Abstract
Bio/chemoinformatics tools can be deployed to compare antimicrobial agents aiming to select an efficient nose-to-brain formulation targeting the meningitis disease by utilizing the differences in the main structural, topological and electronic descriptors of the drugs. Cefotaxime and ceftriaxone were compared at the formulation level (by comparing the loading in gelatin and tripalmitin matrices as bases for the formation of nanoparticulate systems), at the biopharmaceutical level (through the interaction with mucin and the P-gp efflux pumps) and at the therapeutic level (through studying the interaction with S. pneumoniae bacterial receptors). GROMACS v4.6.5 software package was used to carry-out all-atom molecular dynamics simulations. Higher affinity of ceftriaxone was observed compared to cefotaxime on the investigated biopharmaceutical and therapeutic macromolecules. Both drugs showed successful docking on mucin, P-gp efflux pump and S. pneumoniae PBP1a and 2b; but ceftriaxone showed higher affinity to the P-gp efflux pump proteins and higher docking on mucin. Ceftriaxone showed less out-of-matrix diffusion and higher entrapment on the gelatin and the tripalmitin matrices. Accordingly, Ceftriaxone gelatin nanospheres or tripalmitin solid lipid nanoparticles may pose a more feasible and efficient nose-to-brain formulation targeting the meningitis disease compared to the cefotaxime counterparts.
Subject terms: Computational biology and bioinformatics, Medical research, Nanoscience and technology
Introduction
Meningitis is a serious infection or inflammation of the meninges that can be caused by a wide variety of infectious agents1. Viruses account for up to half of cases while fungi (typically cryptococci) are less frequently detected, representing < 10% of cases2. Bacterial meningitis is considered the most severe form of this disease. The etiologic agents responsible for bacterial meningitis vary by age group. Among neonates, most cases are due to group B Streptococcus agalactiae, Escherichia coli, and Listeria monocytogenes. Although Haemophilus (H.) influenzae is implicated in bacterial meningitis in all age groups, it is predominant in children < 5 years of age3. However, the most common causes of bacterial meningitis are Streptococcus (S.) pneumoniae and Neisseria (N.) meningitidis, together accounting for approximately one-quarter of the cases. Pneumococcal meningitis is in general more common than meningococcal meningitis in children < 5 years of age and in the elderly (≥ 65 years of age), whereas meningococcal meningitis is more frequent among older children, adolescents and young adults2,4. Despite the existence of antibiotic therapies, acute bacterial meningitis causes significant morbidity and mortality. Survivors are prone to permanent consequences including brain damage, hearing loss, and learning disabilities3. Therefore, suspected cases should be treated with antibiotics as quickly as possible, even before the diagnosis can be confirmed, as a delay can result in a greater chance of adverse clinical outcomes.
The choice of antibiotic depends on the organism isolated. In most cases the initial treatment has to be empirical, but nonetheless based on epidemiological knowledge of the commonest organisms for each age group and local antibiotic resistance patterns5. Empirical antibiotic therapy should be bactericidal and achieve adequate cerebrospinal fluid (CSF) levels4. In most cases, a broad spectrum cephalosporin, especially cefotaxime (adults 2 g every 6 h; children 50 mg/kg every 6 h) or ceftriaxone (adults, 4 g/day; children, 50 mg/kg, to maximum 2 g/day single dose) is the most appropriate empirical choice. These cover N. meningitides, S. pneumoniae, and H. influenzae and penetrate CSF well5. International guidelines on the duration of treatment recommend 7–10-day treatment for H. influenzae or N. meningitidis meningitis and a 10–14-day treatment for S. pneumoniae meningitis4. Both ceftriaxone and cefotaxime are effective in the treatment of bacterial meningitis, but ceftriaxone has the advantage of being administered as a single daily dose6. Therefore, there is a need to compare the effectiveness of ceftriaxone and cefotaxime in the treatment of bacterial meningitis.
Cefotaxime (C16H17N5O7S2) and ceftriaxone (C18H18N8O7S3) are third generation cephalosporin antibiotics with broad spectrum bactericidal activity against Gram positive and Gram negative bacteria. They cross the blood–brain barrier (BBB) and reach therapeutic concentrations in the central nervous system (CNS). Their bactericidal activity results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs). PBPs are membrane-anchored enzymes which participate in the terminal stages of assembling the bacterial cell wall, and in reshaping the cell wall during cell division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. (National Center for Biotechnology Information. PubChem Database. Ceftriaxone, CID = 5479530, Cefotaxime, CID = 5742673 https://pubchem.ncbi.nlm.nih.gov/compound (accessed on Dec. 16, 2019).
PBPs are targets for β-lactam antibiotics7. These proteins are classified based upon their molecular weights and conserved domain structures. Class A high-molecular-weight (HMW) PBPs are bifunctional proteins with transglycosylase (TG) and transpeptidase (TP) activities. They are believed to be the physiologically important enzymes that catalyze the final stages of peptidoglycan synthesis. Class B HMW PBPs are monofunctional TPs. Class C, or low-molecular-weight (LMW) PBPs are d,d-carboxypeptidases or d,d endopeptidases8. S. pneumoniae, possesses three class A PBPs (PBP1a, PBP1b, and PBP2a), two class B PBPs (PBP2x and PBP2b), and one class C PBP (PBP3) with d,d-carboxypeptidase activity9,10 and PBP2x possesses a C-terminal extension consisting of two PBP- and serine/threonine kinase-associated (PASTA) domains each containing one α-helix and three β-strands11.
The two class B PBPs (PBP2x and PBP2b) are individually essential in S. pneumoniae, performing critical roles in septal and peripheral peptidoglycan synthesis, respectively, and they are therefore likely critical targets for the β-lactam antibiotics12. β-Lactam antibiotics exert their antibacterial effect through covalent interactions with PBPs, thus blocking the terminal step in cell wall biosynthesis. β-Lactam resistance in S. pneumoniae is usually caused by amino acid substitutions in the penicillin-binding domains of 1 or more of its 6 PBPs, resulting from point mutations or mosaic genes following recombination13. Altered PBP1a, PBP2x and PBP2b are the most important PBPs for β-lactam resistance among clinical pneumococcal isolates14,15. Results of genetic and molecular analyses suggested that PBP2a contributed to cefotaxime resistance in some clinical isolates and laboratory strains of S. pneumoniae16.
For successful treatment of meningitis, effective antibiotic levels should be maintained in the brain all through the treatment period. Despite their great potency, systemic delivery of cephalosporins is associated with the potential risk of causing severe systemic side effects17. Therefore, there is demand for a patient compliant method to deliver cephalosporins to the brain. It is well known that there exists a direct nose-to-brain pathway via the olfactory region that could deliver drugs directly to the brain, bypassing the BBB. This route has been investigated as a potential route for delivery of several therapeutic agents via mechanisms that are still not clearly understood18. A key advantage of the nose-to-brain route is the possibility of reducing plasma exposure19, thus eliminating peripheral side effects. Intranasal delivery is also non-invasive, allows frequent administration and is less expensive than parenteral or oral therapy. Unlike parenteral formulations, nasal drops can be self-administered and do not require physician supervision during administration. Therefore, intranasal delivery of cephalosporins could be developed as a potential treatment approach for bacterial meningitis. The key factors that determine the efficacy of delivery via this route include the following: delivery to the olfactory area of the nose rather than the respiratory region, penetration enhancement of the active ingredient through the nasal epithelia, a longer retention time at the nasal mucosal surface, and a reduction in drug metabolism in the nasal cavity20.
Bioinformatics is an interdisciplinary science that represents the convergence of genomics, biotechnology, computer science and information technology21. It encompasses analysis and interpretation of data, modelling of biological phenomenon, development and implementation of computational algorithms and software tools in an effort to facilitate an understanding of the biological processes. Computational biology became increasingly important in various areas such as characterization of genes, determining structural and physiochemical properties of proteins, phylogenetic analyses, comparative or homology modeling, functional site location, characterization of active site for binding, docking of lead molecules into receptor binding sites, protein–protein interactions, molecular simulations, as well as drug designing22. Bioinformatics has several applications pertaining to pharmacy in the areas of drug discovery; designing and development; product/formulation designing; as well as pharmacokinetics and pharmacology23. Deploying genomics and proteomics, potential drug targets are identified by elucidating the interaction at the molecular level of a disease24. Molecular docking could predict the structure of intermolecular complex found between two molecules and predict the affinity between these biomolecules or receptors and potential drug candidate to find the best orientation of the ligand which would form a complex with overall minimum energy25.
In this study, a comparison between cefotaxime and ceftriaxone as third generation cephalosporins antibiotics against bacterial meningitis was performed at the formulation level (by comparing the loading in gelatin and tripalmitin matrices as bases for the formation of nanoparticulate systems), at the biopharmaceutical level (through the interaction with mucin and the P-gp efflux pumps) and at the therapeutic level (through studying the interaction with S. pneumonia bacterial receptors).
Methodology
Construction of the virtual carrier using molecular dynamics simulations
GROMACS26 v4.6.5 software package was used to carry-out all-atom molecular dynamics simulations. The atom typing and assignment of parameters and charges of the gelatin and tripalmitin matrices27 were carried-out online (https://cgenff.paramchem.org/) according to CHARMM general force field (CgenFF). To prepare the gelatin system, 48 peptide molecules were constructed, with 18 amino acids in each molecule. The primary sequence of the peptides was AGPRGQ(Hyp)GPAGPDGQ(Hyp)GP. On the other hand, the tripalmitin system contained 64 molecules of tripalmitin. The two matrices were then subjected to a molecular dynamics run, with full periodic boundary conditions, a time step of 2 fs, and a cut-off distance for Van der Waal’s and electrostatic interactions of 1 nm. PME was used to calculate electrostatic interactions and LINCS algorithm was used to constrain all bonds. The systems were equilibrated for 7 ns at 298 K using a v-rescale thermostat at a pressure of 1 bar using a Berendsen barostat.
Obtaining the target peptides and proteins virtual matrices
The crystal structure of the relevant nose-to-brain delivery and the therapeutic targets related to the bacterial cell wall receptors were obtained from the protein data bank (http://www.rcsb.org). The following codes: 2ACM and 3G6I corresponded to Mucin and P-gp efflux-pump receptors, respectively. 26CW and 2WAD corresponded to the protein binding proteins 1A and 2B, respectively. The polar hydrogens were added to the obtained pdb files using MOE version 2014.0901 (Chemical Computing Group Inc., Montreal, Canada).
Preparing the drugs chemical structures for docking
The isomeric SMILES corresponding to the chemical structures of the studied antibiotics; cefotaxime and ceftriaxone were obtained using PubChem. The corresponding 3D chemical structures were generated using the builder function of MOE version 2014.0901 (Chemical Computing Group Inc., Montreal, Canada). Further, energy minimization was carried out for all the investigated molecules using MMFF94x forcefield of the same software28,29.
Docking of the investigated drugs on the investigated carrier
The docking analysis was employed using MOE version 2014.0901 (Chemical Computing Group Inc., Montreal, Canada). The pdb file of the protein nanoparticles matrix was imported to MOE where the identification of the binding site was performed using MOE's “Site finder” tool30 to be ready for docking using the "triangle matcher” as a placement method.
This software creates dummy atoms around the docking target atoms. These dummy atoms are considered the docking positions. The London ΔG and ASE scores were utilized for calculating the binding energies scoring values. The London ΔG scoring function estimates the free energy of binding of the ligand from a given pose. The functional form is a sum of terms:
where c represents the average gain/loss of rotational and translational entropy; Eflex is the energy due to the loss of flexibility of the ligand (calculated from ligand topology only); fHB measures geometric imperfections of hydrogen bonds and takes a value in [0,1]; cHB is the energy of an ideal hydrogen bond; fM measures geometric imperfections of metal ligations and takes a value in [0,1]; cM is the energy of an ideal metal ligation; and Di is the desolvation energy of atom i. The difference in desolvation energies is calculated according to the formula
where A and B are the protein and/or ligand volumes with atom i belonging to volume B; Ri is the solvation radius of atom i (taken as the OPLS-AA van der Waals sigma parameter plus 0.5 Å); and ci is the desolvation coefficient of atom i. The coefficients {c, cHB, cM, ci} were fitted from ~ 400 X-ray crystal structures of protein–ligand complexes with available experimental pKi data. Atoms are categorized into about a dozen atom types for the assignment of the ci coefficients. The triple integrals are approximated using Generalized Born integral formulas. Like all commonly used scoring functions, lower binding energies (ΔG, kcal/mole) scores indicate more favourable interactions.
Calculating the main descriptors of the investigated drugs
In order to explain the differences in docking scores observed for the studied drugs, some crucial constitutional, electronic and topological descriptors were calculated for the studied drugs. The selected descriptors were the molecular weight, xLogP, topological polar surface area, number of H-atoms donors and acceptors and finally the fragment complexity. The descriptors were calculated using Bioclipse version 2.6 (Bioclipse project, Uppsala University, Sweden) using the molecules mol files generated using Chem3D Ultra version 10 (Cambridgesoft, Perkin Elmer, Akron, Ohio).
Results and discussion
The gelatin and tripalmitin have been rationally selected as the nanoparticulate matrices material for loading the investigated drugs due to their proven successful compatibility with the olfactory nerves and regions, their successful penetration through the drug brain barrier and their efficiency (especially gelatin) on loading several hydrophilic and hydrophobic drugs31–33. The successful construction of the gelatin and tripalmitin virtual matrices using the adopted molecular dynamics method was obtained after following the same protocols of the authors previous studies34–37. Table 1 demonstrates the results of docking the investigated drugs; cefotaxime and ceftriaxone on the selected carriers. The higher score of ceftriaxone on the gelatin matrix could be attributed to its more hydrophilic nature demonstrated by its lower Log P (− 1.59), higher total polar surface area (506.48) and higher number of h-bond donors and acceptors (5 and 9, respectively) that can interact with the gelatin carboxylic and amino groups38,39 compared to the cefotaxime counterparts. Table 2 depicts the SMILES (that were needed for docking experiments, electronic, constitutional and topological physico-chemical descriptors) of the two studied antibiotics. Moreover, the less molecular flexibility and higher molecular weight of ceftriaxone lead to less out-of-matrix diffusion and higher entrapment of this molecule whether on the gelatin or the tripalmitin matrices40–42. Therefore, these factors would play a very important role in deciding the best drug–carrier pair43.
Table 1.
Docking binding energy (ΔG) values after docking of the investigated drugs on the nose-to-brain related macromolecules.
| Macromolecule (carrier/protein)—PDB code | Binding energy (kcal/mole) | |
|---|---|---|
| Cefotaxime | Ceftriaxone | |
| Gelatin matrix (gelatin nanospheres) | − 8.53 ± 0.4 | − 11.68 ± 0.5 |
| Tripalmitin matrix (solid lipid nanoparticles) | − 9.60 ± 0.2 | − 10.77 ± 0.4 |
| Mucin—2ACM | − 11.67 ± 0.2 | − 11.70 ± 0.2 |
| P-gp efflux pump—3G61 | − 8.95 ± 0.3 | − 10.22 ± 0.4 |
| Protein binding protein 1A (PBP-1A) 26CW | − 12.32 ± 0.1 | − 13.18 ± 0.2 |
| Protein binding protein 2B (PBP-2B) 2WAD | − 12.11 ± 0.2 | − 14.35 ± 0.03 |
Table 2.
Main physico-chemical descriptors of the investigated drugs.
| Molecule | SMILES | Total polar surface area | Number of H-bond acceptors | Number of H-bond donors | Molecular flexibility | logP (o/w) | Molecular weight |
|---|---|---|---|---|---|---|---|
| Cefotaxime | s1cc(nc1N)C(= NOC)C(= O)NC1C2SCC(COC(= O)C) = C(N2C1 = O)C(O) = O | 381.41 | 7 | 4 | 5.95 | − 0.64 | 455.472 |
| Ceftriaxone | s1cc(nc1N)C(= NOC)C(= O)NC1C2SCC(CSC3 = NC(= O)C(= O)NN3C) = C(N2C1 = O)C(O) = O | 506.48 | 9 | 5 | − 1.161 | − 1.59 | 554.589 |
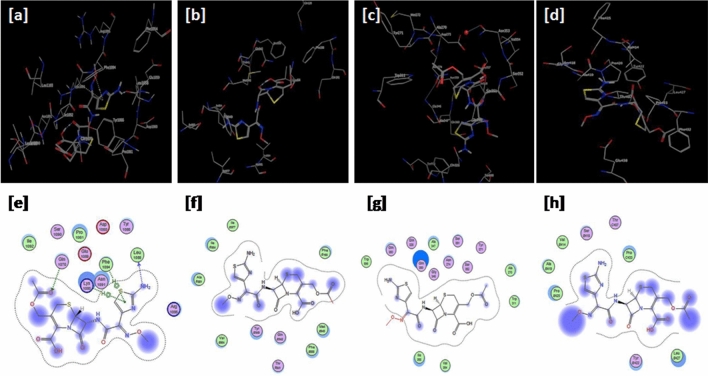
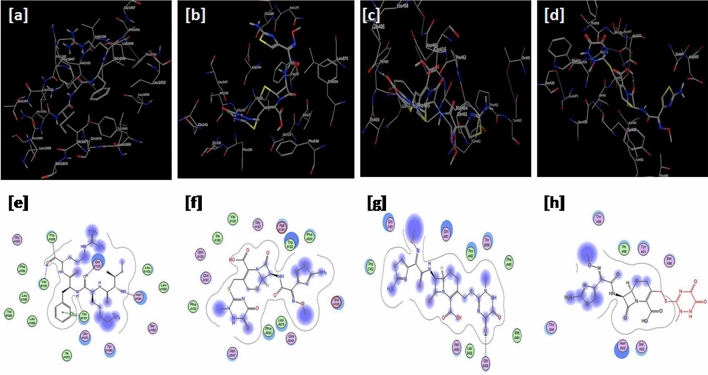
Figure 1 also depicts the successful docking of cefotaxime on the macromolecules viz. the proteins and peptides relevant to the brain delivery (e.g. mucin and P-gp efflux pump) or the meningitis disease bacterial therapeutic targets (e.g. S. pneumoniae PBPa and 2b). Figure 2 demonstrates the successful docking of ceftriaxone on the same targets.
Figure 1.
Docking results of cefotaxime on (a–e) 2ACM, (b–f) 3G61, (c–g) 2C6W and (d–h) 2WAD. Upper and lower panels represent 3D and 2D images, respectively.
Figure 2.
Docking results of ceftriaxone on (a–e) 2ACM, (b–f) 3G61, (c–g) 2C6W and (d–h) 2WAD. Upper and lower panels represent 3D and 2D images, respectively.
Table 1 shows the obtained binding energies after docking of the two antibiotics on the investigated biopharmaceutical and therapeutic macromolecules. The recorded results showed the higher affinity of ceftriaxone compared to cefotaxime. On the delivery level, the docking results of the two drugs on mucin as an indicator of the ability of the antibiotic for better mucoadhesion in the olfactory region was in favor of ceftriaxone. Furthermore, higher affinity of the ceftriaxone to the P-gp efflux pump proteins (responsible for the expulsion of drugs outside the brain cells and the blood–brain barrier) was observed as revealed by the lower binding energy scores. This finding warrants the usage of a carrier system such as gelatin nanospheres that can circumvent this usual biopharmaceutical hindrance. The interpretation of these results can also be ascribed to the differences in the main structural, topological and electronic descriptors of the two investigated drugs (Table 2) as previously discussed by Metwally and Hathout36,44.
Recently, the authors have proven the contribution of these main descriptors of drugs on the affinity and hence the loading of drugs on different carriers (Tripalmitin solid lipid nanoparticles, protein and PLGA nanoparticles)36 where the drug molecular weight, total polar surface area, and molecular flexibility have shown great influences of the drugs to different proteins and receptors40,45.
It should be noticed that the difference in the number of hydrogen bond donors and acceptors between the two closely related chemical structures should have contributed to the superior interaction of ceftriaxone over cefotaxime with the investigated bacterial proteins indicating more potency.
The obtained molecular docking experiments results on the selected biological protein receptors may explain the effectiveness of both drugs in meningitis treatment at clinical studies but in less doses and dosing frequency in case of ceftriaxone. In a well-conducted clinical study on 82 children, the effectiveness and safety of the two-antibiotics were evaluated in the short-term treatment of primary bacterial meningitis using a prospective, randomized, multicenter study design. Ceftriaxone was effective at a single dose (100 mg/kg on the first day followed by 75 mg/kg/day) while cefotaxime needed four divided doses (200 mg/kg/day) per day for 4–7 days6.
In light of the obtained results, it can be concluded that the ceftriaxone nose-to-brain delivery should pose a more feasible and efficient therapy to the S. pneumoniae related meningitis disease as compared to cefotaxime.
Conclusion and future perspective
In the current work, the use of several bio/chemoinformatics tools have proven that ceftriaxone gelatin nanospheres or tripalmitin solid lipid nanoparticles may pose better nose-to-brain formulation targeting the meningitis disease compared to the cefotaxime counterparts. The current study could find a comprehensive solution to the usual debate about the feasibility of performing extensive researches on anti-microbials and/or biosimilars aiming for better alternatives considering all aspects and points of views; formulation, biopharmaceutical or therapeutic. This kind of research would also offer a new platform in medicines design which can save formulators and pharmacists huge time and resources spent on wet-lab experimentation. Moreover, the probability of errors and inaccurate results obtained from biological experiments are relatively reduced.
Author contributions
R.M.H. performed the conceptualization and the docking experiments, wrote the main text, interpreted and discussed the results. S.G.A. and G.S.E. wrote the main manuscript text, interpreted and discussed the results. R.M.H. performed the figures and tables. A.A.M. performed the molecular modelling, interpreted and discussed the results. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sherihan G. Abdelhamid and Ghadir S. El-Housseiny.
References
- 1.Troendle M, Pettigrew A. A systematic review of cases of meningitis in the absence of cerebrospinal fluid pleocytosis on lumbar puncture. BMC Infect. Dis. 2019;19:692. doi: 10.1186/s12879-019-4204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths MJ, McGill F, Solomon T. Management of acute meningitis. Clin. Med. (Lond.) 2018;18:164–169. doi: 10.7861/clinmedicine.18-2-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oordt-Speets AM, Bolijn R, van Hoorn RC, Bhavsar A, Kyaw MH. Global etiology of bacterial meningitis: A systematic review and meta-analysis. PLoS One. 2018;13:e0198772. doi: 10.1371/journal.pone.0198772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Brouwer BDM, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat. Rev. Dis. Primers. 2016;2:16074. doi: 10.1038/nrdp.2016.74. [DOI] [PubMed] [Google Scholar]
- 5.El BH, Laundy M, Booy R. Diagnosis and treatment of bacterial meningitis. Arch. Dis. Child. 2003;88:615–620. doi: 10.1136/adc.88.7.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz H, Hofmann T, Noack R, Edwards DJ, Stoeckel K. Prospective comparison of ceftriaxone and cefotaxime for the short-term treatment of bacterial meningitis in children. Chemotherapy. 1998;44:142–147. doi: 10.1159/000007106. [DOI] [PubMed] [Google Scholar]
- 7.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 8.Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A. Penicillin binding proteins: Key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol. Rev. 2006;30:673–691. doi: 10.1111/j.1574-6976.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 9.Land AD, Tsui HC, Kocaoglu O, Vella SA, Shaw SL, Keen SK, Sham LT, Carlson EE, Winkler ME. Requirement of essential Pbp2x and GpsB for septal ring closure in Streptococcus pneumoniae D39. Mol Microbiol. 2013;90:939–955. doi: 10.1111/mmi.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massidda O, Novakova L, Vollmer W. From models to pathogens: How much have we learned about Streptococcus pneumoniae cell division? Environ. Microbiol. 2013;15:3133–3157. doi: 10.1111/1462-2920.12189. [DOI] [PubMed] [Google Scholar]
- 11.Hakenbeck R, Bruckner R, Denapaite D, Maurer P. Molecular mechanisms of beta-lactam resistance in Streptococcus pneumoniae. Future. Microbiol. 2012;7:395–410. doi: 10.2217/fmb.12.2. [DOI] [PubMed] [Google Scholar]
- 12.Tsui HT, Boersma MJ, Vella SA, Kocaoglu O, Kuru E, Peceny JK, Carlson EE, VanNieuwenhze MS, Brun YV, Shaw SL, Winkler ME. Pbp2x localizes separately from Pbp2b and other peptidoglycan synthesis proteins during later stages of cell division of Streptococcus pneumoniae D39. Mol Microbiol. 2014;94:21–40. doi: 10.1111/mmi.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakenbeck R. beta-lactam-resistant Streptococcus pneumoniae: Epidemiology and evolutionary mechanism. Chemotherapy. 1999;45:83–94. doi: 10.1159/000007170. [DOI] [PubMed] [Google Scholar]
- 14.Kosowska K, Jacobs MR, Bajaksouzian S, Koeth L, Appelbaum PC. Alterations of penicillin-binding proteins 1A, 2X, and 2B in Streptococcus pneumoniae isolates for which amoxicillin MICs are higher than penicillin MICs. Antimicrob. Agents Chemother. 2004;48:4020–4022. doi: 10.1128/AAC.48.10.4020-4022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai K, Davies TA, Jacobs MR, Appelbaum PC. Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrob. Agents Chemother. 2002;46:1273–1280. doi: 10.1128/AAC.46.5.1273-1280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichmann P, Konig A, Marton A, Hakenbeck R. Penicillin-binding proteins as resistance determinants in clinical isolates of Streptococcus pneumoniae. Microb. Drug Resist. 1996;2:177–181. doi: 10.1089/mdr.1996.2.177. [DOI] [PubMed] [Google Scholar]
- 17.Viladrich PF, Cabellos C, Pallares R, Tubau F, Martinez-Lacasa J, Linares J, Gudiol F. High doses of cefotaxime in treatment of adult meningitis due to Streptococcus pneumoniae with decreased susceptibilities to broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 1996;40:218–220. doi: 10.1128/AAC.40.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson LR, Frey WH. Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J Neuroimmune. Pharmacol. 2007;2:81–86. doi: 10.1007/s11481-006-9039-x. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey L, Iannitelli A, Garrett NL, Moger J, Imbert I, King T, Porreca F, Soundararajan R, Lalatsa A, Schatzlein AG, Uchegbu IF. Nanoparticulate peptide delivery exclusively to the brain produces tolerance free analgesia. J. Control Release. 2018;270:135–144. doi: 10.1016/j.jconrel.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Xiong G, Tsang WC, Schñtzlein AG, Uchegbu IF. Nose-to-brain delivery. J. Pharmacol. Exp. Ther. 2019;370:593. doi: 10.1124/jpet.119.258152. [DOI] [PubMed] [Google Scholar]
- 21.Heo GE, Kang KY, Song M, Lee JH. Analyzing the field of bioinformatics with the multi-faceted topic modeling technique. BMC Bioinform. 2017;18:251. doi: 10.1186/s12859-017-1640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefranc MP, Giudicelli V, Regnier L, Duroux P. IMGT, a system and an ontology that bridge biological and computational spheres in bioinformatics. Brief. Bioinform. 2008;9:263–275. doi: 10.1093/bib/bbn014. [DOI] [PubMed] [Google Scholar]
- 23.Hathout RM, El-Ahmady SH, Metwally AA. Curcumin or bisdemethoxycurcumin for nose-to-brain treatment of Alzheimer disease? A bio/chemo-informatics case study. Nat. Prod. Res. 2018;32:2873–2881. doi: 10.1080/14786419.2017.1385017. [DOI] [PubMed] [Google Scholar]
- 24.Gill SK, Christopher AF, Gupta V, Bansal P. Emerging role of bioinformatics tools and software in evolution of clinical research. Perspect. Clin. Res. 2016;7:115–122. doi: 10.4103/2229-3485.184782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmaso V, Moro S. Bridging molecular docking to molecular dynamics in exploring ligand–protein recognition process: An overview. Front. Pharmacol. 2018;9:923. doi: 10.3389/fphar.2018.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pronk S, Éll SÉP, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29:845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, Mackerell AD. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gooding M, Adigbli D, Edith Chan AW, Melander RJ, MacRobert AJ, Selwood DL. A bifurcated proteoglycan binding small molecule carrier for siRNA delivery. Chem. Biol. Drug Des. 2014;84:24–35. doi: 10.1111/cbdd.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costache AD, Sheihet L, Knight DD, Kohn J. Modeling of polymer-drug interactions in biodegradable tyrosine-based nanospheres using molecular dynamics simulations and docking. NSTI-Nanotech. 2009;2009(2):76–78. [Google Scholar]
- 30.Elhefnawi M, ElGamacy M, Fares M. Multiple virtual screening approaches for finding new hepatitis C virus RNA-dependent RNA polymerase inhibitors: Structure-based screens and molecular dynamics for the pursue of new poly pharmacological inhibitors. BMC Bioinform. 2012;13(Suppl 17):S5. doi: 10.1186/1471-2105-13-S17-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakeri S, Ashrafizadeh M, Zarrabi A, Roghanian R, Afshar EG, Pardakhty A, Mohammadinejad R, Kumar A, Thakur VK. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines. 2020;8:20. doi: 10.3390/biomedicines8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He H, Yao J, Zhang Y, Chen Y, Wang K, Lee RJ, Yu B, Zhang X. Solid lipid nanoparticles as a drug delivery system to across the blood–brain barrier. Biochem. Biophys. Res. Commun. 2019;519:385–390. doi: 10.1016/j.bbrc.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 33.El-Gizawy SA, El-Maghraby GM, Hedaya AA. Formulation of acyclovir-loaded solid lipid nanoparticles: 2. Brain targeting and pharmacokinetic study. Pharm. Dev. Technol. 2019;24:1299–1307. doi: 10.1080/10837450.2019.1667386. [DOI] [PubMed] [Google Scholar]
- 34.Metwally AA, El-Ahmady SH, Hathout RM. Selecting optimum protein nano-carriers for natural polyphenols using chemoinformatics tools. Phytomedicine. 2016;23:1764–1770. doi: 10.1016/j.phymed.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 35.Hathout RM, Metwally AA. Towards better modelling of drug-loading in solid lipid nanoparticles: Molecular dynamics, docking experiments and Gaussian Processes machine learning. Eur. J. Pharm. Biopharm. 2016;108:262–268. doi: 10.1016/j.ejpb.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Metwally AA, Hathout RM. Computer-assisted drug formulation design: Novel approach in drug delivery. Mol. Pharm. 2015;12:2800–2810. doi: 10.1021/mp500740d. [DOI] [PubMed] [Google Scholar]
- 37.Hathout RM, Metwally AA, Woodman TJ, Hardy JG. Prediction of drug loading in the gelatin matrix using computational methods. ACS Omega. 2020;5(3):1549–1556. doi: 10.1021/acsomega.9b03487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ossama M, Hathout RM, Attia DA, Mortada ND. Enhanced allicin cytotoxicity on HEPG-2 cells using glycyrrhetinic acid surface-decorated gelatin nanoparticles. ACS Omega. 2019;4:11293–11300. doi: 10.1021/acsomega.9b01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hathout RM, Metwally AA. Gelatin nanoparticles. Methods Mol. Biol. 2000;2019:71–78. doi: 10.1007/978-1-4939-9516-5_6. [DOI] [PubMed] [Google Scholar]
- 40.Hill M, Cunningham RN, Hathout RM, Johnston C, Hardy JG, Migaud ME. Formulation of antimicrobial tobramycin loaded PLGA nanoparticles via complexation with AOT. J. Funct. Biomater. 2019;10(2):26. doi: 10.3390/jfb10020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah SAA, Firlak M, Berrow SR, Halcovitch NR, Baldock SJ, Yousafzai BM, Hathout RM, Hardy JG. Electrochemically enhanced drug delivery using polypyrrole films. Mater. Basel. 2018;11:20. doi: 10.3390/ma11071123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hathout RM, Woodman TJ. Applications of NMR in the characterization of pharmaceutical microemulsions. J. Control. Release. 2012;161:62–72. doi: 10.1016/j.jconrel.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 43.Ramezanpour M, Leung SS, Gado-Magnero KH, Bashe BY, Thewalt J, Tieleman DP. Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim. Biophys. Acta. 2016;1858:1688–1709. doi: 10.1016/j.bbamem.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 44.Metwally AA, Hathout RM. Replacing microemulsion formulations experimental solubility studies with in-silico methods comprising molecular dynamics and docking experiments. Chem. Eng. Res. Des. 2015;104:453–456. doi: 10.1016/j.cherd.2015.09.003. [DOI] [Google Scholar]
- 45.Hathout, R. M., Abdelhamid, S. G. & Metwally, A. A. Chloroquine and hydroxychloroquine for combating COVID-19: Investigating efficacy and hypothesizing new formulations using Bio/chemoinformatics tools. Inform. Med. Unlocked21, 100446 (2020). [DOI] [PMC free article] [PubMed]