Abstract
Background
A high hepatitis B virus (HBV) load is a common exclusion criterion in hepatocellular carcinoma (HCC) clinical trials for anti-programmed cell death (PD)-1 immunotherapy. However, the validity of this criterion is barely verified. This study aimed to evaluate the impact of baseline HBV DNA levels and antiviral therapy on the oncological outcomes and liver functions of patients with HCC receiving anti-PD-1 immunotherapy.
Methods
We reviewed HCC trials related to anti-PD-(L)1 immunotherapy and whether they ruled out patients with increased HBV loads on clinicaltrials.gov. Then, for this retrospective study, we enrolled 253 HCC patients treated with anti-PD-1 blockade in our institution. Baseline information was compared between patients with low and high HBV loads. Overall survival (OS) and progression-free survival (PFS) were compared, and univariate and multivariate analyses were applied to identify potential risk factors for oncological outcomes and hepatic impairment.
Results
Among 76 HCC clinical trials including 13,927 patients receiving anti-PD-(L)1 blockade, 41 (53.9%) excluded patients with relatively high baseline HBV loads. The PFS and OS did not differ significantly between patients with baseline HBV loads ≤ 2000 IU/mL and those with viral loads >2000 IU/mL (p=0.615 and 0.982). The incidence of hepatic impairment showed no association with the baseline HBV load (p=0.319). Patients receiving antiviral therapy had a better OS than those without antiviral therapy in the high baseline HBV load group (p= 0.001).
Conclusion
High HBV loads did not compromise the clinical outcomes of HCC patients receiving anti-PD-1 blockade. Antiviral therapy could improve the OS of HCC patients with high HBV loads.
Keywords: hepatocellular carcinoma, immunotherapy, hepatitis B virus, viral load
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most common malignancy worldwide, and its incidence continues to increase.1,2 Approximately 33% of HCC patients are infected with hepatitis B virus (HBV).3 The microenvironment of HBV-related HCC is more immunosuppressive than that of non-viral-related HCC due to the enrichment of regulatory T cells, which can be partly attributed to the upregulation expression of programmed death-1 (PD-1) expression.4 Studies have shown that the HBV load is positively correlated with the expression of PD-1 on T cells.4–6 Previous studies identified high HBV DNA concentration as an independent risk factor for poor survival and early recurrence after curative resection in HCC patients.7–9 Moreover, higher HBV DNA levels are associated with a poorer response to transarterial chemoembolization (TACE) and sorafenib.10–13 Studies have also reported that antiviral treatment can prolong the survival of HCC patients with HBV infection.7,10,14
To date, few studies have reported the impact of HBV load on immunotherapy for HCC patients. The emerging immune checkpoint inhibitors (ICIs) blocking the PD-1 pathway have revolutionized the treatment modalities for advanced HCC, which can achieve objective response rates of 17% to 20% as monotherapy.15,16 However, the Phase III trial of Pembrolizumab failed to achieve its primary endpoints, indicating the potential necessity for identifying subgroups of patients most likely benefiting from ICIs.17 Combination therapies generally showed better effects, and oncologists have been taking efforts to explore the best therapeutic methods and indications.18–21 A series of clinical trials were registered, and we noticed that researchers focused predominantly on advanced HCC. Late-stage HCC patients are more likely to have impaired liver function and decreased general condition, and researchers should perform a more comprehensive and strict assessment of the included patients.22,23 The current HCC clinical trials using anti-PD-1 blockade therapy usually list increased HBV levels as an exclusion criterion, requiring patients with HBV to have a baseline HBV load up to 100–2000 IU/mL.15–17 The underlying concern is that patients with high HBV loads might suffer from HBV reactivation and hepatic impairment during anti-PD-1 immunotherapy. However, the validity of these criteria is barely proved. Furthermore, whether antiviral therapy can improve the efficacy and safety of anti-PD-1 blockade remains unclear.
This study aimed to explore whether the baseline HBV DNA level and antiviral therapy had impacts on the prognostic outcomes of HCC patients receiving anti-PD-1 immunotherapy and to evaluate the effect of viral load and antiviral treatment on hepatic impairment. With the increasing application of anti-PD-1 immunotherapy, the above results can provide evidence for more appropriate patient selection and avoidance of potential adverse effects.
Methods
Summary of Enrolled Registered Clinical Trials
We reviewed HCC clinical trials associated with anti-PD-(L)1 immunotherapy published on ClinicalTrials.gov to assess the current status of clinical trials associated with anti-PD-1 immunotherapy in HCC patients to clarify the necessity of our study. Whether baseline HBV DNA levels were adopted as exclusion criteria were recorded. A detailed description of the selected enrolled trials is shown in Supplement Figure 1. The clinical trial registration numbers of enrolled trials are listed in Supplementary Table 1.
Patient Selection
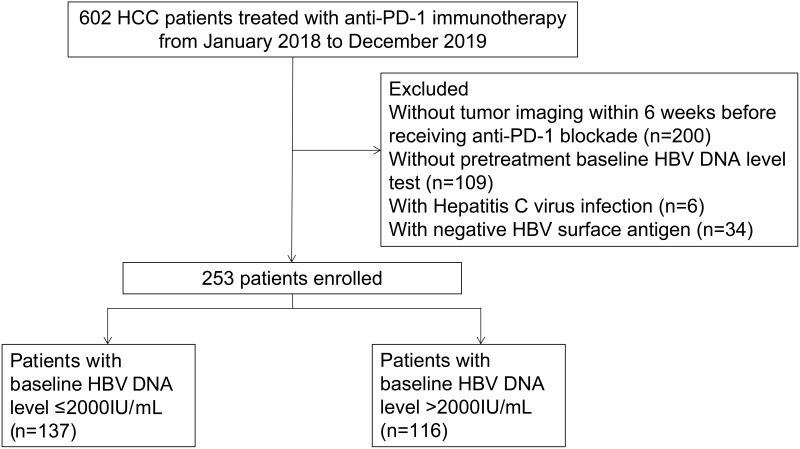
In this retrospective study, to investigate the necessity of these exclusion criteria, we reviewed HCC patients receiving anti-PD-1 immunotherapy in Sun Yat-sen University Cancer Center from January 1, 2018 to December 20, 2019. In total, 602 patients were enrolled according to the following inclusion criteria: 1) having clinical and/or pathological diagnostic records of HCC in our institution; 2) age at diagnosis ≥ 18 years old; and 3) having information on hepatitis virus infection. Patients were excluded for 1) not having pretreatment baseline HBV DNA tests; 2) without tumor imaging within six weeks before receiving anti-PD-1 blockade; 3) with hepatitis C virus infection; or 4) with negative HBV surface antigen. Finally, we enrolled 253 patients for analyses. HBV loads were measured by a real-time viral polymerase chain reaction in our institution. The patients were treated with anti-PD-1 blockades according to the standard drug instructions, which are listed in Supplementary Table 2. The study flow chart is shown in Figure 1. This study was performed in accordance with the ethical standards of the 1964 Helsinki declaration and was approved by the institutional review board of Sun Yat-sen University Cancer Center.
Figure 1.
The flowchart of patients enrolled in this study.
Clinical Endpoints
The primary endpoint was overall survival (OS), which was defined as the time from the first medication of anti-PD-1 immunotherapy to death. Progression-free survival (PFS) was defined as time from the first medication of anti-PD-1 immunotherapy to the first report of progressive disease according to the modified Response Evaluation Criteria in Solid Tumors. According to the Common Terminology Criteria for Adverse Events (AEs) (CTCAE v5.0), hepatic impairment was defined as a five-fold or greater increase of alanine aminotransferase or aspartate aminotransferase than the upper limit of normal value (baseline was normal), or a five-fold or greater increase of ALT/AST than the baseline (baseline was abnormal).24 HBV reactivation was defined according to the American Association for the Study of Liver Disease 2018 Hepatitis B guidance. For patients with positive HBsAg, HBV reactivation can be diagnosed as one of the following: (1) a ≥ 2 log (100-fold) increase in HBV DNA compared to the baseline level; (2) HBV DNA ≥ 3 log (1000) IU/mL in a patient with previously undetectable level.25
Statistical Analysis
A two-independent t-test was adopted for comparing variables distributed normally. Chi-square or Fisher’s exact tests were used for comparing categorical variables. OS and PFS were compared by the Kaplan–Meier method with the Log-rank test. A multivariate Cox regression model was performed to assess potentially significant variables in the univariate analysis. Binary logistic regression analysis was adopted to evaluate the association between hepatic impairment and potential factors, including age, gender, HBsAg status, HBeAg status, the Barcelona Clinic Liver Cancer (BCLC) stage, alpha-fetoprotein (AFP), albumin-bilirubin (ALBI) grade, cirrhosis and treatment modality. A two-tailed P value < 0.05 was considered statistically significant. The analyses were performed with IBM SPSS, version 26.0 and R software version 3.3.2.
Results
Characteristics of HCC Clinical Trials and Exclusion Criteria of HBV DNA Level
We identified 76 HCC clinical trials that enrolled 13,927 HCC patients receiving anti-PD-(L)1 blockade. Table 1 lists the detailed characteristics of clinical trials and relevant HBV DNA exclusion criteria. A total of 41 trials (53.9%) excluded patients with relatively high baseline HBV DNA levels, 26.8% and 95.1% of which excluded patients with baseline levels greater than 100 IU/mL and 2000 IU/mL, respectively. There was no significant correlation between the exclusion criteria of baseline HBV loads and other characteristics of trials, including phase of study, stage of HCC, treatment modality and sample size (p=0.061, 0.252, 0.154 and 0.499).
Table 1.
Characteristics of HCC Clinical Trials Associated with Anti-PD-1 or PD-L1 Immunotherapy
| Characteristics | Number of Trials (%) |
|---|---|
| Total | 76 |
| Phase of study | |
| I | 9 (11.8) |
| II | 47 (61.8) |
| III | 16 (21.1) |
| Not Applicable | 4 (5.3) |
| Stage of HCC | |
| Early | 10 (13.2) |
| Local Advanced | 25 (32.9) |
| Advanced | 41 (53.9) |
| Treatment modality | |
| ICI alone | 16 (21.1) |
| ICI with local treatments | 19 (25.0) |
| ICI with targeted drugs | 41 (53.9) |
| Types of endpoints | |
| Survival | 71 (93.4) |
| Response | 61 (80.3) |
| Adverse events/Toxicity | 45 (59.2) |
| Sample Size | |
| Median (Range) | 50 (12–1723) |
| < 100 | 52 (68.4) |
| 100–500 | 15 (19.7) |
| >500 | 9 (11.8) |
| Cutoff values of exclusion criterion for HBV loads | |
| 0–100 | 11 (14.5) |
| 100–2000 | 28 (36.8) |
| >2000 | 2 (2.6) |
| None | 35 (46.1) |
Abbreviation: ICI, immune checkpoint inhibitors including anti-PD-1 or PD-L1 blockade in this study.
Patient Characteristics
The clinical characteristics of the 253 enrolled HCC patients are summarized in Table 2. There was no significant difference in age or gender between patients with low (baseline viral load ≤ 2000IU/mL) and high (baseline viral load > 2000IU/mL) HBV DVA levels. Patients with higher viral load tended to have positive HBeAg and to receive antiviral therapy (p=0.141 and 0.057). The proportion of BCLC stage and the AFP level was similar between the two groups. A higher HBV load was associated with a higher ALBI grade (p=0.034). The incidence of cirrhosis was slightly higher in patients with high viral loads. Among the whole cohort, 31 patients received anti-PD-1 immunotherapy alone, 69 patients received immunotherapy combined with targeted drugs, 65 patients received immunotherapy combined with local treatment including hepatic arterial infusion or TACE, and 88 patients received immunotherapy combined with targeted drugs and local treatment. Higher viral load had no significant relation with treatment modality (p=0.057).
Table 2.
Baseline Characteristics of Patients with High and Low Baseline HBV DNA Level
| Characteristics | DNA Level ≤2000 IU/mL | DNA Level >2000 IU/mL | P value |
|---|---|---|---|
| Sample size | 137 | 116 | |
| Age (years) | 50.69±11.86 | 50.72±11.96 | 0.988 |
| Gender | 0.998 | ||
| Male | 117 (85.4) | 100 (86.2) | |
| Female | 20 (14.6) | 16 (13.8) | |
| HBeAg | 0.141 | ||
| Positive | 26 (19.0) | 32 (27.6) | |
| Negative | 111 (81.0) | 84 (72.4) | |
| BCLC stage | 0.611 | ||
| B | 34(24.8) | 33 (28.4) | |
| C | 103 (75.2) | 83 (71.6) | |
| AFP, ng/mL | 0.262 | ||
| ≤200 | 59 (43.1) | 41 (35.3) | |
| >200 | 78 (56.9) | 75 (64.7) | |
| a ALBI grade | 0.034 | ||
| I | 68 (49.6) | 40 (34.5) | |
| II | 68 (49.6) | 73 (62.9) | |
| III | 1 (0.7) | 3 (2.6) | |
| Cirrhosis | 0.454 | ||
| Yes | 47 (34.3) | 46 (39.7) | |
| No | 90 (65.7) | 70 (60.3) | |
| Treatment modality | 0.019 | ||
| b AP alone | 23 (16.8) | 8 (6.9) | |
| AP withc TD | 42 (30.7) | 27 (23.3) | |
| AP withd LT | 33 (24.1) | 32 (27.6) | |
| AP with TD and LT | 39 (28.5) | 49 (42.2) | |
| Antiviral therapy | 0.057 | ||
| Yes | 104 (75.9) | 100 (86.2) | |
| No | 33 (24.1) | 16 (13.8) |
Abbreviations: aALBI, albumin-bilirubin grade; bAP, anti-PD-1 immunotherapy; cTD, targeted drugs; dLT, local treatments.
Survival Outcomes
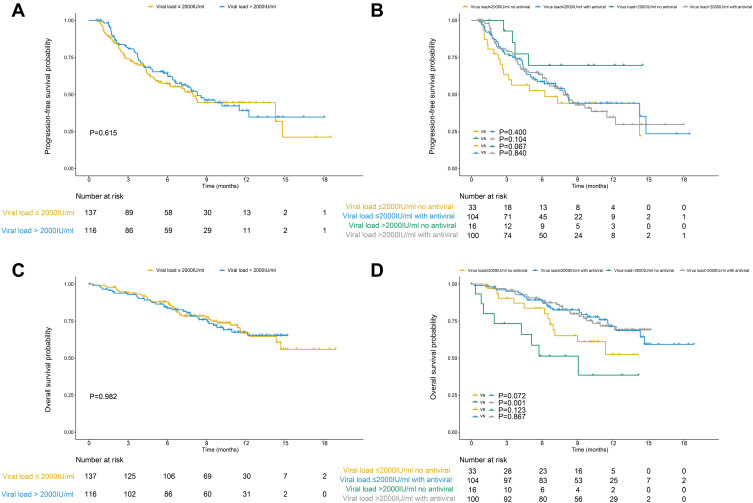
The median follow-up time was 10.2 months. During treatment, 121 (47.8%) patients suffered progressive disease. The median PFS time was 5.5 months. There was no significant difference in PFS between patients with a baseline HBV DNA level ≤ 2000IU/mL and those with a DNA level > 2000IU/mL (p=0.615). The survival curves are plotted in Figure 2A. The PFS was also similar when the cutoff value was set to 0 IU/mL or 100 IU/mL (p=0.485 and 0.842) (Supplementary Figures 2A and 3A). The only significant factor related to PFS was gender in the univariate analysis (p=0.028), but no significant factor was identified in the multivariate analysis.
Figure 2.
The survival curves of patients with baseline HBV DNA level ≤2000IU/mL and those with DNA level > 2000IU/mL after receiving anti-PD-1 immunotherapy. There was no significant in the progression-free survival (PFS) (A) or overall survival (OS) (C) between groups with low and high baseline viral loads. Antiviral therapy could not improve the PFS (B), but it could prolong the OS of HCC patients with high baseline viral load (D).
During follow-up, 70 (27.7%) patients died. The median survival time was 9.1 months. No significant association was found between the high baseline viral load and OS (p=0.982). The survival curves are shown in Figure 2C. A similar tendency was found when the cutoff value was set to 0 IU/mL or 100 IU/mL (p=0.551 and 0.744) (Supplementary Figures 2C and 3C). According to multivariate analysis, the OS was significantly related to treatment modality, BCLC stage, serum AFP level and antiviral therapy (p=0.005, 0.032, 0.036 and 0.025).
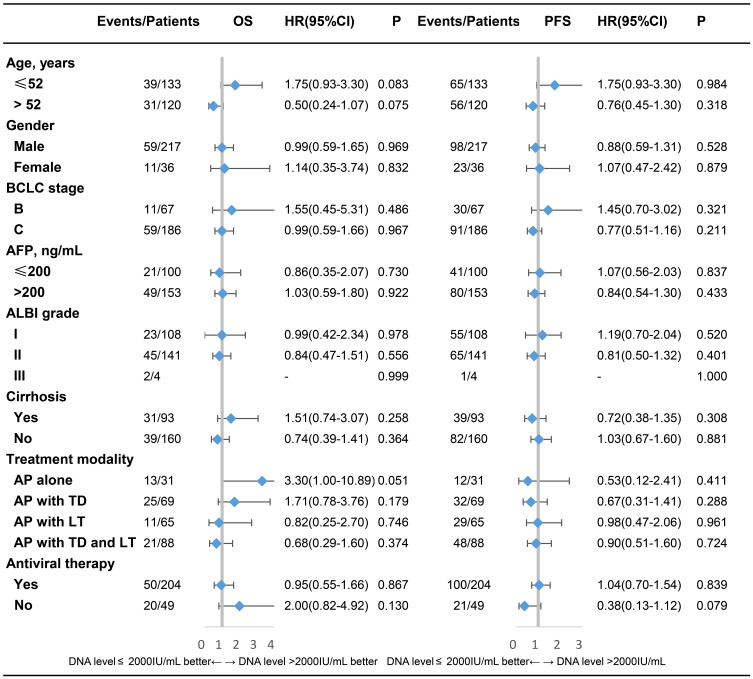
The impact of baseline viral load on PFS and OS was evaluated in subgroups stratified by different variables. Detailed information is shown in Figure 3. No interaction effect was found between baseline viral load and each variable in PFS or OS. The baseline HBV DNA level did not significantly affect PFS or OS in each subgroup.
Figure 3.
Subgroup analysis of the overall survival (OS) and progression-free survival (PFS) in groups stratified by each variable.
HBV Reactivation and Hepatic Impairment After Receiving Anti-PD-1 Immunotherapy
Among 253 patients, 143 (56.5%) received viral load monitoring after anti-PD-1 blockade, and two (1.4%) patients suffered from HBV reactivation. Both patients had low baseline viral load, and one patient with positive HBeAg received antiviral therapy. Neither of them had hepatic impairment during treatment. A total of 54 (21.3%) patients suffered hepatic impairment, while the baseline viral load had no significant impact on the incidence of hepatic impairment (p=0.319). In the multivariate analysis, an increasing number of treatment modalities, more advanced BCLC stage and cirrhosis related to a higher incidence of hepatic impairment (p=0.001, 0.043 and 0.014). The occurrence of hepatic impairment did not significantly affect the PFS or OS (p=0.071 and 0.323).
Impact of Antiviral Therapy on the Clinical Outcomes of HCC Patients
Antiviral therapy did not improve the PFS in groups with low or high HBV DNA levels (Figure 2B, Supplementary Figures 2B and 3B). However, patients receiving antiviral therapy had better survival than those who did not receive antiviral therapy in the group with high baseline HBV DNA levels (p=0.001) (Figure 2D, Supplementary Figures 2D and 3D). There was no significant difference in the incidence rates of hepatic impairment between patients treated with or without antiviral therapy in groups with either low or high HBV loads (p=0.707 and 0.476).
Discussion
To our knowledge, this is the first study to systemically evaluate the impact of baseline HBV loads in HCC patients receiving anti-PD-1 immunotherapy. We found that the baseline viral load had no significant impact on the prognostic outcomes or rates of hepatic impairment in HCC patients treated with anti-PD-1 blockade. Receiving antiviral therapy could prolong the OS of patients with high viral loads. These findings have clinical values since most HCC patients are infected with HBV, especially in endemic regions.26
In the Asian cohort of the CheckMate-040 study, the survival was similar among patients with different HCC etiologies.27 Similarly, the KETNOTE-224 study reported comparable efficacy of pembrolizumab in HCC patients with and without hepatic virus infection.16 Yau et al evaluated the efficacy of Nivolumab plus Ipilimumab in patients with advanced HCC, and the OS was similar between patients with and without HBV infection (22.8 months vs 22.2 months).19 The rates of AEs were similar between HCC patients with HBV/HCV infection and those without during treatment with nivolumab or pembrolizumab.15,16 However, HCC patients with HBV were required to have a baseline HBV load < 100 IU/mL for eligibility in these studies, and whether the baseline viral load had an impact on clinical outcomes was not assessed. According to our results, most (53.9%) HCC clinical trials excluded patients with relatively high baseline HBV loads. However, evidence is scarce on whether different viral loads affect the efficacy and safety of anti-PD-1 blockade. Inconsistent and stringent exclusion criteria, especially for the HBV DNA level, can exclude a considerable number of patients from enrollment. Considering that HBV is one of the major etiologies of HCC, this exclusion criterion may limit the efficiency and generalizability of trials.
Although viral loads are reported to affect the efficacy of ICIs in some malignancies such as gastric and anal squamous carcinoma, our study found no significant association between survival and HBV DNA level in HCC patients.28,29 Publications have reported that the expression of PD-1 is positively correlated with HBV DNA levels, and ICIs can enhance the antiviral immunity.5,30 Therefore, viral antigens in the tumor immune microenvironment may interfere with the antitumor effects of ICIs. However, unlike other viruses, HBV can integrate into the genome of both HCC cells and hepatocytes, which may potentially impede virus-specific immune cells from discriminating infected tumor cells and hepatocytes. The antitumor ability of ICIs may depend on other carcinogenetic processes rather than on HBV-related cascades.31 Ho et al have found that viral etiology has no effect on the tumor immune microenvironment, and viral status should not be adopted as a criterion for ICI medication.31 Since viral status had no impact on the efficacy of ICIs, it can be inferred that viral load may not compromise the efficacy of anti-PD-1 blockade, which was supported by our data.
Although physicians are concerned about whether anti-PD-1 blockade has a significant influence on HBV reactivation and hepatic impairment, our study provides the first statistical analysis accordingly. The incidence rate of HBV reactivation was 1.4% in our study, which was similar to the incidence rate (1.6%) reported by Zhang et al.32 Although blocking PD-1 can improve the antiviral immunity of HBV-specific CD8+ T cells, it can also promote the proliferation of T regulatory cells which may increase immunosuppression and lead to HBV reactivation.33,34 Since the theoretical influence of blocking PD-1 on HBV remains contradictory, whether a higher viral load may increase the risk of HBV reactivation should be further explored. The CheckMate-040 and KETNOTE-224 studies have shown that the rates of AEs were similar across virus etiologies, but the sample size of HCC patients with HBV infection is limited in these trials.15–17 Patients with viral hepatitis can also tolerate ICIs in other cancers such as melanoma and non-small cell lung cancer.35–37 However, these reports are mainly case series. In addition, previous studies mainly focused on the status of HBV infection but did not specifically evaluate the impact of viral load.38,39 Our study became the first systemic assessment of the safety of anti-PD-1 immunotherapy in patients with HBV-related HCC with adequate number of patients, and we demonstrated similar rates of hepatic impairment between patients with low and high baseline HBV DNA levels.
This study also found that antiviral therapy could prolong the OS of HCC patients receiving anti-PD-1 immunotherapy whose baseline viral loads were relatively high. Studies have reported that antiviral therapy can prolong the survival of HCC patients receiving hepatectomy or sorafenib.7,10 Receiving antiviral therapy can partly relieve or delay the deterioration of liver function to improve the survival. Intriguingly, one of our patients suffering from HBV reactivation had undetectable baseline HBV DNA levels and did not receive antiviral therapy during treatment. This indicates that patients with positive HBsAg may need to receive antiviral therapy during anti-PD-1 immunotherapy, regardless of the HBV DNA level. Although antiviral therapy can improve the clinical outcomes of HCC patients, the survival of advanced HCC patients is still relatively poor. Potential assisting targets should be explored for precise therapy in HCC patients in terms of microRNAs, DNA methylation and the tumor microenvironment.40 In addition to ICIs, other modalities of immunotherapy, such as dendritic cell vaccination and immunomodulatory treatments, may assist current clinical practice strategies for improving the outcomes of HCC patients.41
There are several limitations in this study except for its retrospective nature. First, some baseline characteristics were not well balanced between groups with different viral loads, so we assessed the impact of baseline viral loads in subgroups stratified by each variable. Second, all the patients in this study only received anti-PD-1 blockade, and whether our results can apply to other ICIs needs further exploration. Third, HCC patients with hepatitis C virus were excluded from this study, and the influence of viral loads on these patients remains unclear. Fourth, as a retrospective study, further multicenter validation is expected.
Conclusions
In conclusion, HBV loads did not affect the clinical outcomes of HCC patients receiving anti-PD-1 blockade therapy. Antiviral therapy could improve the OS of patients with high HBV loads. Patients with positive HBsAg should receive antiviral therapy regardless of their viral loads.
Acknowledgments
The authors thank American Journal Experts for English language polishing.
Funding Statement
This study received no funding support.
Abbreviations
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; PD-1, programmed death-1; TACE, transarterial chemoembolization; ICIs, immune checkpoint inhibitors; OS, overall survival; PFS, progression-free survival; AEs, adverse events; BCLC, the Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; ALBI, albumin-bilirubin.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author Yaojun Zhang on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the institutional review board of Sun Yat-sen University Cancer Center as a retrospective study, and the requirement for informed consent was waived. All procedures performed in studies involving human participants were in accordance with the ethical standards of the1964 Helsinki declaration and its later amendments.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–261. doi: 10.1016/j.jhep.2019.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim CJ, Lee YH, Pan L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68(5):916–927. doi: 10.1136/gutjnl-2018-316510 [DOI] [PubMed] [Google Scholar]
- 5.Hou FQ, Wu XJ, Wang Y, et al. Rapid downregulation of programmed death-1 and interferon-gamma-inducible protein-10 expression is associated with favourable outcome during antiviral treatment of chronic hepatitis B. J Viral Hepat. 2013;20(Suppl 1):18–26. doi: 10.1111/jvh.12060 [DOI] [PubMed] [Google Scholar]
- 6.Peng G, Luo B, Li J, et al. Hepatitis B e-antigen persistency is associated with the properties of HBV-specific CD8 T cells in CHB patients. J Clin Immunol. 2011;31(2):195–204. doi: 10.1007/s10875-010-9483-5 [DOI] [PubMed] [Google Scholar]
- 7.Chen JL, Lin XJ, Zhou Q, Shi M, Li SP, Lao XM. Association of HBV DNA replication with antiviral treatment outcomes in the patients with early-stage HBV-related hepatocellular carcinoma undergoing curative resection. Chin J Cancer. 2016;35:28. doi: 10.1186/s40880-016-0089-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn W, Paik YH, Kim JM, et al. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol. 2014;21(7):2429–2435. doi: 10.1245/s10434-014-3621-x [DOI] [PubMed] [Google Scholar]
- 9.Li ZL, Yan WT, Zhang J, et al. Identification of actual 10-year survival after hepatectomy of HBV-related hepatocellular carcinoma: a multicenter study. J Gastrointest Surg. 2019;23(2):288–296. doi: 10.1007/s11605-018-4006-4 [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Wen F, Li J, et al. A high baseline HBV load and antiviral therapy affect the survival of patients with advanced HBV-related HCC treated with sorafenib. Liver Int. 2015;35(9):2147–2154. doi: 10.1111/liv.12805 [DOI] [PubMed] [Google Scholar]
- 11.Yu SJ, Lee JH, Jang ES, et al. Hepatocellular carcinoma: high hepatitis B viral load and mortality in patients treated with transarterial chemoembolization. Radiology. 2013;267(2):638–647. doi: 10.1148/radiol.13121498 [DOI] [PubMed] [Google Scholar]
- 12.Lim S, Han J, Kim GM, Han KH, Choi HJ. Hepatitis B viral load predicts survival in hepatocellular carcinoma patients treated with sorafenib. J Gastroenterol Hepatol. 2015;30(6):1024–1031. doi: 10.1111/jgh.12898 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Zhong X, Chen ZH, et al. Hepatitis B virus DNA negativity acts as a favorable prognostic factor in hepatocellular carcinoma patients. Asian Pac J Cancer Prev. 2014;15(22):9635–9641. doi: 10.7314/APJCP.2014.15.22.9635 [DOI] [PubMed] [Google Scholar]
- 14.Wang ZY, Tao QF, Wang ZH, et al. Antiviral therapy improves post-operative survival outcomes in patients with HBV-related hepatocellular carcinoma of less than 3cm - A retrospective cohort study. Am J Surg. 2020;219(4):717–725. doi: 10.1016/j.amjsurg.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 15.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 17.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- 18.Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72(2):307–319. doi: 10.1016/j.jhep.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 19.Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020. doi: 10.1001/jamaoncol.2020.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu XD, Sun HC. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol. 2019;12(1):110. doi: 10.1186/s13045-019-0794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol. 2019;9:1157. doi: 10.3389/fonc.2019.01157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253(3):453–469. doi: 10.1097/SLA.0b013e31820d944f [DOI] [PubMed] [Google Scholar]
- 23.Rich NE, Yopp AC, Singal AG. Medical management of hepatocellular carcinoma. J Oncol Pract. 2017;13(6):356–364. doi: 10.1200/JOP.2017.022996 [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Common Terminology Criteria for Adverse Events. November 2017.
- 25.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142(12):2471–2477. doi: 10.1002/ijc.31280 [DOI] [PubMed] [Google Scholar]
- 27.Yau T, Hsu C, Kim TY, et al. Nivolumab in advanced hepatocellular carcinoma: sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71(3):543–552. doi: 10.1016/j.jhep.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Zhang F, Zhou N, et al. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: a systematic review and meta-analysis. Oncoimmunology. 2019;8(5):e1581547. doi: 10.1080/2162402X.2019.1581547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balermpas P, Martin D, Wieland U, et al. Human papilloma virus load and PD-1/PD-L1, CD8(+) and FOXP3 in anal cancer patients treated with chemoradiotherapy: rationale for immunotherapy. Oncoimmunology. 2017;6(3):e1288331. doi: 10.1080/2162402X.2017.1288331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–88. doi: 10.1016/j.jhep.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 31.Ho WJ, Danilova L, Lim SJ, et al. Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma. J Immunother Cancer. 2020;8(1):e000394. doi: 10.1136/jitc-2019-000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7(1):322. doi: 10.1186/s40425-019-0808-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45(4):963–970. doi: 10.1016/j.molimm.2007.07.038 [DOI] [PubMed] [Google Scholar]
- 34.Trehanpati N, Vyas AK. Immune regulation by T regulatory cells in hepatitis B virus-related inflammation and cancer. Scand J Immunol. 2017;85(3):175–181. doi: 10.1111/sji.12524 [DOI] [PubMed] [Google Scholar]
- 35.Pertejo-Fernandez A, Ricciuti B, Hammond SP, et al. Safety and efficacy of immune checkpoint inhibitors in patients with non-small cell lung cancer and hepatitis B or hepatitis C infection. Lung Cancer. 2020;145:181–185. doi: 10.1016/j.lungcan.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 36.Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: a case series. Melanoma Res. 2018;28(2):155–158. doi: 10.1097/CMR.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 37.Tio M, Rai R, Ezeoke OM, et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer. 2018;104:137–144. doi: 10.1016/j.ejca.2018.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah NJ, Al-Shbool G, Blackburn M, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer. 2019;7(1):353. doi: 10.1186/s40425-019-0771-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapia Rico G, Chan MM, Loo KF. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): a review of the available evidence. Cancer Treat Rev. 2020;86:102011. doi: 10.1016/j.ctrv.2020.102011 [DOI] [PubMed] [Google Scholar]
- 40.Gnoni A, Santini D, Scartozzi M, et al. Hepatocellular carcinoma treatment over sorafenib: epigenetics, microRNAs and microenvironment. Is there a light at the end of the tunnel? Expert Opin Ther Targets. 2015;19(12):1623–1635. doi: 10.1517/14728222.2015.1071354 [DOI] [PubMed] [Google Scholar]
- 41.Longo V, Gnoni A, Casadei Gardini A, et al. Immunotherapeutic approaches for hepatocellular carcinoma. Oncotarget. 2017;8(20):33897–33910. doi: 10.18632/oncotarget.15406 [DOI] [PMC free article] [PubMed] [Google Scholar]