Abstract
Background
Rumen microbiota in ruminants are vital for sustaining good rumen ecology, health, and productivity. Currently, limited information is available regarding the response of yaks (Bos grunniens) to fluctuating environments, especially the rumen microbiome. To address this, we investigated the diet, rumen bacterial community, and volatile fatty acids (VFA) of rumen fluid of yaks raised in the great Qinghai-Tibet plateau (QTP) at 2800 (low altitude, L), 3700 (middle altitude, M), and 4700 m (high altitude, H) above sea level.
Results
The results showed that despite a partial diet overlap, H yaks harbored higher fibrous fractious contents than the M and L grazing yaks. Bacteria including Christensenellaceae_R-7_group, Ruminococcus_1, Romboutsia, Alloprevotella, Eubacterium coprostanoligenes, Clostridium, Streptococcus, and Treponema were found to be enriched in the rumen of yaks grazing at H. They also showed higher rumen microbial diversity and total VFA concentrations than those shown by yaks at M and L. Principal coordinates analysis (PCoA) on weighted UniFrac distances revealed that the bacterial community structure of rumen differed between the three altitudes. Moreover, Tax4fun metagenome estimation revealed that microbial genes associated with energy requirement and carbohydrate metabolic fate were overexpressed in the rumen microbiota of H yaks.
Conclusions
Collectively, our results revealed that H yaks had a stronger herbage fermenting ability via rumen microbial fermentation. Their enhanced ability of utilizing herbage may be partly owing to a microbiota adaptation for more energy requirements in the harsh H environment, such as lower temperature and the risk of hypoxia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-020-02054-5.
Keywords: Yak, Rumen microbiota, VFA profiles, Fermenting ability, High altitude
Background
The rumen is a complicated microbial ecosystem harboring compartment, hosting abundant bacteria, protozoa, and fungi, that play vital roles in ruminants [1]. Ruminants depend on their rumen microbes for degradation of structural carbohydrates (cellulose, hemicellulose, and lignin) in herbage, and synthesis of rumen volatile fatty acids (VFA) and microbial proteins synthesis as energy and protein sources [2]. A previous study suggested that the rumen is considered to be free of microbes after birth, but is soon contaminated with microbes from the dam and surrounding environment [3]. The rumen microbial consortium provides useful functions for the host, such as food fermentation [4], immunity regulation [5], disease preventive measures [6], energy balance [7], and physical development [8]. Some experiments have shown that fluctuation of the rumen microbial consortium can lead to a shift in its function [4, 9]; nevertheless, it is still unknown how rumen microbiota composition and function influence response to various environmental factors.
The Qinghai-Tibet Plateau (QTP), located in the southwest part of China and known as the Earth’s third pole, is the highest and largest plateau on the planet. The major land use on this plateau has been for grazing livestock since ancient times. It has been reported that more than 15 million yaks (Bos grunniens) are raised on the QTP, accounting for approximately 90% of the total yak population worldwide [10]. Yaks are one of the world’s most treasured domesticated livestock. They are an iconic symbol of Tibet and high elevation because of their ability to thrive in an extremely harsh living environment [2]. Under traditional management practices, yaks commonly graze in a full-grazing system with herbage as the only feed [4]. Yaks play an essential function in the alpine ecosystem in various ways, including enhancing plant diversity through creation of micro-habitats, providing a livelihood for local herdsmen, enhancing soil structure, and promoting the material circulation and energy flow in the ecosystem [10]. These unique characteristics result in an attractive system explore the adaptation process of yaks to altitudinal gradients. First, because the main characteristics of high altitude areas are low pressure, low oxygen, low temperature, high sunlight, and climate dynamics [11], the ecological mechanisms associated with rumen microbiota consortium could respond in a different manner compared with those of low altitude yaks. Second, yaks have adapted well to high altitude environments through long-term evolution [9]. However, the adjustment of microbial communities to high altitude areas is still difficult to explain, as the rumen flora is related to nutrition and metabolism of the host. Finally, yaks are ruminants and their rumen are usually considered free of microbes at birth; hence, the rumen microorganisms of calves would be obtained from the environment and mother’s milk [2]. Therefore, the composition of yak rumen flora is affected by both the host and the environmental conditions. This makes investigations more complicated compared to non-host environmental microbial communities.
Previous studies have studied the relationship between yak rumen microbiota and host factors (e.g., age, health status, rumen region) and the environment (e.g., diet, environmental microbes) [2, 12–14]. High-altitude grazing animals, such as Tibetan sheep and yak, harbor similar rumen microbiota but different microbial interactions when compared with their low-altitude relatives [9]. There is little data on how high-altitude yaks acclimate to harsh conditions, such as cold climate and hypoxic environments, from the perspective of rumen microbiota. Therefore, we explored how the rumen microbiota responds to the adaptation process of yaks to high-altitude environments. In this study, we investigated the yak diet profile, rumen microbiota composition, and VFA profiles at different altitudes. We addressed three critical questions: (1) Do the yaks have different rumen microbiota diversity and fermentation ability (VFA profiles) at different elevations? (2) Is there a link between rumen microbiota and VFAs? (3) Does rumen microbiota composition and function covary at and due to different elevations? Our results are significant for studying microbiota adaptation to higher energy demands of the associated hosts under harsh conditions such as cold climate and hypoxic high-altitude environmental conditions.
Results
Chemical composition of herbage samples
The nutrient composition of mixed edible herbage samples collected from the three altitudes of the QTP is presented in Table S1. The neutral detergent fiber (NDF) content increased with altitude (P < 0.05). However, the acid detergent fiber (ADF) content was the highest (P < 0.05) in H and the lowest in M (P < 0.05). There were no significant differences in the contents of dry matter (DM), ether extract (EE), crude protein (CP), and organic matter (OM) (P > 0.05) in herbage from the three altitudes.
Rumen fermentation parameters
There were no significant differences in yak rumen pH, NH3-N, isobutyrate, valerate, and isovalerate concentrations between the three altitudes (P > 0.05; Table 1), whereas, the rumen total VFA (TVFA) concentration increased with altitude (P < 0.05). The proportions of acetate and propionate in H were higher than in M and L (P < 0.05). The proportion of butyrate at low and middle altitudes was higher than at high altitude (P < 0.05).
Table 1.
Effects of herbage from the three elevations on ruminal fermentation in yaks
| Item | Altitude d | SEM e | P value | ||
|---|---|---|---|---|---|
| L | M | H | |||
| pH | 6.76 | 6.73 | 6.69 | 0.0234 | 0.4585 |
| NH3-N (mg/L) | 79.43 | 75.14 | 73.04 | 1.5017 | 0.2125 |
| TVFA (mmol/L) | 21.31c | 37.10b | 40.64a | 1.5587 | 0.0026 |
| Acetate (%) | 72.53b | 73.32b | 77.88a | 0.5994 | 0.0060 |
| Propionate (%) | 11.53b | 12.14b | 13.78a | 0.3456 | 0.0179 |
| Butyrate (%) | 11.62a | 11.87a | 4.84b | 0.6264 | 0.0013 |
| Isobutyrate (%) | 1.45 | 1.47 | 1.51 | 0.0525 | 0.9170 |
| Valerate (%) | 1.26 | 1.14 | 1.13 | 0.0482 | 0.5054 |
| Isovalerate (%) | 0.81 | 0.83 | 0.85 | 0.0343 | 0.9101 |
a,b,c Values in the same row with different superscript letters differ significantly (P < 0.05)
d L, 2800 m; M, 3700 m; H, 4700 m
e Standard error of the mean
Composition of bacterial population found in rumen fluid
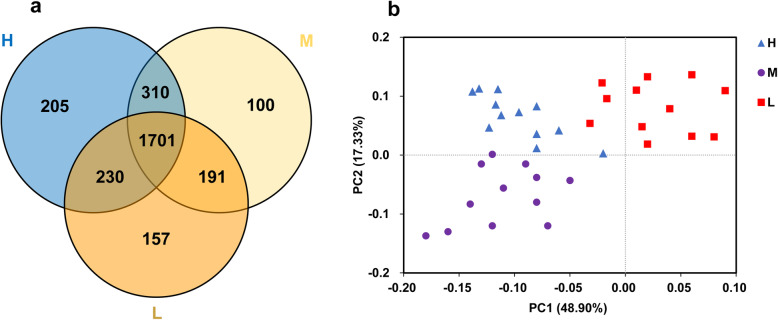
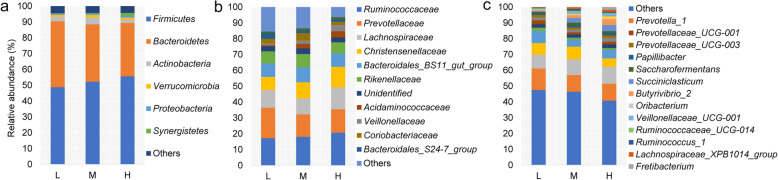
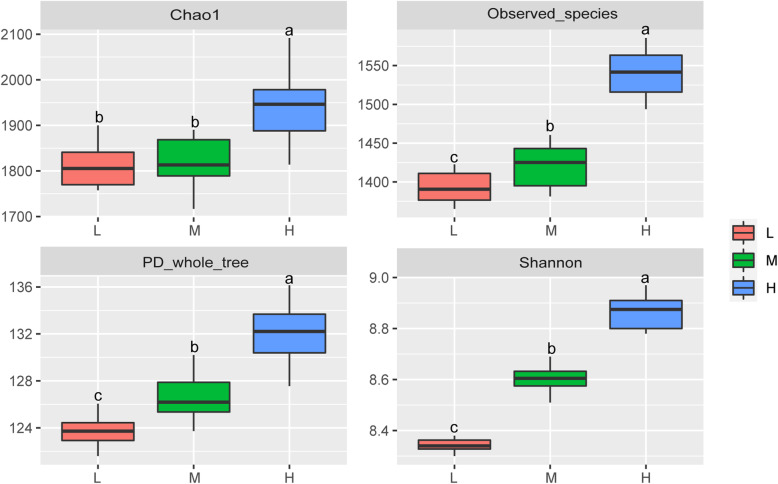
A total of 2,833,238 raw reads were obtained from 36 samples, with an average of 78,701 reads for each sample (minimum, 45,511; maximum, 113,622). Using these sequences, we identified 2894 OTUs based on 97% nucleotide sequence identification between total reads. A total of 1701 OTUs were shared between samples from different elevations; the total OTUs of the high, middle, and low altitude samples were 2446, 2302, and 2279, respectively (Fig. 1a). Taxonomic analysis of the reads revealed 22 bacterial phyla. Firmicutes and Bacteroidetes were the predominant phyla, accounting for 52.31 and 37.08% of the total sequences, respectively (Fig. 2a). Actinobacteria, Verrucomicrobia, and Proteobacteria represented 3.27, 1.36, and 1.01% of the total sequences, respectively. The alpha diversity index analysis is shown in Fig. 3. The community diversity indices (Shannon index) enhanced with altitude (P < 0.05). The community richness counts (Chao 1 estimator) at high altitude were greater than at middle and low altitudes (P < 0.05). PCoA plots based on weighted UniFrac distance metric revealed the differences in microbial diversity between samples from the three elevations (Fig. 1b).
Fig. 1.
Differences in bacterial community richness and OTUs at different elevations. Venn diagram (a) showing the different and similar OTUs at the three elevations. Principal coordinate analysis (PCoA) (b) of the yak ruminal microbiota at the three elevations. The PCoA plots were constructed using the weighted Unifrac method. L, 2800 m; M, 3700 m; H, 4700 m
Fig. 2.
Bacterial comparisons of the rumen in yaks grazing pastures at the three elevations (L, 2800 m; M, 3700 m; H, 4700 m). The relative abundance of (a) bacterial phyla, (b) family, and (c) genus were obtained to be > 1%
Fig. 3.
Community richness estimates and diversity indices for the three elevations. a, b, c Boxes with different superscripts differ significantly (P < 0.05). L, 2800 m; M, 3700 m; H, 4700 m
The effects of altitude on the prevalence of certain bacterial phyla (average relative abundance ≥1% for at least one elevation) in yak rumen are presented in Table S2. The relative abundance of Firmicutes increased (P < 0.05), whereas the proportion of Bacteroidetes decreased (P < 0.05) with altitude. Yaks at the middle altitude had a higher relative abundance of Actinobacteria (P < 0.05) compared with yaks at high and low altitudes. At the family level, Ruminococcaceae (18.86%), Prevotellaceae (15.04%), Christensenellaceae (13.69%), and Lachnospiraceae (10.04%) were the dominant families; other families included Bacteroidales_BS11_gut_group (8.74%), Rikenellaceae (7.72%), and Coriobacteriaceae (3.19%) (Fig. 2b). The relative abundances of Ruminococcaceae and Christensenellaceae increased with altitude, while the relative abundance of Prevotellaceae decreased with altitude (Table S3). The relative abundances of the Coriobacteriaceae and Rikenellaceae families in M were higher than the abundancies in H and L (P < 0.05). At the genus profile level, 208 taxa were identified, and the proportions of 25 genera (average relative abundance ≥0.1% for at least one elevation) differed between the three altitudes (Table S4). Among these, Prevotella_1 (11.57%) was the most dominant genus, followed by Christensenellaceae_R-7_group (9.87%), Rikenellaceae_RC9_gut_group (6.83%), and Ruminococcaceae_NK4A214_group (5.83%) (Fig. 2c). The relative abundances of Ruminococcus_1, Christensenellaceae_R-7_group, Romboutsia, Alloprevotella, E. coprostanoligenes, Clostridium, Treponema, and Streptococcus increased with altitude (P < 0.05), however, the proportions of Prevotellaceae_UCG-001, Succiniclasticum, Butyrivibrio_2, and Lachnospiraceae_XPB1014_group decreased (P < 0.05). The relative abundances of the genera, Rikenellaceae_RC9_gut_group, Oribacterium, Saccharofermentans, and Ruminococcaceae_UCG-014, at the middle altitude were higher than at the low and high altitudes (P < 0.05).
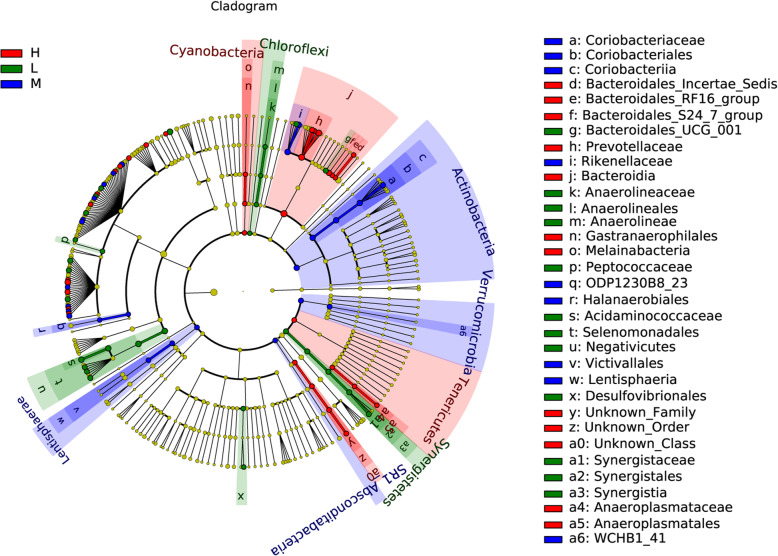
We also performed LEfSe (Linear discriminant analysis Effect Size) to detect variations in the bacterial taxa composition. Figure 4 depicts a representative cladogram of the structure of the predominant microbiome, showing the most remarkable differences in taxa among the different elevations. The data indicated that twelve clades were more abundant in the L group, nine clades were more abundant in the M group, and twelve clades were more abundant in the H group. The different bacterial taxa from the three elevations are shown in Fig. S1. Moreover, when the microbial communities were compared in the context of different elevations, the most differentially abundant bacterial genera in L were Christensenellaceae_R-7_group and Ruminococcaceae_UCG-010, Butyrivibrio_2; Ruminococcus_1 was more abundant in M, while Prevotella_1 and Ruminococcaceae_NK4A214_group were more abundant in H. Of note, the genera Butyrivibrio_2 and Prevotella_1 were the most differentiated among communities, with an absolute LDA score factor of ~ 5.
Fig. 4.
LEfSe (Linear discriminant analysis Effect Size) cladogram comparing microbial communities among the three elevations. Differences are represented by the color of the group where taxa are most abundant; Red: taxa abundant in H, Green: taxa abundant in L, Blue: taxa abundant in M. L, 2800 m; M, 3700 m; H, 4700 m
Correlations between bacterial communities and rumen fermentation parameters
We analyzed the correlation between the relative abundance of rumen bacteria genera and fermentation parameters through correlation analysis (Fig. 5). The abundances of the rumen bacterial genera and the rumen NH3-N and TVFA concentrations were closely related to each other if P < 0.05. The NH3-N concentration was positively correlated with the relative abundances of genera Ruminococcaceae_NK4A214_group and Ruminococcaceae_UCG-005, and were reversely correlated with Butyrivibrio_2 abundance. The acetate molar proportion was positively correlated with the relative abundance of genera Veillonellaceae_UCG-001, while Alloprevotella was negatively correlated with Prevotella_1, Rikenellaceae_RC9_gut_group, and Prevotellaceae_UCG-001 abundances. The propionate molar proportion was positively correlated with Alloprevotella and Succiniclasticum and was reversely correlated with Prevotella_1 and Prevotellaceae_UCG-001 abundances. The butyrate molar proportion was positively correlated with Butyrivibrio_2, Ruminococcaceae_UCG-010, together with Christensenellaceae_R-7_group, and was negatively associated with Prevotella_1 abundance. The isobutyrate molar proportion was positively correlated with Ruminococcaceae_UCG-010 and was reversely associated with Ruminococcaceae_NK4A214_group abundance. The valerate molar proportion was positively associated with Christensenellaceae_R-7_group, Butyrivibrio_2, and Lachnospiraceae_XPB1014_group, and was negatively correlated with Rikenellaceae_RC9_gut_group abundance. The TVFA concentration was directly associated with the relative abundances of the genera Christensenellaceae_R-7_group, Succiniclasticum, Butyrivibrio_2, and Alloprevotella, and was negatively correlated with Prevotella_1 and Prevotellaceae_UCG-001 abundances.
Fig. 5.
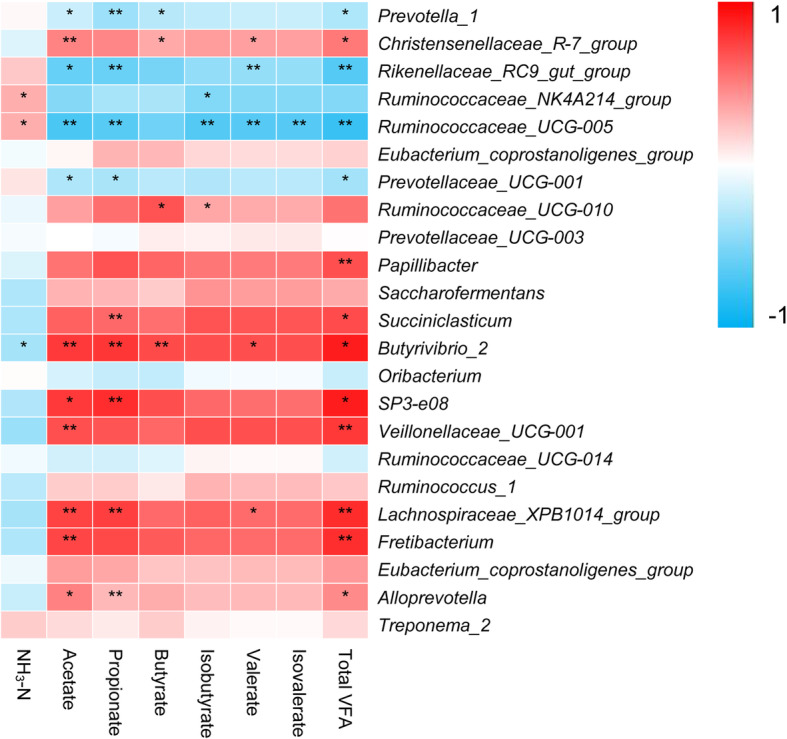
Correlation between the relative abundances of rumen bacteria and fermentation parameters. “*” and “**” indicate the significance level at 0.05 and 0.01, respectively
Tax4Fun gene function estimation
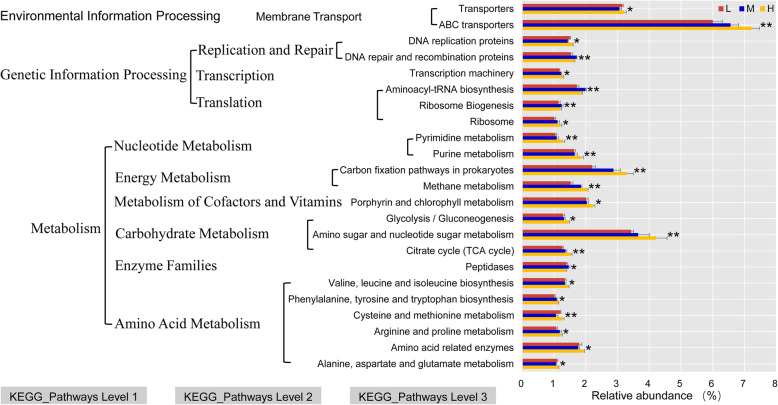
Tax4Fun was used to predict the function of the rumen microbiota of yaks from three altitudes. Interestingly, the relative abundance of ABC transporters (6.63%) was the highest at all the three altitudes, and amino sugar and nucleotide sugar metabolism (3.77%) was the second-most abundant. The Tax4Fun predictive software enriched 46 predominant pathways (relative abundances > 1%) in the level 3 KEGG pathways. Among these, 23 pathways showed significant differences at high, middle, and low altitudes (P < 0.05) (Fig. 6). Notably, the relative abundances of carbohydrate and energy metabolism gene categories significantly increased with altitude (P < 0.05).
Fig. 6.
Functional predictions for rumen microbiota with significantly different KEGG pathways (P < 0.05) for the three elevations (H, M, and L). KEGG pathways at Level 1, Level 2, and Level 3 are represented. L, 2800 m; M, 3700 m; H, 4700 m. “*” and “**” indicate the significance level at 0.05 and 0.01, respectively
Discussion
Biodiversity is imperative in promoting the sustainability and productivity of numerous ecosystems [15]. Therefore, high microbial diversity is closely related to strong metabolic ability and stability [11]. The diversity of human intestinal microbiota improves the fermentation efficiency of dietary fiber and promotes stability of the intestinal ecosystem [16]. In addition, the increase in diversity of human gut microbes also reflects better health and stronger metabolic capacity [17]. In this study, rumen microbial diversity of yaks increased with altitude, and was comparable to the gastrointestinal tract (GIT) microbial diversity of other mammals, such as pikas [11] and rhesus macaques [18]. Therefore, we speculate that the rumen communities of high-altitude yaks may have a higher ability to use high-fiber herbage to help them meet their energy needs in cold and high-altitude habitats.
This study revealed that the phyla Firmicutes and Bacteroidetes were the predominant bacteria in the rumen of yaks. The two phyla in the current study were also found to be abundant in the GIT of yaks [1, 2, 4], sheep [19], goats [20], bovine animals [21], and pikas [11], indicating an ecological and functional role of Firmicutes and Bacteroidetes in the mammalian GIT. Huang and Li (2017) [22] reported that the relative abundance of Firmicutes in yaks was higher than that of Bacteroidetes during grazing. The same conclusion was made in this study. Firmicutes perform essential functions in energy conversion and harvesting [23], whereas Bacteroidetes are responsible for protein hydrolysis, carbohydrate degradation, and fermentation of amino acids to acetate [24]. In the current study, the relative abundance of Firmicutes increased with altitude, whereas the proportion of Bacteroidetes decreased. The increase in Firmicutes and the ratio of Firmicutes to Bacteroidetes in rumen indicates that yaks grazing in high elevation pastures may possess better herbage energy utilization ability and increased resistance to cold stress. Similar findings were also found in mice exposed to cold environments. The non-shivering thermogenesis and energy harvest of these mice increased, signaling that high altitude yaks may have a higher energy harvest and consumption [25].
At the family level, the relative abundances of Ruminococcaceae and Christensenellaceae increased with altitude, while the relative abundance of Prevotellaceae decreased. In an earlier study, Ruminococcaceae played essential roles in cellulose degradation in the GIT [26]. Through rumen fermentation, cellulose can be degraded by microorganisms into VFA, which is an essential energy source for epithelial cells and can provide 60–75% of the required metabolic energy to the host [27]. In black howler monkeys, the abundance of Ruminococcaceae increased during periods of energy deprivation and seemed to compensate for the decrease in energy intake [28]. In high-altitude habitats, yaks suffering from low oxygen and cold environments need more energy to maintain their metabolic balance, but the available food sources are limited [10]. Therefore, an increased abundance of Ruminococcaceae may lead to increased energy utilization efficiency to support yaks living in cold and high-altitude environments. Christensenellaceae can naturally secrete α-arabinosidase, β-galactosidase, and β-glucosidase, which are also closely related to feed efficiency [29]; hence, it is speculated that these bacteria are vital for the adaptation of high-altitude yaks to the harsh environment on the QTP. Stewart et al. (2018) [30] identified rumen uncultured genomes with large amounts of polysaccharide utilization loci associated with the Prevotellaceae family that contain proteins capable of binding and degrading a variety of carbohydrate substrates. Li and Zhao (2015) [31] revealed that the relative abundance of Prevotellaceae was decreased in the gut of Han Chinese living in Tibet. These results suggest a relevant role of Prevotellaceae in the high-altitude adaptation. However, a previous study showed that the relative abundance of Prevotellaceae was increased in the gut of Plateau pika (4431 m elevation) [32]. These differences imply that various species may have an adaptable gut microbiota composition as an adaptation to high-altitude environments. In addition, dietary composition is another key factor affecting the GIT microbiota [13]; therefore, comparing the rumen microbiota of yaks fed the same diet at different altitudes may further enhance the understanding of the role of microbiota in host adaptation.
Importantly, the relative abundances of Ruminococcus_1, Christensenellaceae_R-7_group, Romboutsia, Alloprevotella, E. coprostanoligenes, Clostridium, Treponema, and Streptococcus clearly increased with altitude, demonstrating that these microorganisms in the rumen may adapt well to low temperature and low oxygen environments. The microorganisms that were enhanced in the high-altitude yaks may be involved in performing important functions for the host. For example, Christensenellaceae_R-7_group and Ruminococcus_1 contain genes for essential cellulase and hemicellulase secreting enzymes [33, 34] that may improve the yak’s ability to degrade plant cellulose and obtain energy from indigestible polysaccharides. The genus Romboutsia has a variety of metabolic abilities, which can be engaged in the fermentation of carbohydrates and the utilization of single amino acids [35]. Alloprevotella is reported to be closely related to decreased cardiovascular disease risk [36], and can produce moderate concentrations of rumen acetate, but major amounts of succinate [37]. This result is inconsistent with the present study showing a positive correlation between the relative abundance of Alloprevotella and acetate and propionate concentrations. E. coprostanoligenes is documented to have a cholesterol- removing capability [38]. Additionally, a number of organisms in the genus Clostridium are associated with cellulose degradation and nitrogen fixation [39]. This study demonstrated that some potential and relevant probiotics were significantly enhanced in the high-altitude grazing yaks. These genera in the rumen may help yaks improve metabolic capability and maintain host health to consequently tolerate to the harsh environments at higher elevations. Nevertheless, more investigations are warranted to clearly evaluate the ecological functions of these bacteria in yak rumen.
In addition to the observed potential probiotics, we found that a potentially pathogenic bacteria, Treponema, also increased with elevation. Some species of Treponema have been detected in the genital and oral tracts of humans [40], the rumen of yaks [41], and the gut of rhesus macaques [18]. They can cause various diseases in these organisms, such as syphilis, yaws, and papillomatous digital dermatitis [18, 42]. Despite the potential risk of these pathogens, their functional roles in yaks still remain unclear and deserve further investigation.
Along with the potential roles of probiotics and pathogens, it was found that commensal bacteria were enriched in the rumen of high-altitude yaks. Streptococcus is a common group of bacteria found in human skin, mouths, throats, and intestines [43]. The genus Streptococcus has also been detected in pika mouths [32] and gut [11], indicating that this genus may be widely distributed in mammalian host microbiota. Additionally, some species of this genus have been found to be related to human pyruvate metabolism [44]. Therefore, we speculate that they play a key role in the rumen of high-altitude yaks.
In the current study, some bacteria, including Christensenellaceae_R-7_group, Ruminococcaceae_UCG.010, Succiniclasticum, Butyrivibrio_2, and Alloprevotella, were found closely associated with VFAs. It is difficult to conclude which genus was responsible for the specific VFA due to the complex interactions among microbes, such as resource competition [45] and cross-feeding [46], but these bacteria likely play key roles in the fermentation of herbage structural carbohydrates in the yak rumen.
In addition to variations of yak rumen microbiomes, VFAs exhibited remarkable fluctuations in response to altitude. VFAs are produced by rumen microorganisms fermenting plant cellulose and other carbohydrates, and play an essential role in ruminant growth and immunity [47]. VFAs formed in the rumen are largely absorbed across the host’s ruminal epithelium [9, 48]. Thus, the high altitude yaks might possess the ability to more efficiently transport and absorb VFAs than those grazing at low altitude. Acetic and propionate acid could activate the GPR43 and GPR41 receptors to produce PYY and GLP-1 hormones, which can increase glucose utilization for body energy [49]. It is worth noting that the main VFA (acetic acid) in the rumen may reduce the abundance of Escherichia coli, thereby maintaining rumen health [50]. This research showed that TVFA concentration and the proportions of acetate and propionate significantly increased with altitude, indicating that high altitude yaks were more capable to thrive under the harsh conditions of Tibetan pastures compared to those at the middle and low altitudes. Thorough assessment of plasma VFA levels are needed to reinforce the finding related to VFA transport in high-altitude ruminants. Such analyses may provide valuable insights for understanding how changes in the microbiota and host genes are related to each other.
Microorganisms have an impact on the body’s immunity, degradation and absorption of nutrients, and even enzyme metabolism [5]. In the current study, we utilized Tax4Fun to predict the function of the yak rumen microbial community. Our data indicates that the estimated gene functional profiles of yak rumen microbiomes were significantly impacted by altitude. Most dramatically, in KEGG pathways level 2, those genes involved in carbohydrate and energy metabolism were enhanced in the high altitude yaks. This indicates that the rumen microbiota of the high-altitude yaks produce a large amount of VFAs to provide the host with additional energy, helping the host to maximize the use of nutrients and indigestible plant components, such as cellulose. Although high altitude yaks ingest higher fiber herbage as compared to middle and low altitude yaks, they may more efficiently degrade high-fiber herbage due to improved rumen microbial diversity and function. Thus, the enhanced ability of high-altitude yaks to utilize herbages may be a kind of microbiota adaptation for more energy requirements in cold and hypoxic high-altitude environments. Among the KOs, high-altitude yaks showed enrichment in the carbon fixation pathways of prokaryotes; this was consistent with the highly efficient formation of VFAs [51]. Notably, ABC transporters were the most expressed pathway in membrane transport and directly participate in the production of ATP. Furthermore, Hamana et al. (2012) [52] revealed that ABC transport function is a barrier to protect ruminants from the invasion of toxic substances. In this study, ABC transporters were expressed at a significantly higher level in high altitude yaks. In a high-altitude environment, low oxygen and high ultraviolet radiation may cause DNA and protein damage, and genes related to replication and repair may help reduce damage to biomolecules. Therefore, this pathway may help yaks adapt to high-altitude environments. However, our results were only based on the predicted metagenomics, and may not represent the actual function of rumen bacteria. Further studies should be conducted to directly sequence the yak rumen metagenome to explore the roles of these genes in yak environmental adaptability. Future studies using metagenome analysis are also needed to explore the roles of these gene functions in yak environmental adaptability.
Conclusions
Our results showed that some potential probiotics, including Christensenellaceae_R-7_group, Ruminococcus_1, Romboutsia, Alloprevotella, E. coprostanoligenes, and Clostridium, were enriched in the rumen of high-altitude yaks. Shifts in the rumen microbiomes were caused by a high-altitude environment characterized by cold temperatures, hypoxia, and the production of high-fiber herbage. Moreover, rumen microbial diversity and herbage fermenting ability of yaks increased with elevation; therefore, in high altitude yaks, these should be considered as microbiota adaptation to partially meet the higher energy requirements needed for survival in the harsh cold and hypoxic environment.
Methods
Animals and sample collection
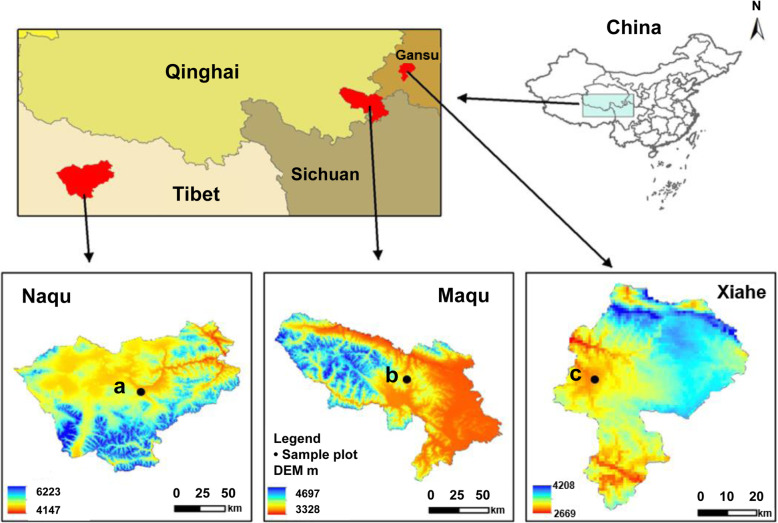
Three districts in the middle part of the northwestern region of QTP of China were selected as experimental sites (Fig. 7): Sangke Township (latitude 34°17′36″N, longitude 102°18′31″E; altitude 2800 m), Xiahe County, Gansu Province; Manrima village (latitude 33°40′4″N, longitude 101°52′12″E; altitude 3700 m), Maqu County, Gansu Province; and Gulu Township (latitude 30°58′68″N, longitude 91°37′34″E; altitude 4700 m), Nagqu Prefecture, Tibetan Autonomous Region. These three locations are typical areas for raising the yaks on the QTP, with mean annual air temperatures of 1.2 °C, − 0.5 °C, and − 1.5 °C, respectively, and a mean annual precipitation of 620 mm, 518 mm, and 422 mm, respectively. The vegetation consisted of typical alpine meadows. The main edible herbage species and the proportions are presented in Table S5. At the study sites, yaks commonly grazed in a full-grazing system with herbage as the only feed [10]. We selected 12 healthy yaks 5 years of age with an average initial body weight of 284.38 ± 8.36 kg from each sampling site. Rumen fluid samples and herbage samples were collected during the summer in mid July at Sangke Township, late July at Manrima village, and early August at Gulu Township. Ten-day intervals were set so that the herbage would be approximately in the same phenological stage.
Fig. 7.
Sampling sites of herbage and yaks from 3 different altitudes in the Qinghai-Tibet plateau. a, Sangke Township (Xiahe County, Gansu Province, 2800 m); b, Manrima village (Maqu County, Gansu Province, 3700 m); c, Gulu Township (Naqu Prefecture, Tibet Autonomous Region, 4700 m)
Sampling of plants and analyses of chemical composition
A total of 20 randomly selected quadrats (50 cm × 50 cm) were chosen from the vegetation on which the yaks grazed. Mixed herbage samples were collected in the quadrats, inedible herbage was removed, and only edible herbage was retained. The samples were placed in a 60 °C oven for 24 h to a constant weight, ground using a mill, and passed through a 1 mm sieve for further chemical analysis. The dry matter (DM) of the herbage was prepared by subjecting the samples for dry matter determination in an air-flow oven at 65 °C for 72 h [53]. Nitrogen content was determined by the Kjeldahl method and crude protein (CP) concentration was calculated as 6.25 × N. Ether extract (EE) was measured by the weight loss of dry matter after 8 h of extraction with ether in a Soxhlet extractor [53]. The fibrous fractions of neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were analyzed using the methods outlined by Van Soest et al. (1991) [54].
Sampling of rumen digesta and analyses of fermentation parameters
Twelve yaks of similar average body weight in each sampling site were selected for collection of ruminal content (liquid and particulate forage material), which was sampled in the morning before grazing. All animals in this experiment continued to graze on natural grass after collecting rumen fluid and were in good health condition. Samples (approximately 50 mL) were collected using an oral stomach tube as described by Fan et al. (2020) [55]. The first 50 mL of rumen fluid was discarded to avoid contamination from previous animas or its own saliva [4]; this was followed by collection of 50 mL rumen fluid from each animal, and immediate pH measurement by pH meter (Model 144 PB-10, Sartorius Co., Germany). The rumen contents were filtered with four layers of woven gauze and divided into two portions for analysis of ruminal fermentation parameters and for DNA extraction. For analysis of VFA concentrations, the filtrate was thawed and centrifuged at 1000×g for 15 min and then analyzed using gas chromatography (chromatograph SP-3420A, Beifenrili Analyzer Associates, Beijing, China) as described by Erwin et al. (1961) [56]. The concentration of NH3-N in the rumen was later analyzed using a specific visible spectrophotometry device (UV-VIS8500, Tianmei, Shanghai, China) [57].
DNA extraction, sequencing, sequence processing, and analysis
Bacterial DNA was prepared and extracted from the digesta using an E.Z.N.A.® Stool DNA kit (Omega Bio-TEK, Norcross, GA, USA). The concentration and purity of the extracted DNA were detected using an ultra-microspectrophotometer (NanoDrop 2000C, Thermo Scientific, USA). The V3-V4 region of the 16S rRNA was amplified using primers 338F (5-ACTCCTACGGGAGGCAGCAG-3) and 806R (5- GGACTACHVGGGTWTCTAAT-3) [58]. The barcode of the unique eight-base sequence of each sample was added to each primer for sample identification and determination. PCR was conducted in triplicates as follows: an initial denaturing step at 94 °C for 5 min, followed by 28 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s, and a final extension at 72 °C for 7 min. Amplicons were extracted from 2% agarose gels, purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions, and quantified using the QuantiFluor™-ST system (Promega, Madison, WI, USA). Purified amplicons were pooled in equimolar concentrations and paired-end sequenced (2 × 300 bp) on an Illumina MiSeq PE300 platform (Illumina, Inc., San Diego, CA, USA) according to the standard protocols. The processing of the sequencing data was mainly performed using QIIME 1.9.0 software [59]. The original sequences were sorted based on their unique sample barcodes and were trimmed for sequence quality using the QIIME pipeline (length > 300 bp, average base quality score > 30) [60]. Chimera sequences were removed with the UCHIME algorithm. These effective tags were clustered into operational taxonomic units (OTUs) based on a sequence similarity threshold of 97% using UPARSE (version 7.0) [61]. Representative sequences were classified into organisms using RDP classifier (version 2.2) based on the SILVA (SSU123) database [62]. Alpha diversity analysis was performed by calculating the Chao1 index, Shannon index, phylogenetic diversity index (PD_whole_tree), and observed species index (observed_species) using QIIME (version 1.9.0). The PCoA with weighted UniFrac distance matrices and the analysis of similarity in QIIME were used to estimate differences in bacterial communities between samples [63].
Statistical analysis
The chemical composition of herbage, ruminal fermentation parameters, relative abundance of bacteria, and the alpha diversity indices were analyzed using a completely randomized design by one-way analysis of variance (SAS Institute Inc., version 9.2, USA). Significant difference was declared at P < 0.05. Pearson correlation coefficients between bacterial consortium and rumen fermentation end-products parameters were calculated using the PROCCORR procedure of SAS 9.2 with a heatmap format as described by Pan et al. (2017) [64]. Briefly, only those bacterial taxa with an abundance > 0.1% of the total consortium in at least one ruminal sample were used in the analysis. The abundances of bacterial consortium at the genus level and ruminal parameters were considered to be correlated with each other for correlation coefficient values (|r|) ≥ 0.55 and P < 0.05 [65]. Tax4fun software was used to compare the species compositions obtained from the 16S sequencing data and then to infer the functional gene composition of samples. The functional composition was predicted from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database [66].
Supplementary Information
Additional file 1: Table S1. Nutrient composition of herbage at different altitudes
Additional file 2: Table S2. Comparison of the dominant phyla (average relative abundance ≥1% for at least one altitude) within the rumen
Additional file 3: Table S3. Comparison of the dominant families (average relative abundance ≥1% for at least one altitude) within the rumen
Additional file 4: Table S4. Comparison of the dominant genera (average relative abundance ≥0.1% for at least one altitude) within the rumen
Additional file 5: Table S5. Major species and proportion of edible herbage at different altitudes
Additional file 6: Figure S1. Histogram of LDA scores computed for each taxon ranging from phylum to genus. L, 2800 m; M, 3700 m; H, 4700 m; LDA, linear discriminant analysis
Acknowledgements
We would like to extend our sincere gratitude to Shulin Wang and Xiongxiong Cui for their assistance in sample collection. We would also like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- OTUs
Operational taxonomic units
- PCoA
Principal coordinate analysis
- QIIME
Quantitative insights into microbial ecology
- VFA
Volatile fatty acids
- NH3-N
Ammonia nitrogen
- PCR
Polymerase chain reaction
- DNA
Deoxyribonucleic acid
- rRNA
Ribosomal ribonucleic acid
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LEfSe
Linear discriminant analysis Effect Size
- LDA
Linear discriminant analysis
Authors’ contributions
The experiment was conceived and designed by FJH, and performed by QSF. Data analysis was performed by QSF. Paper writing were conducted by QSF. All authors have helped in revision and approved the final manuscript.
Funding
This research was supported by grants from the Program for Strategic Priority Research Program of Chinese Academy of Sciences (XDA20100102), the Second Tibetan Plateau Scientific Expedition and Research: Grassland Ecosystem and Ecological Animal Husbandry (2019QZKK0302), and Innovative Research Team of Ministry of Education (IRT_17R50). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All trial procedures are strictly in accordance with the regulations for the management of experimental field approved by Lanzhou University (Nos.: 2010–1 and 2010–2), and all procedures for handling and caring for animals are in conformity with China’s regulations on the protection and use of laboratory animals and are approved by the Chinese Zoological Society. I obtained written informed consent to use the animals in my study from the owner(s) of the animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou Z, Fang L, Meng Q, Li SL, Chai ST, Liu SJ, et al. Assessment of ruminal bacterial and archaeal community structure in yak (Bos grunniens) Front Microbiol. 2017;8:179. doi: 10.3389/fmicb.2017.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu C, Wu H, Liu SJ, Chai ST, Meng QX, Zhou ZM. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front Microbiol. 2019;10:1116. doi: 10.3389/fmicb.2019.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jami E, Israel A, Kotser A, Mizrahi I. Exploring the bovine rumen bacterial community from birth to adulthood. Isme J. 2013;7(6):1069–1079. doi: 10.1038/ismej.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma L, Xu SX, Liu HJ, Xu TW, Hu LY, Zhao N, et al. Yak rumen microbial diversity at different forage growth stages of an alpine meadow on the Qinghai-Tibet plateau. PeerJ. 2019;7:e7645. doi: 10.7287/peerj.7645v0.2/reviews/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin R, Nauta AJ, Amor KB, Knippels LMJ, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes. 2010;1(4):367–382. doi: 10.3920/BM2010.0027. [DOI] [PubMed] [Google Scholar]
- 6.Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8(2):110–120. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shabat SKB, Sasson G, Doronfaigenboim A, Durman T, Yaacoby S, Miller MEB, et al. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016;10(12):2958–2972. doi: 10.1038/ismej.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer F, Backhed F. The gut microbiota-masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZG, Xu DM, Wang L, Hao JJ, Wang JF, Zhou X, et al. Convergent evolution of rumen microbiomes in high-altitude mammals. Curr Biol. 2016;26(14):1873–1879. doi: 10.1016/j.cub.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Fan QS, Wanapat M, Hou FJ. Mineral nutritional status of yaks (Bos Grunniens) grazing on the Qinghai-Tibetan plateau. Animals. 2019;9(7):468. doi: 10.3390/ani9070468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Zhou R, Zhu JX, Huang XD, Qu JP. Environmental filtering increases with elevation for the assembly of gut microbiota in wild pikas. Microb Biotechnol. 2019;12(5):976–992. doi: 10.1111/1751-7915.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LZ, Wang ZS, Xue B, Wu D, Peng QH. Comparison of rumen archaeal diversity in adult and elderly yaks (Bos grunniens) using 16S rRNA gene high-throughput sequencing. J Integr Agr. 2017;16(5):1130–7. 10.1016/S2095-3119(16)61454-5.
- 13.Hu R, Zou H, Wang Z, Cao BH, Peng QH, Jing XP, et al. Nutritional interventions improved rumen functions and promoted compensatory growth of growth-retarded yaks as revealed by integrated transcripts and microbiome analyses. Front Microbiol. 2019;10:318. doi: 10.3389/fmicb.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi FY, Guo N, Degen AA, Salem AZM, Lopez S, Kholif AM. Effects of level of feed intake and season on digestibility of dietary components, efficiency of microbial protein synthesis, rumen fermentation and ruminal microbiota in yaks. Anim Feed Sci Technol. 2010;259:114359. doi: 10.1016/j.anifeedsci.2019.114359. [DOI] [Google Scholar]
- 15.Li H, Qu JP, Li TT, Wirth SH, Zhang YM, Zhao XQ, et al. Diet simplification selects for high gut microbial diversity and strong fermenting ability in high-altitude pikas. Appl Microbiol Biotechnol. 2018;102(15):6739–6751. doi: 10.1007/s00253-018-9097-z. [DOI] [PubMed] [Google Scholar]
- 16.Tap J, Furet JP, Bensaada M, Philippe C, Roth H, Rabot S, et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol. 2015;17(12):4954–4964. doi: 10.1111/1462-2920.13006. [DOI] [PubMed] [Google Scholar]
- 17.Clarke SF, Murphy EF, Osullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Yao Y, Dong M, Xia T, Li D, Xie M, et al. Characterisation of the gut microbial community of rhesus macaques in high-altitude environments. BMC Microbiol. 2020;20(1):68. doi: 10.1186/s12866-020-01747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui XX, Wang ZF, Yan TH, Chang SH, Wang H, Hou FJ. Rumen bacterial diversity of Tibetan sheep (Ovis aries) associated with different forage types on the Qinghai-Tibetan plateau. Can J Microbiol. 2019;65(12):859–869. doi: 10.1139/cjm-2019-0154. [DOI] [PubMed] [Google Scholar]
- 20.Lei Y, Zhang K, Guo M, Li GW, Li C, Li BB, et al. Exploring the spatial-temporal microbiota of compound stomachs in a pre-weaned goat model. Front Microbiol. 2018;9:1846. doi: 10.3389/fmicb.2018.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi YL, Zeng SQ, Zhang R, Diao QY, Tu Y. Effects of dietary energy levels on rumen bacterial community composition in Holstein heifers under the same forage to concentrate ratio condition. BMC Microbiol. 2018;18:69. doi: 10.1186/s12866-018-1213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Li Y, Luo Y. Bacterial community in the rumen of Tibetan sheep and Gansu alpine fine-wool sheep grazing on the Qinghai-Tibetan plateau. China J Gen and Appl Microbiol. 2017;63:122–130. doi: 10.2323/jgam.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 24.Thomas F, Hehemann JH, Rebuffet E, Czjzek M, Michel G. Environmental and gut bacteroidetes: the food connection. Front Microbiol. 2011;2:93. doi: 10.3389/fmicb.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevalier C, Stojanovic O, Colin DJ, Suarezzamorano N, Tarallo V, Veyratdurebex C, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163(6):1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Biddle A, Stewart L, Blanchard J, Leschine S. Untanglingthegenetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5(3):627–640. doi: 10.3390/d5030627. [DOI] [Google Scholar]
- 27.Bergman EN. Energy contributions of volatile fatty-acids from the gastrointestinal-tract in various species. Physiol Rev. 1990;70(2):567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 28.Amato KR, Leigh SR, Kent A, Mackie RI, Yeoman CJ, Stumpf RM, et al. The gut microbiota appears to compensate for seasonal diet variation in the wild black howler monkey (Alouatta pigra) Microb Ecol. 2015;69(2):434–443. doi: 10.1007/s00248-014-0554-7. [DOI] [PubMed] [Google Scholar]
- 29.Perea K, Perz K, Olivo SK, Williams AF, Lachman MM, Ishaq SL, et al. Feed efficiency phenotypes in lambs involve changes in ruminal, colonic, and small-intestine-located microbiota. J Anim Sci. 2017;95(6):2585–2592. doi: 10.2527/jas.2016.1222. [DOI] [PubMed] [Google Scholar]
- 30.Stewart RD, Auffret MD, Warr A, Wiser AH, Press MO, Langford KW, et al. Assembly of 913 microbial genomes from metagenomic sequencing of the cow rumen. Nat Commun. 2018;9(1):870. doi: 10.1038/s41467-018-03317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Zhao X. Comparative analyses of fecal microbiota in Tibetan and Chinese Han living at low or high altitude by barcoded 454 pyrosequencing. Sci Rep. 2015;5(1):14682. doi: 10.1038/srep14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Li T, Berasategui A, Rui JP, Zhang X, Li CN, et al. Gut region influences the diversity and interactions of bacterial communities in pikas (Ochotona curzoniae and Ochotona daurica) FEMS Microbiol Ecol. 2017;93(12):fix149. doi: 10.1093/femsec/fix149. [DOI] [PubMed] [Google Scholar]
- 33.Dai X, Tian Y, Li J, Su XY, Wang XW, Zhao SG, et al. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in cow rumen. Appl Environ Microbiol. 2014;81(4):1375–1386. doi: 10.1128/AEM.03682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escobar JS, Klotz B, Valdes BE, Agudelo GM. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. 2014;14(1):311. doi: 10.1186/s12866-014-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerritsen J. The genus Romboutsia: Genomic and Functional Characterization of Novel Bacteria Dedicated to Life in the Intestinal Tract. Ph.D. thesis. Wageningen: Wageningen University; 2015. [Google Scholar]
- 36.Kelly TN, Bazzano LA, Ajami NJ, He H, Zhao JY, Petrosino JF, et al. Gut microbiome associates with lifetime cardiovascular disease risk profile among Bogalusa heart study participants. Circ Res. 2016;119(8):956–964. doi: 10.1161/CIRCRESAHA.116.309219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Downes J, Dewhirst FE, Tanner ACR, Wade WG. Description of Alloprevotella rava gen. Nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. Nov., comb. nov. Int J Syst Evol Microbiol. 2013;63(4):1214–1218. doi: 10.1099/ijs.0.041376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freier TA, Beitz DC, Li L, Hartman PA. Characterization of Eubacterium coprostanoligenes sp. nov., a cholesterol-reducing anaerobe. Int J Syst Bacteriol. 1994;44(1):137–142. doi: 10.1099/00207713-44-1-137. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L, Wu Q, Dai J, Zhang SN, Wei FW. Evidence of cellulose metabolism by the giant panda gut microbiome. PNAS. 2011;108(43):17714–17719. doi: 10.1073/pnas.1017956108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans NJ, Brown JM, Demirkan I, Murray RD, Vink WD, Blowey R, et al. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet Microbiol. 2008;130(1):141–150. doi: 10.1016/j.vetmic.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Klevenhusen F, Petri RM, Kleefisch MT, Khiaosa-Ard R, Metzler-Zebeli BU, Zebeli Q. Changes in fibre-adherent and fluid-associated microbial communities and fermentation profiles in the rumen of cattle fed diets differing in hay quality and concentrate amount. FEMS Microbiol Ecol. 2017;93(9):fix100. doi: 10.1093/femsec/fix100. [DOI] [PubMed] [Google Scholar]
- 42.Nordhoff M, Moter A, Schrank K, Wieler LH. High prevalence of treponemes in bovine digital dermatitis-a molecular epidemiology. Vet Microbiol. 2008;131(3):293–300. doi: 10.1016/j.vetmic.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 43.Chaffanel F, Charron-Bourgoin F, Soligot C, Kebouchi M, Bertin S, Payot S, et al. Surface proteins involved in the adhesion of Streptococcus salivarius to human intestinal epithelial cells. Appl Microbiol Biotechnol. 2018;102(6):2851–2865. doi: 10.1007/s00253-018-8794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorth P, Turner KH, Gumus P, Nizam N, Buduneli N, Whiteley M. Metatranscriptomics of the human oral microbiome during health and disease. MBio. 2014;5(2):e01012–e01014. doi: 10.1128/mBio.01012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. PNAS. 2009;106(14):5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens R, Newgard CB, et al. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem. 2010;285(29):22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui ZH, Wu SR, Liu SJ, Sun L, Feng YZ, Cao YC, et al. From maternal grazing to barn feeding during pre-weaning period: altered gastrointestinal microbiota contributes to change the development and function of the rumen and intestine of yak calves. Front Microbio. 2020;11:485. doi: 10.3389/fmicb.2020.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naeem A, Drackley JK, Stamey J, Loor JJ. Role of metabolic and cellular proliferation genes in ruminal development in response to enhanced plane of nutrition in neonatal Holstein calves. J Dairy Sci. 2012;95(4):1807–1820. doi: 10.3168/jds.2011-4709. [DOI] [PubMed] [Google Scholar]
- 49.Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud D, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong XF, Zhou XL, Lian GQ, Blachier F, Liu G, Tan B, et al. Dietary supplementation with chitooligosaccharides alters gut microbiota and modifies intestinal luminal metabolites in weaned Huanjiang mini-piglets. Livest Sci. 2014;160:97–101. doi: 10.1016/j.livsci.2013.11.023. [DOI] [Google Scholar]
- 51.Fuchs G. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu Rev Microbiol. 2011;65(1):631–658. doi: 10.1146/annurev-micro-090110-102801. [DOI] [PubMed] [Google Scholar]
- 52.Hamana K, Itoh T, Sakamoto M, Hayashi H. Covalently linked polyamines in the cell wall peptidoglycan of the anaerobes belonging to the order Selenomonadales. J Gen and Appl Microbiol. 2012;58(4):339–347. doi: 10.2323/jgam.58.339. [DOI] [PubMed] [Google Scholar]
- 53.AOAC . Official methods of analysis (15th ed) Washington, D.C.: Association of Official Analytical Chemists; 1990. [Google Scholar]
- 54.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74(10):3583–97. 10.3168/jds.S0022-030291)78551-2. [DOI] [PubMed]
- 55.Fan QS, Wanapat M, Hou FJ. Chemical composition of milk and rumen microbiome diversity of yak, impacting by herbage grown at different phenological periods on the Qinghai-Tibet Plateau. Animals. 2020;10(6):1030. 10.3390/ani10061030. [DOI] [PMC free article] [PubMed]
- 56.Erwin ES, Marco GJ, Emery EM. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J Dairy Sci. 1961;44(9):1768–71. 10.3168/jds.S0022-0302(61)89956-6.
- 57.Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro medial. J Dairy Sci 1980;63(1):64–75. 10.3168/jds.S0022-0302(80)82888-8. [DOI] [PubMed]
- 58.Dennis KL, Wang YW, Blatner NR, Wang SY, Saadalla A, Trudeau E, et al. Adenomatous polyps are driven by microbe-instigated focal inflammation and are controlled by IL-10-producing T cells. Cancer Res. 2013;73(19):5905–5913. doi: 10.1158/0008-5472.CAN-13-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microb. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microb. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan XH, Xue FG, Nan XM, Tang ZW, Wang K, Beckers Y, et al. Illumina sequencing approach to characterize thiamine metabolism related bacteria and the impacts of thiamine supplementation on ruminal microbiota in dairy cows fed high-grain diets. Front Microbiol. 2017;8:1818. doi: 10.1371/journal.pone.0198225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang WM, Li C, Li FD, Wang XJ, Zhang XX, Liu T, et al. Effects of early feeding on the host rumen transcriptome and bacterial diversity in lambs. Sci Rep. 2016;6(1):32479. doi: 10.1038/srep32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minoru K, Susumu G, Yoko S, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:109–114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Nutrient composition of herbage at different altitudes
Additional file 2: Table S2. Comparison of the dominant phyla (average relative abundance ≥1% for at least one altitude) within the rumen
Additional file 3: Table S3. Comparison of the dominant families (average relative abundance ≥1% for at least one altitude) within the rumen
Additional file 4: Table S4. Comparison of the dominant genera (average relative abundance ≥0.1% for at least one altitude) within the rumen
Additional file 5: Table S5. Major species and proportion of edible herbage at different altitudes
Additional file 6: Figure S1. Histogram of LDA scores computed for each taxon ranging from phylum to genus. L, 2800 m; M, 3700 m; H, 4700 m; LDA, linear discriminant analysis
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.