recurrent read-through fusion transcripts in breast cancer. Breast Cancer Res Treat. 2014;146(2):287–97. doi: 10.1007/s10549-014-3019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tang YC, Ho SC, Tan E, Ng AWT, McPherson JR, Goh GYL, et al. Functional genomics identifies specific vulnerabilities in PTEN-deficient breast cancer. Breast Cancer Res. 2018;20(1):22. [DOI] [PMC free article] [PubMed]; Tang YC, Ho SC, Tan E, Ng AWT, McPherson JR, Goh GYL. et al. Functional genomics identifies specific vulnerabilities in PTEN-deficient breast cancer. Breast Cancer Res. 2018;20(1):22. doi: 10.1186/s13058-018-0949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang F, Gao Y, Tang L, Ning K, Geng N, Zhang H, et al. A novel PAK4-CEBPB-CLDN4 axis involving in breast cancer cell migration and invasion. Biochem Biophys Res Commun. 2019;511(2):404–8. [DOI] [PubMed]; Wang F, Gao Y, Tang L, Ning K, Geng N, Zhang H. et al. A novel PAK4-CEBPB-CLDN4 axis involving in breast cancer cell migration and invasion. Biochem Biophys Res Commun. 2019;511(2):404–8. doi: 10.1016/j.bbrc.2019.02.070. [DOI] [PubMed] [Google Scholar]
- [84].Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–75. [DOI] [PubMed]; Baselga J, Swain SM.. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- [85].Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100(15):8933–8. [DOI] [PMC free article] [PubMed]; Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE.. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100(15):8933–8. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yang W, Itoh F, Ohya H, Kishimoto F, Tanaka A, Nakano N, et al. Interference of E2-2-mediated effect in endothelial cells by FAM96B through its limited expression of E2-2. Cancer Sci. 2011;102(10):1808–14. [DOI] [PubMed]; Yang W, Itoh F, Ohya H, Kishimoto F, Tanaka A, Nakano N. et al. Interference of E2-2-mediated effect in endothelial cells by FAM96B through its limited expression of E2-2. Cancer Sci. 2011;102(10):1808–14. doi: 10.1111/j.1349-7006.2011.02022.x. [DOI] [PubMed] [Google Scholar]
- [87].Oberst MD, Chen LY, Kiyomiya K, Williams CA, Lee MS, Johnson MD, et al. HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol. 2005;289(2):C462–70. [DOI] [PubMed]; Oberst MD, Chen LY, Kiyomiya K, Williams CA, Lee MS, Johnson MD. et al. HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol. 2005;289(2):C462–70. doi: 10.1152/ajpcell.00076.2005. [DOI] [PubMed] [Google Scholar]
- [88].Parr C, Jiang WG. Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells. Int J Cancer. 2006;119(5):1176–83. [DOI] [PubMed]; Parr C, Jiang WG.. Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells. Int J Cancer. 2006;119(5):1176–83. doi: 10.1002/ijc.21881. [DOI] [PubMed] [Google Scholar]
- [89].Parr C, Watkins G, Mansel RE, Jiang WG. The hepatocyte growth factor regulatory factors in human breast cancer. Clin Cancer Res. 2004;10(1 Pt 1):202-11. [DOI] [PubMed]; Parr C, Watkins G, Mansel RE, Jiang WG.. The hepatocyte growth factor regulatory factors in human breast cancer. Clin Cancer Res. 2004;10(1 Pt 1):202–11.. doi: 10.1158/1078-0432.ccr-0553-3. [DOI] [PubMed] [Google Scholar]
- [90].Berlato C, Chan KV, Price AM, Canosa M, Scibetta AG, Hurst HC. Alternative TFAP2A isoforms have distinct activities in breast cancer. Breast Cancer Res. 2011;13(2):R23. [DOI] [PMC free article] [PubMed]; Berlato C, Chan KV, Price AM, Canosa M, Scibetta AG, Hurst HC.. Alternative TFAP2A isoforms have distinct activities in breast cancer. Breast Cancer Res. 2011;13(2):R23. doi: 10.1186/bcr2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhou B, Guo H, Tang J. Long non-coding RNA TFAP2A-AS1 inhibits cell proliferation and invasion in breast cancer via miR-933/SMAD2. Med Sci Monit. 2019;25:1242–53. [DOI] [PMC free article] [PubMed]; Zhou B, Guo H, Tang J. Long non-coding RNA TFAP2A-AS1 inhibits cell proliferation and invasion in breast cancer via miR-933/SMAD2. Med Sci Monit. 2019;25:1242–53. doi: 10.12659/MSM.912421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Xu J, Zheng J, Wang J, Shao J. miR-876-5p suppresses breast cancer progression through targeting TFAP2A. Exp Ther Med. 2019;18(2):1458–64. [DOI] [PMC free article] [PubMed]; Xu J, Zheng J, Wang J, Shao J.. miR-876-5p suppresses breast cancer progression through targeting TFAP2A. Exp Ther Med. 2019;18(2):1458–64. doi: 10.3892/etm.2019.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shou J, Lai Y, Xu J, Huang J. Prognostic value of FOXA1 in breast cancer: a systematic review and meta-analysis. Breast. 2016;27:35–43. [DOI] [PubMed]; Shou J, Lai Y, Xu J, Huang J.. Prognostic value of FOXA1 in breast cancer: a systematic review and meta-analysis. Breast. 2016;27:35–43. doi: 10.1016/j.breast.2016.02.009. [DOI] [PubMed] [Google Scholar]
- [94].Xu Y, Qin L, Sun T, Wu H, He T, Yang Z, et al. Twist1 promotes breast cancer invasion and metastasis by silencing Foxa1 expression. Oncogene. 2017;36(8):1157–66. [DOI] [PMC free article] [PubMed]; Xu Y, Qin L, Sun T, Wu H, He T, Yang Z. et al. Twist1 promotes breast cancer invasion and metastasis by silencing Foxa1 expression. Oncogene. 2017;36(8):1157–66. doi: 10.1038/onc.2016.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tian Y, Wang L, Jia M, Lu T, Ruan Y, Wu Z, et al. Association of oligodendrocytes differentiation regulator gene DUSP15 with autism. World J Biol Psychiatry. 2017;18(2):143–50. [DOI] [PubMed]; Tian Y, Wang L, Jia M, Lu T, Ruan Y, Wu Z. et al. Association of oligodendrocytes differentiation regulator gene DUSP15 with autism. World J Biol Psychiatry. 2017;18(2):143–50. doi: 10.1080/15622975.2016.1178395. [DOI] [PubMed] [Google Scholar]
- [96].Maiti P, Kim HJ, Tu YT, Barrientos A. Human GTPBP10 is required for mitoribosome maturation. Nucleic Acids Res. 2018;46(21):11423–37. [DOI] [PMC free article] [PubMed]; Maiti P, Kim HJ, Tu YT, Barrientos A.. Human GTPBP10 is required for mitoribosome maturation. Nucleic Acids Res. 2018;46(21):11423–37. doi: 10.1093/nar/gky938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lavdovskaia E, Kolander E, Steube E, Mai MM, Urlaub H, Richter-Dennerlein R. The human Obg protein GTPBP10 is involved in mitoribosomal biogenesis. Nucleic Acids Res. 2018;46(16):8471–82. [DOI] [PMC free article] [PubMed]; Lavdovskaia E, Kolander E, Steube E, Mai MM, Urlaub H, Richter-Dennerlein R.. The human Obg protein GTPBP10 is involved in mitoribosomal biogenesis. Nucleic Acids Res. 2018;46(16):8471–82. doi: 10.1093/nar/gky701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kong DS, Kim J, Lee IH, Kim ST, Seol HJ, Lee JI, et al. Integrative radiogenomic analysis for multicentric radiophenotype in glioblastoma. Oncotarget. 2016;7(10):11526–38. [DOI] [PMC free article] [PubMed]; Kong DS, Kim J, Lee IH, Kim ST, Seol HJ, Lee JI. et al. Integrative radiogenomic analysis for multicentric radiophenotype in glioblastoma. Oncotarget. 2016;7(10):11526–38. doi: 10.18632/oncotarget.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]