Abstract
Background:
The utility of dermal regeneration templates for treating high-risk diabetic foot wounds is unclear. The authors report wound healing and major amputation outcomes among a cohort of diabetic patients with complex diabetic foot wounds treated in a multidisciplinary setting.
Methods:
All patients with complex diabetic foot wounds treated with a dermal regeneration template (March of 2013 to February of 2019) were captured in a prospective institutional database. Wound severity was classified according to the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system to determine limb salvage prognosis at baseline. Wound healing and major amputation rates were reported using Kaplan-Meier analyses. A stepwise Cox proportional hazards model was used to identify independent characteristics associated with wound healing.
Results:
Eighty-five patients with 107 complex diabetic foot wounds were treated (mean age, 61.2 ± 3.3 years; 63.5 percent male and 61.2 percent African American). Most diabetic foot wounds were high-risk (wound, ischemia, and foot infection stage 3 or 4, 93.5 percent), corresponding to a predicted 25 to 50 percent risk of major amputation at 1 year. Dermal regeneration template use resulted in successful wound granulation in 66.7 percent of cases, with a mean time to complete wound healing of 198 ± 18 days. Twelve- and 18-month wound healing rates were 79.0 ± 5.0 percent and 93.0 ± 3.3 percent, respectively. Major amputation was required in 11.2 percent of patients. Independent predictors of poor wound healing included lack of bone involvement, higher WIfI stage, and prior dermal regeneration template failure.
Conclusion:
Application of a dermal regeneration template to complex diabetic foot wounds at high risk for major amputation results in good wound healing and excellent limb salvage outcomes among diabetic patients treated in a multidisciplinary setting. (Plast. Reconstr. Surg. 146: 893, 2020.)
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, IV.
Diabetic foot ulcers are one of the most prevalent, costly, and debilitating complications of diabetes mellitus. The 5-year risk of death in adults with diabetes with a diabetic foot ulcer is estimated to be 2.5 times greater compared with those without.1 With a high risk of recalcitrance and infection, proper control can be difficult to achieve.2 Lower extremity amputation is often inevitable as the disease continues to progress3 but is itself linked with postoperative decreased quality of life and increased 5-year mortality risk.1,2 Thus, there is great interest in identifying effective treatment strategies in the management of diabetic foot ulcers.
Integra Dermal Regeneration Template (Integra LifeSciences Holdings Corp., Plainsboro, N.J.) is a synthetic collagen bilayer matrix that serves as a dermal template to promote endogenous wound healing. It is constructed of two layers, the outer being a thin silicone film that acts similar to epidermis, and the inner being a complex matrix of cross-linked fibers that acts as a scaffold for regenerating dermal skin cells.4 Initially approved by the U.S. Food and Drug Administration in 1996 for burn injuries,5,6 the dermal regeneration template has since been applied to enhance wound healing in a variety of settings.7–10 Recently, the use of dermal regeneration templates and other collagen-based wound dressings has been reported to be an effective tool in the management of diabetic foot ulcers.11,12 For example, Iorio et al. demonstrated efficacy using a dermal regeneration template for the reconstruction and closure of diabetic foot ulcer at low risk for amputation.5 However, the utility of dermal regeneration templates for treating high-risk diabetic foot ulcers and complex diabetic foot wounds is less clear, and there are limited studies examining the specific elements that contribute to its efficacy in a large cohort. The aim of our study was to report wound healing and major amputation outcomes among a cohort of diabetic patients with complex postsurgical diabetic foot wounds, and to characterize the patient and disease factors associated with diabetic foot wounds healing using a dermal regeneration template.
PATIENTS AND METHODS
Study Cohort
We included all patients who underwent débridement of a diabetic foot ulcer or gangrene resulting in a complex postsurgical diabetic foot wound that was treated with a dermal regeneration template from March of 2013 to February of 2019 by the Johns Hopkins Hospital Multidisciplinary Limb Preservation Team. Our team consults on all inpatient and outpatient diabetic foot ulcers in our hospital system, and thus treats a wide range of wounds, from simple to complex. Patients without diabetic foot wounds, those treated with débridement and primary closure, and those treated with negative-pressure wound therapy without dermal regeneration template placement were excluded from the analysis.
The Johns Hopkins Medicine Institutional Review Board approved the study. All patients treated by the Johns Hopkins Hospital Multidisciplinary Limb Preservation Team sign informed consent to allow their information to be entered into a secure database. Patient demographic information, comorbidities, wound characteristics, treatment details, and long-term outcomes are entered into the database in a prospective fashion.
Multidisciplinary Limb Preservation Team
Our multidisciplinary limb preservation team has been described previously,13 and consists of a vascular surgeon, a surgical podiatrist, an endocrinologist, a dedicated physician assistant, a wound care nurse, and a prosthetist. There is also a plastic surgery attending physician on the team who is involved on an inpatient basis as needed. Infectious diseases and orthopedic foot and ankle consultants are also engaged as necessary on a case-by-case basis.
Briefly, all patients presenting with a diabetic foot ulcer or gangrene are evaluated by all members of the team on their initial presentation, and diagnostic studies including noninvasive vascular laboratory studies and radiographs of the affected foot are obtained. Magnetic resonance imaging is used on a case-by-case basis if necessary to determine the presence of osteomyelitis when the radiographs are unclear. When the vascular laboratory studies show evidence of peripheral artery disease, lower extremity revascularization is performed first except in cases of advanced infection. Following this, wound débridement to healthy tissue and/or bone is performed. In cases of large defects where primary closure of the wound is not possible following débridement, a dermal regeneration template cut to the size of the wound is applied. Free flaps are used infrequently in our patient population because of heavy calcific disease of the tibial vessels that is common among adults with diabetes. Following placement of the dermal regeneration template, the wound is dressed either with a negative-pressure wound therapy device or a bolster dressing. A silver restore contact layer is placed between the wound and the dressing in both cases. We primarily use negative-pressure wound therapy except in cases with surrounding soft-tissue maceration or concern for ongoing hemostasis that may lead to hematoma formation.
All patients are initially treated with broad-spectrum antibiotics, which are ultimately tailored based on their intraoperative wound cultures. Intraoperative cultures of both predebridement and postdebridement tissue and bone are sent to provide complete information about the organisms involved. For those patients with osteomyelitis, all wounds involving the metatarsal head or more proximally are treated with 6 weeks of parenteral antibiotics that are prescribed in accordance with infectious diseases recommendations. Wounds with osteomyelitis limited to the phalanx or metatarsophalangeal joint and those with only soft-tissue involvement are treated with 2 weeks of oral antibiotics.
Following débridement and dermal regeneration template application, patients are discharged to a rehabilitation center or to home with home nursing, depending on the functional status of the patient and their ability to manage their prescribed antibiotics and wound care at home. Dressing changes are performed at the facility or by a trained home nurse three times per week for both negative-pressure wound therapy and bolster dressings. The wounds are offloaded to allow for the best outcome possible. Plantar wounds are offloaded with non-weight-bearing status until sutures are removed and we have confirmed that the dermal regeneration template has incorporated into the wound. Subsequently, offloading is achieved with boots or wedge shoes that are provided to limit joint mobility and motion of the graft. Hyperbaric oxygen is prescribed only to patients with ischemia caused by microvascular compromise or macrovascular compromise without an option for lower extremity revascularization. Patients must have an improvement in their transcutaneous oxygen pressure measurements with hyperbaric oxygen treatment to qualify for therapy. After 3 to 4 weeks, depending on the status of the wound, the silicone layer of the dermal regeneration template is removed and local wound care is begun. The choice of local wound care products varies from visit to visit based on the characteristics of the wound. This is continued until secondary closure is achieved or, in patients with larger wounds, formation of a vascularized wound neodermis with punctate bleeding is observed and split-thickness skin grafts are applied.
During the postoperative period, all patients are followed closely by the multidisciplinary limb preservation team. Initially, they are evaluated in our multidisciplinary clinic on a weekly or biweekly basis. As the wound progresses, visits are spaced out accordingly. Once wound healing is achieved, patients are monitored with outpatient surveillance visits according to the Comprehensive Foot Examination and Risk Assessment Guidelines.14,15
Wound Definitions and Outcomes
On initial presentation, all wounds were graded according to the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system16 based on provider consensus. The WIfI score has been shown to correlate with both wound healing and 1-year major amputation risk in multiple settings.13,16–22 Based on the initial WIfI description, estimated 1-year major amputation rates are 3 percent for WIfI stage 1 wounds, 8 percent for stage 2 wounds, 25 percent for stage 3 wounds, and 50 percent for stage 4 wounds.16
The primary outcomes of the study were dermal regeneration template success and complete wound healing. Dermal regeneration template success was defined as successful complete granulation of the wound. Wound healing was defined as complete epithelialization with the restoration of sustained functional and anatomical continuity for 6 weeks after complete healing.23,24
Statistical Analysis
All data are summarized as mean ± SEM or number (percent) as appropriate. Baseline demographic and comorbidity data are reported on a per-patient basis. Wound and treatment characteristics and wound outcomes are reported on a per-wound basis. Dermal regeneration template success and major amputation rates are descriptive, and reported in a binary fashion. Wound healing rates were reported using Kaplan-Meier analyses with life tables. We then used univariable and multivariable Cox proportional hazards models to assess the association of various wound characteristics with wound healing. The covariates included in the multivariable model were chosen using a forward stepwise multivariable model with p (enter) = 0.20 and p (leave) = 0.10. Analyses were performed using Stata version 14.0 (StataCorp, College Station, Texas). A significance level of p < 0.05 was used for all comparisons.
RESULTS
Study Cohort
Eighty-five patients with complex postsurgical diabetic foot wounds were treated during the study period (Table 1). Mean patient age was 61.2 ± 3.3 years, 54 (63.5 percent) were male, and 52 (61.2 percent) were African American. The majority of patients [n = 80 (94.1 percent)] had type 2 diabetes, and the mean baseline hemoglobin A1c value was 8.94 ± 0.33 percent. Nearly all patients [n = 84 (98.8 percent)] had loss of peripheral sensation, and retinopathy affected 26 (30.6 percent). Hypertension [n = 76 (89.4 percent)], peripheral artery disease [n = 59 (69.4 percent)], and dyslipidemia [n = 58 (68.2 percent)] were the most common comorbidities, followed by coronary artery disease [n = 30 (35.3 percent)], chronic kidney disease [n = 23 (27.1 percent)], and congestive heart failure [n = 18 (21.2 percent)].
Table 1.
Demographics and Baseline Characteristics of Complex Diabetic Foot Wound Patients Treated with a Dermal Regeneration Template
| Variable | Overall (%) |
|---|---|
| No. | 85 |
| Mean age ± SD, yr | 61.2 ± 3.3 |
| Male sex | 54 (63.5) |
| Race | |
| White | 30 (35.3) |
| African American | 52 (61.2) |
| Other/unknown | 3 (3.5) |
| Insurance status | |
| Medicare/Medicaid | 65 (76.5) |
| Private/self-pay | 19 (22.4) |
| Other | 1 (1.2) |
| Type of DM | |
| Type 1 | 5 (5.9) |
| Type 2 | 80 (94.1) |
| Mean baseline hemoglobin A1c ± SD, % | 8.94 ± 0.33 |
| Comorbidities | |
| HTN | 76 (89.4) |
| Dyslipidemia | 58 (68.2) |
| CAD | 30 (35.3) |
| CHF | 18 (21.2) |
| CVD | 12 (14.1) |
| PAD | 59 (69.4) |
| CKD | 23 (27.1) |
| Dialysis | 16 (18.8) |
| Retinopathy | 26 (30.6) |
| LOPS | 84 (98.8) |
| Gastroparesis | 4 (4.7) |
| COPD | 4 (4.7) |
| Renal transplant | 9 (10.6) |
| Smoking status | |
| Current | 16 (18.8) |
| Former | 29 (34.1) |
| Never | 40 (47.1) |
DM, diabetes mellitus; HTN, hypertension; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cerebrovascular disease; PAD, peripheral arterial disease; CKD, chronic kidney disease; LOPS, loss of protective sensation; COPD, chronic obstructive pulmonary disease.
Wound and Treatment Characteristics
A dermal regeneration template was used to treat 107 complex diabetic foot wounds overall (Table 2). The mean wound area and depth were 30.9 ± 2.8 cm2 and 1.24 ± 0.08 cm, respectively. Approximately half [n = 49 (45.8 percent)] of dermal regeneration template applications involved wounds on the forefoot, followed by the heel [n = 25 (23.4 percent)], midfoot [n = 21 (19.6 percent)], ankle [n = 3 (5.6)], and lower leg/Achilles tendon [n = 6 (5.6 percent)]. Bone involvement because of acute or chronic osteomyelitis occurred in 76 (71.7 percent). Nearly all of the diabetic foot wounds treated were at high risk for major amputation; 71 (66.4 percent) were WIfI stage 4 wounds, 29 (27.1 percent) were stage 3 wounds, and only seven (6.5 percent) were stage 1 or 2 wounds.
Table 2.
Wound and Treatment Characteristics of Complex Diabetic Foot Wound Patients Treated with a Dermal Regeneration Template
| Variable | Wound (%) |
|---|---|
| No. | 107 |
| Wound characteristics | |
| Mean area ± SD, cm2 | 30.9 ± 2.8 |
| Mean depth ± SD, cm | 1.24 ±0.08 |
| Wound location | |
| Leg | 3 (2.8) |
| Ankle | 6 (5.6) |
| Achilles | 3 (2.8) |
| Heel | 25 (23.4) |
| Midfoot | 21 (19.6) |
| Forefoot | 49 (45.8) |
| Bone involvement | |
| Yes | 76 (71.7) |
| No | 30 (28.3) |
| WIfI stage | |
| 1 | 3 (2.8) |
| 2 | 4 (3.7) |
| 3 | 29 (27.1) |
| 4 | 71 (66.4) |
| Treatment characteristics | |
| Plantar dermal regeneration template location | 34 (31.8) |
| Dressing | |
| NPWT | 88 (82.2) |
| Bolster | 19 (17.8) |
| Hyperbaric oxygen | |
| Yes | 12 (11.2) |
| No | 95 (88.8) |
| Split-thickness skin graft | 16 (15.1) |
WIfI, Wound, Ischemia, and foot Infection; NPWT, negative-pressure wound therapy.
In most cases [n = 88 (82.2 percent)], a negative-pressure wound therapy dressing was used postoperatively. The remaining 19 patients (17.8 percent) received a bolster dressing. Hyperbaric oxygen was prescribed in only 12 cases (11.2 percent).
Wound Outcomes
The overall success rate for all initial dermal regeneration template applications was 66.7 percent (n = 72) (Table 3). The majority of wounds [n = 87 (81.3 percent)] received only one dermal regeneration template application, although 17 (15.9 percent) received two and three (2.8 percent) received three applications. The mean time to complete wound healing was 198 ± 18 days overall. Sixteen wounds (15.1 percent) underwent split-thickness skin graft placement at a mean time of 83.3 ± 14.3 days. Based on Kaplan-Meier analysis, the estimated 12-month and 18-month wound healing rates were 79.0 ± 5.0 percent and 93.0 ± 3.3 percent, respectively (Fig. 1). Among those wounds that underwent multiple dermal regeneration template applications, the estimated 12-month wound healing rate was 67.4 ± 13.1 percent. Major amputations were required for only 12 wounds (11.2 percent).
Table 3.
Wound Outcomes for Complex Diabetic Foot Wound Patients Treated with a Dermal Regeneration Template
| Variable | No. (%) |
|---|---|
| No. of wounds | 107 |
| Dermal regeneration template success | 72 (66.7) |
| No. of dermal regeneration template applications | |
| 1 | 87 (81.3) |
| 2 | 17 (15.9) |
| 3 | 3 (2.8) |
| Major amputation | 12 (11.2) |
| Mean time to wound healing ± SD, days | 198 ± 18 |
| Mean time to split-thickness skin graft ± SD, days | 83.3 ± 14.3 |
| Mean 12-mo wound healing ± SD, % | 79.0 ± 5.0 |
| Mean 18-mo wound healing ± SD, % | 93.0 ± 3.3 |
Fig. 1.
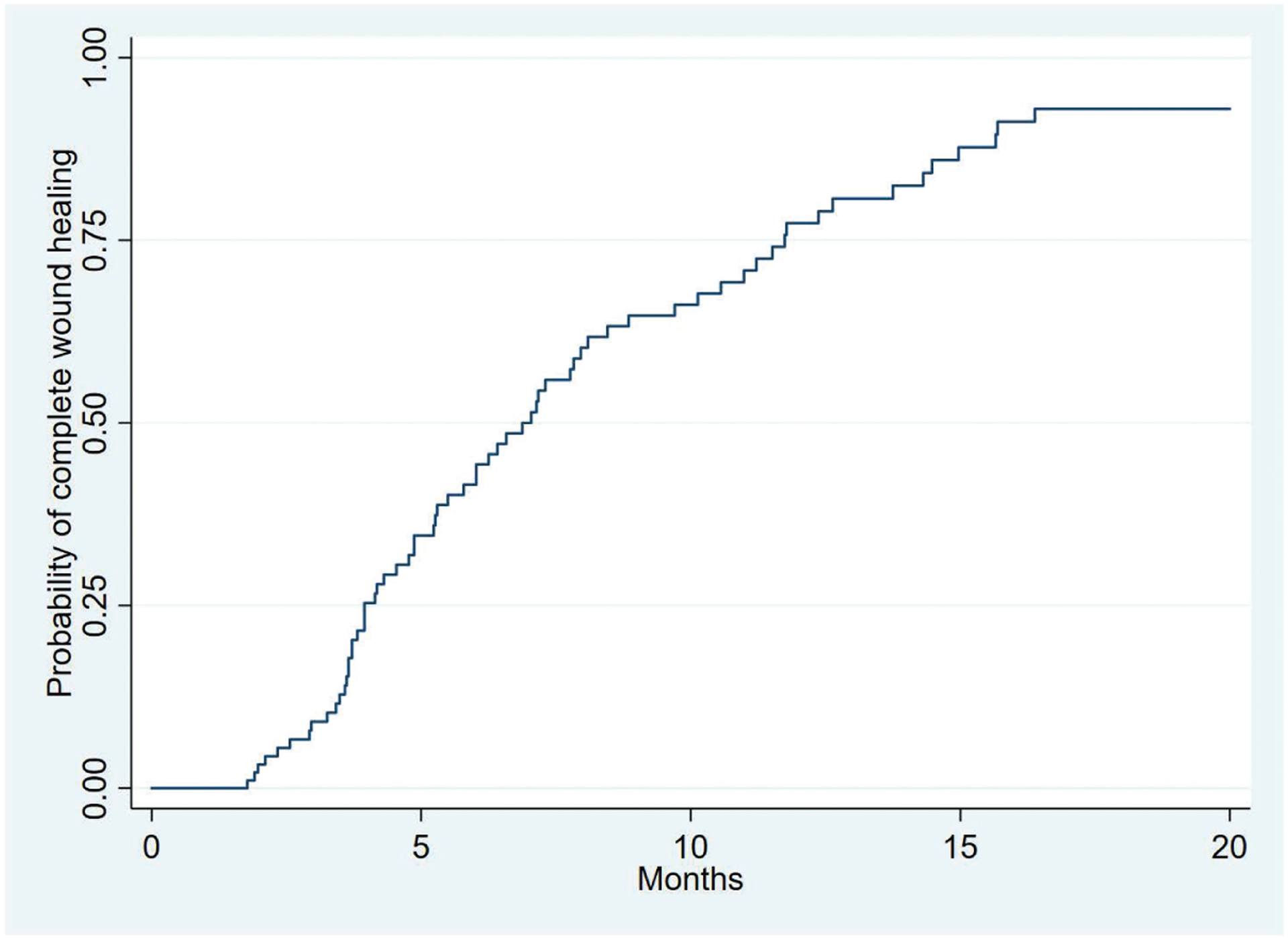
Kaplan-Meier curve demonstrating complete wound healing following dermal regeneration template application for the treatment of complex diabetic foot wounds. The standard error of the curve is less than 10 percent for all reported values.
Factors Associated with Wound Healing following Dermal Regeneration Template Application
Based on univariable Cox proportional hazards models (Table 4), wound characteristics that were significantly associated with wound healing included forefoot location (hazard ratio, 5.25; 95 percent CI, 1.22 to 22.6) and bone involvement (hazard ratio, 1.86; 95 percent CI, 1.04 to 3.33). Wounds located on the heel had a slightly higher likelihood of healing (hazard ratio, 4.08; 95 percent CI, 0.88 to 19.0), whereas larger wounds had a slightly lower likelihood of healing (hazard ratio, 0.99 per cm2; 95 percent CI, 0.98 to 1.00 per cm2), although these were not statistically significant. No treatment characteristics, including dressing type or use of hyperbaric oxygen, were significantly associated with wound healing (all, p ≥ 0.16) (Table 4).
Table 4.
Univariable Analysis of Factors Associated with Wound Healing
| Variable | HR (95% CI) | P |
|---|---|---|
| Wound characteristics | ||
| Mean area (per cm2) | 0.99 (0.98–1.00) | 0.06 |
| Wound location | ||
| Leg/ankle | 2.66 (0.53–13.4) | 0.24 |
| Achilles | Ref | — |
| Heel | 4.08 (0.88–19.0) | 0.07 |
| Midfoot | 2.83 (0.61–13.2) | 0.19 |
| Forefoot | 5.25 (1.22–22.6) | 0.03 |
| Bone involvement | ||
| Yes | 1.86 (1.04–3.33) | 0.04 |
| No | Ref | — |
| WIfI stage | ||
| 1 | Ref | — |
| 2 | 1.08 (0.19–6.01) | 0.94 |
| 3 | 0.88 (0.20–3.84) | 0.86 |
| 4 | 0.73 (0.18–3.04) | 0.67 |
| Treatment characteristics | ||
| Plantar dermal regeneration template location | 0.67 (0.38–1.18) | 0.16 |
| Dressing | ||
| NPWT | 0.84 (0.40–1.77) | 0.64 |
| Bolster | Ref | |
| Hyperbaric oxygen | ||
| Yes | 0.84 (0.40–1.78) | 0.63 |
| No | Ref | |
| Split-thickness skin graft | 1.51 (0.82–2.77) | 0.19 |
| Prior dermal regeneration template failure | 0.73 (0.42–1.26) | 0.26 |
HR, hazard ratio; Ref, reference; WIfI, Wound, Ischemia, and foot Infection; NPWT, negative-pressure wound therapy.
Based on stepwise multivariable Cox proportional hazards modeling (Table 5), bone involvement was independently associated with a higher likelihood of wound healing (hazard ratio, 2.36; 95 percent CI, 1.26–4.42), whereas increasing WIfI stage was associated with a lower likelihood of wound healing (hazard ratio, 0.69 per stage increase; 95 percent CI, 0.47 to 0.99). Prior dermal regeneration template failure was also associated with a lower likelihood of wound healing (hazard ratio, 0.61; 95 percent CI, 0.35 to 1.07), although this was not statistically significant because of wide confidence intervals.
Table 5.
Multivariable Analysis of Factors Associated with Wound Healing
| Variable | HR (95% CI) | P |
|---|---|---|
| Bone involvement | 2.36 (1.26–4.42) | 0.008 |
| WIfI stage (per stage increase) | 0.69 (0.47–0.99) | 0.049 |
| Prior dermal regeneration template failure | 0.61 (0.35–1.07) | 0.08 |
HR, hazard ratio; WIfI, Wound, Ischemia, and foot Infection.
DISCUSSION
A dermal regeneration template is a collagen-based dressing that may be a valuable tool in limb-salvage therapy for diabetic foot ulcers and complex diabetic foot wounds. Although previously shown to have good results when applied to diabetic foot wounds at low-risk for amputation,5 the efficacy of dermal regeneration templates for treating complex diabetic foot wounds at high risk for amputation is less clear. In the current study, we quantified dermal regeneration template success rates, wound healing times, and major amputation rates for 107 complex postsurgical diabetic foot wounds treated with a dermal regeneration template in a multidisciplinary setting. Despite more than 90 percent of the treated diabetic foot wounds being WIfI stage 3 and 4 wounds, dermal regeneration template placement was successful in 67 percent of cases, and only 11 percent needed subsequent major amputation. Twelve-month wound healing rates were nearly 80 percent, and 18-month wound healing rates were 93 percent. Considered together, these data suggest that a dermal regeneration template can be applied successfully to high-risk diabetic foot wounds in select patients.
The rate of success we report with dermal regeneration template use in this study is higher than previously described.5,11,12,25 For example, Iorio et al. reported limb-salvage rates of 83 percent and 46 percent for diabetic foot ulcers at low-risk and high-risk for major amputation, respectively.5 In that study, amputation risk assessment was based on positive bone cultures following débridement or the presence of severe peripheral vascular disease. In our study, osteomyelitis was common (72 percent), as was peripheral artery disease (69 percent). Furthermore, we used an internationally validated risk validation tool—the WIfI classification system16—to quantify risk of major amputation. Despite 66 percent of the diabetic foot wounds we treated having an estimated major amputation risk of 50 percent by 1 year,16 we achieved limb salvage in 89 percent of cases overall. Our data demonstrate a substantial decrease in need for major amputation among high-risk wounds, perhaps because of our use of an aggressive revascularization approach and a multidisciplinary care model where patients reap the benefit of more frequent follow-up and coordinated care by experts in a variety of pertinent specialties.13,20
Notably, Driver et al. reported 51 percent complete wound closure by 12 weeks (84 days) in a large randomized controlled trial designed to evaluate the safety and efficacy of dermal regeneration templates for the treatment of nonhealing diabetic foot ulcers.25 We report a higher overall dermal regeneration template success rate of 67 percent, but at a longer mean wound healing time of 198 days. However, the trial excluded wounds with osteomyelitis and inadequate vascular perfusion, and was limited to wounds between 1 and 12 cm2 in area.25 The mean wound area in our study was 31 cm2, which was more than twice the size. The wound healing times that we report are consistent with existing literature regarding high-risk diabetic foot wounds.20 Although longer wound healing times may be costly, our group has previously demonstrated that even prolonged wound healing in advanced stage wounds can be profitable and achieve good outcomes for the patient.26
Using multivariable analysis, we demonstrate that the utility of a dermal regeneration template for treating complex diabetic foot wounds is highly influenced by wound properties. WIfI stages 3 and 4 have been associated with negative outcomes in multiple prior studies, including poor wound healing and increased rates of major amputation.13,16–22 This is consistent with our finding that increasing WIfI stage is associated with a lower likelihood of wound healing, and may be attributable to factors such as compromised vascular supply and increased bacterial contamination in higher risk wounds. Prior failure to achieve complete granulation with a dermal regeneration template was another weaker predictor of impaired healing. Although we attempted to use multiple dermal regeneration template applications to accomplish healing in some patients, this finding suggests that perhaps other treatment modalities should be considered if this approach has already failed. Given the prolonged wound healing time and the relatively high product cost27 associated with dermal regeneration templates, a more comprehensive cost-to-benefit analysis would be beneficial to fully understand these systemic implications.
Interestingly, we also found that bony involvement was associated with better wound healing with a dermal regeneration template based on our multivariable analysis. This was unexpected given that bone exposure in diabetic foot ulcers has long been considered an indicator of severe disease because of an increased depth of ulceration and increased likelihood of concurrent osteomyelitis,16,28,29 which puts patients at higher risk for nonhealing and major amputation.5,16 Driver et al. specifically excluded diabetic foot ulcers with exposed bone from their randomized controlled trial of dermal regeneration templates because of concerns about impaired wound healing,25 although there has been a subsequent observational study that demonstrated no correlation between bone exposure and limb salvage in all-risk diabetic foot ulcers.5 There are a number of reasons why we may have observed surprisingly good outcomes among patients with complex diabetic foot wounds and bone involvement. First, wounds with bony involvement may be more aggressively débrided in the operating room than soft-tissue wounds. Second, our multidisciplinary limb preservation service typically prescribes 6 weeks of intravenous antibiotic therapy if osteomyelitis is present at the level of the metatarsal or more proximally, which enhances systemic control and limits disease spread in limb-salvaging management. This is prescribed even if clear surgical margins are achieved on the presumption that there is persistent microscopic residual infection. Third, most bone involvement in diabetic foot ulcers occurs in relation to forefoot wounds, which have been shown to have better healing compared to the midfoot and heel in this study and in previous studies.30 Finally, in our experience, soft-tissue diabetic foot wounds extensive enough to warrant dermal regeneration template placement are usually extremely large or associated with a long history of nonhealing attributable to either their location or other patient/disease factors. Therefore, there is likely some selection bias in terms of which soft-tissue wounds we considered for dermal regeneration template placement. Other studies have shown good outcomes with dermal regeneration template application to smaller soft-tissue wounds5,25 that would suggest it may be beneficial to expand our use of this technology to less complex wounds (i.e., WIfI stage 1 or 2) as well.
In our experience, proper postoperative management is essential for ensuring favorable wound healing with a dermal regeneration template. Weight-bearing is a significant challenge, and patients must be offloaded as much as possible to achieve good outcomes.2 With regard to dressing the wound, we use both negative-pressure wound therapy and bolster dressings, with no apparent difference in outcomes between the two. We typically prefer negative-pressure would therapy if the wound is nonedematous, and bolster if there is maceration of the surrounding skin or if the wound overlies a joint space. More investigation is necessary to critically evaluate these observations and optimize the perioperative management of dermal regeneration template therapy.
There are several limitations of our study that should be considered. First, we performed a descriptive cohort analysis reporting our observed outcomes with dermal regeneration templates to date, and thus were unable to generate wound healing comparisons against a control group. However, our data are prospectively collected and, as noted above, our cohort is unique in that we report the successful treatment of very complex postsurgical diabetic foot wounds that would have a high risk of major amputation in most settings. Second, our sample size of 107 diabetic foot wounds is relatively small, even though this is the largest cohort of dermal regeneration template applications in advanced-stage wounds that we are aware of. Third, our multidisciplinary limb preservation service has a history of good wound healing and limb salvage outcomes because of factors previously mentioned, and thus our results may not be applicable to all treatment centers. We have recently published a cost analysis of our multidisciplinary model26,31 but do not currently have any financial data specifically regarding dermal regeneration template use in our cohort. This may be a potential avenue of future study, to further aid in quantifying its utility when compared to other methods of treatment.
CONCLUSIONS
Application of a dermal regeneration template to complex postsurgical diabetic foot wounds at high risk for major amputation results in good wound healing and excellent limb salvage outcomes among diabetic patients treated in a multidisciplinary setting. Wound characteristics including bony involvement; WIfI stage; and prior failed dermal regeneration template applications are significantly associated with wound healing outcomes after risk adjustment. Our data suggest that use of a dermal regeneration template is an effective option for the treatment of complex diabetic foot wounds in select patients.
| CODING PERSPECTIVE | ||
|---|---|---|
| 15275 | Coding perspective provided by Dr. Raymond Janevicius is intended to provide coding guidance. | |
| Application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area up to 100 cm2; first 25 cm2 or less wound surface area | ||
| +15276 | Application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area up to 100 cm2; each additional 25 cm2 wound surface area, or part thereof | |
| 15277 | Application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area greater than or equal to 100 cm2; first 100 cm2 wound surface area, or 1 percent of body area of infants and children | |
| +15278 | Application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area greater than or equal to 100 cm2; each additional 100 cm2 wound surface area, or part thereof, or each additional 1 percent of body area of infants and children, or part thereof | |
| 15004 | Surgical preparation or creation of recipient site by excision of open wounds, burn eschar, or scar (including subcutaneous tissues), or incisional release of scar contracture, face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet and/or multiple digits; first 100 cm2 or 1 percent of body area of infants and children | |
| +15005 | Surgical preparation or creation of recipient site by excision of open wounds, burn eschar, or scar (including subcutaneous tissues), or incisional release of scar contracture, face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet and/or multiple digits; each additional 100 cm2, or part thereof, or each additional 1 percent of body area of infants and children | |
| • Integra is a skin substitute graft and the 1527X series of codes is used. This series of codes is reported by anatomic site and surface area of the grafted area. | ||
| • If the total wound surface area of the grafted area is less than 100 cm2, report codes 15275 and 15276. | ||
| • If the total wound surface area is 100 cm2 or greater, use codes 15277 and 15278. | ||
| • For example, an Integra graft of 70 cm2 (<100 cm2) is reported using the following codes: | ||
| 15275 | First 25 cm2 | |
| 15276 | Additional 25 cm2, or part thereof | |
| 15276 | Additional 25 cm2, or part thereof | |
| • An Integra graft of 150 cm2 (>100 cm2) is reported using the following codes: | ||
| 15277 | First 100 cm2 | |
| 15278 | Additional 100 cm2, or part thereof | |
| • Codes 15276 and 15278 are add-on codes, so modifier 51 is not appended. | ||
| • All skin substitute graft codes have a 0-day global period, so all postoperative visits are separately reportable. | ||
| • Wound excision to prepare the wound for application of Integra is reported with codes 15004 and 15005. These codes are reported by total surface area of surgical preparation. | ||
|
CODING PRINCIPLE: Per Current Procedural Terminology, the surgical preparation codes 15002 through 15005 are reported for “preparing a clean and viable wound surface for placement of an autograft, flap, skin substitute graft or for negative pressure wound therapy.” The intent is “to heal the wound by primary intention” and “appreciable nonviable tissue is removed.” Simple cleansing of the wound or scraping of granulation tissue is considered part of the skin substitute codes and is not separately reportable. | ||
| Disclosure: Dr. Janevicius (janeviciusray@comcast.net) is the president of JCC, a firm specializing in coding consulting services for surgeons, government agencies, attorneys, and other entities. | ||
Disclosure:
Dr. Sherman is a consultant for Integra LifeSciences. The remaining authors have no financial disclosures to report. No funding was received for this article.
REFERENCES
- 1.Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33:1493–1498. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. [DOI] [PubMed] [Google Scholar]
- 3.Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes: The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care 1999;22:1029–1035. [DOI] [PubMed] [Google Scholar]
- 4.Integra LifeSciences Corp. Integra dermal regeneration template. Available at: https://www.integralife.com/integra-dermal-regeneration-template/product/wound-reconstruction-care-inpatient-acute-or-integra-dermal-regeneration-template. Accessed July 24, 2019.
- 5.Iorio ML, Goldstein J, Adams M, Steinberg J, Attinger C. Functional limb salvage in the diabetic patient: The use of a collagen bilayer matrix and risk factors for amputation. Plast Reconstr Surg. 2011;127:260–267. [DOI] [PubMed] [Google Scholar]
- 6.Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: Debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117;72S–109S. [DOI] [PubMed] [Google Scholar]
- 7.De Angelis B, Gentile P, Tati E, et al. One-stage reconstruction of scalp after full-thickness oncologic defects using a dermal regeneration template (Integra). Biomed Res Int. 2015;2015:698385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham GP, Helmer SD, Haan JM, Khandelwal A. The use of Integra dermal regeneration template in the reconstruction of traumatic degloving injuries. J Burn Care Res. 2013;34:261–266. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DQ, Potokar TS, Price P. An objective long-term evaluation of Integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns 2010;36:23–28. [DOI] [PubMed] [Google Scholar]
- 10.Valerio IL, Masters Z, Seavey JG, Balazs GC, Ipsen D, Tintle SM. Use of a dermal regeneration template wound dressing in the treatment of combat-related upper extremity soft tissue injuries. J Hand Surg Am. 2016;41:e453–e460. [DOI] [PubMed] [Google Scholar]
- 11.Holmes C, Wrobel JS, Maceachern MP, Boles BR. Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: A systematic review. Diabetes Metab Syndr Obes. 2013;6:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Mu D, Gao F. Efficacy and safety of acellular dermal matrix in diabetic foot ulcer treatment: A systematic review and meta-analysis. Int J Surg. 2017;40:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Mathioudakis N, Hicks CW, Canner JK, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing but not major amputation in patients with diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg. 2017;65:1698–1705.e1. [DOI] [PubMed] [Google Scholar]
- 14.Boulton AJ, Armstrong DG, Albert SF, et al. ; American Diabetes Association; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31:1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Yoshida S, Sumikawa Y, et al. Rapid healing of intractable diabetic foot ulcers with exposed bones following a novel therapy of exposing bone marrow cells and then grafting epidermal sheets. Br J Dermatol. 2004;151:1019–1028. [DOI] [PubMed] [Google Scholar]
- 16.Mills JL Sr, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: Risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59:220–234.e1–2. [DOI] [PubMed] [Google Scholar]
- 17.Cull DL, Manos G, Hartley MC, et al. An early validation of the Society for Vascular Surgery lower extremity threatened limb classification system. J Vasc Surg. 2014;60:1535–1541. [DOI] [PubMed] [Google Scholar]
- 18.Zhan LX, Branco BC, Armstrong DG, Mills JL Sr. The Society for Vascular Surgery lower extremity threatened limb classification system based on wound, ischemia, and foot infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg. 2015;61:939–944. [DOI] [PubMed] [Google Scholar]
- 19.Beropoulis E, Stavroulakis K, Schwindt A, Stachmann A, Torsello G, Bisdas T. Validation of the Wound, Ischemia, foot Infection (WIfI) classification system in nondiabetic patients treated by endovascular means for critical limb ischemia. J Vasc Surg. 2016;64:95–103. [DOI] [PubMed] [Google Scholar]
- 20.Hicks CW, Canner JK, Mathioudakis N et al. The SVS WIfI classification independently predicts wound healing in diabetic foot ulcers. J Vasc Surg 2017;68:1096–1103. [DOI] [PubMed] [Google Scholar]
- 21.Ward R, Dunn J, Clavijo L, Shavelle D, Rowe V, Woo K. Outcomes of critical limb ischemia in an urban, safety net hospital population with high WIfI amputation scores. Ann Vasc Surg. 2017;38:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson WP, Loretz L, Hanesian C, et al. Society for Vascular Surgery Wound, Ischemia, foot Infection (WIfI) score correlates with the intensity of multimodal limb treatment and patient-centered outcomes in patients with threatened limbs managed in a limb preservation center. J Vasc Surg. 2017;66:488–498.e2. [DOI] [PubMed] [Google Scholar]
- 23.Margolis DJ, Berlin JA, Strom BL. Interobserver agreement, sensitivity, and specificity of a “healed” chronic wound. Wound Repair Regen. 1996;4:335–338. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994;2:165–170. [DOI] [PubMed] [Google Scholar]
- 25.Driver VR, Lavery LA, Reyzelman AM, et al. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 2015;23:891–900. [DOI] [PubMed] [Google Scholar]
- 26.Hicks CW, Canner JK, Karagozlu H, et al. Quantifying the costs and profitability of care for diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg. 2019;70:233–240. [DOI] [PubMed] [Google Scholar]
- 27.Schiavon M, Francescon M, Drigo D, et al. The use of Integra dermal regeneration template versus flaps for reconstruction of full-thickness scalp defects involving the calvaria: A cost-benefit analysis. Aesthetic Plast Surg. 2016;40:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aragón-Sánchez FJ, Cabrera-Galván JJ, Quintana-Marrero Y, et al. Outcomes of surgical treatment of diabetic foot osteomyelitis: A series of 185 patients with histopathological confirmation of bone involvement. Diabetologia 2008;51:1962–1970. [DOI] [PubMed] [Google Scholar]
- 29.Sumpio BE. Foot ulcers. N Engl J Med. 2000;343:787–793. [DOI] [PubMed] [Google Scholar]
- 30.Pickwell KM, Siersma VD, Kars M, Holstein PE, Schaper NC; Eurodiale consortium. Diabetic foot disease: Impact of ulcer location on ulcer healing. Diabetes Metab Res Rev. 2013;29:377–383. [DOI] [PubMed] [Google Scholar]
- 31.Hicks CW, Canner JK, Karagozlu H, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system correlates with cost of care for diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg. 2018;67:1455–1462. [DOI] [PubMed] [Google Scholar]


