Abstract
Objective:
Although postmenopausal women have behavioral and biological risk factors for HIV infection, the activity of pre-exposure prophylaxis agents in older adults has not been well studied.
Design:
We used an ex-vivo approach to compare the tissue concentrations of tenofovir diphosphate (TFVdp) and emtricitabine triphosphate (FTCtp) in cervical tissues from premenopausal and postmenopausal women.
Method:
Cervical explants from 16 premenopausal and 11 postmenopausal women were incubated in 10–300 μg/mL tenofovir or emtricitabine for 24 hours. Explants were then snap frozen in liquid nitrogen and stored until analysis. TFVdp and FTCtp were quantified using tandem liquid chromatography-mass spectrometry.
Results:
Active metabolite concentrations of TFVdp were >9-fold lower in postmenopausal explants (p<0.05). The percentage of TFV converted to TFVdp in preM explants was 0.0038 (BLQ-0.5886) compared to 0.0004 (BLQ-0.0706) in postM explants. The majority of FTCtp concentrations were below the limit of quantification. For both TFVdp and FTCtp there was a a trend for more unquantifiable concentrations in postmenopausal vs premenopausal (TFV: 38% vs 21%, p=0.2; FTC: 71% vs 52%, p=0.2).
Conclusion:
These findings could have implications in the use of nucleotide-based PrEP strategies targeted to older women. If validated in vivo, lower exposures of active nucleoside/tide metabolites could mean postmenopausal women need higher doses of tenofovir-based PrEP to achieve protective efficacy.
Keywords: PrEP, tenofovir, explants, menopause, emtricitabine
Introduction
Pre-exposure prophylaxis (PrEP) with antiretrovirals has proven that with consistent use, the risk of HIV acquisition in high-risk individuals can be reduced. Efficacy was first demonstrated in a 2010 study when daily use of Truvada® (fixed dose oral combination of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC)) reduced HIV infections by 44% in men or transgender women who have sex with men [1]. This finding was later validated in heterosexuals and injection drug users [2–4]. Regulatory agencies from several countries including the United States, Canada, Kenya, and South Africa [5] have approved the use of TDF/FTC for PrEP and the World Health Organization has endorsed and published guidelines for its use [6].
Not all PrEP initiatives, however, have met with success. Two studies in heterosexual women were stopped early for futility based on recommendation from the Data Safety Monitoring Boards [7, 8]. Adherence in these studies when assessed by random blood concentrations suggested < 30% of subjects assigned to the active arms were taking daily doses. Further pharmacologic analyses have demonstrated that the level of adherence needed to achieve protective concentrations differs based on the route of HIV exposure, with stricter adherence to TDF/FTC-based PrEP required for protection from vaginal compared to colorectal acquisition [9, 10].
To date, clinical trials that have evaluated PrEP in women have focused on younger females [7, 8,11]. However, the CDC reports 26% of new HIV diagnoses in the United States are in adults over the age of 45; of these, 29% are in females acquired through heterosexual contact [12]. High-risk behaviors such as unprotected sexual intercourse is common among older adults both in the U.S. [13,14] and in hyper endemic areas such as South Africa [15], possibly due to lack of concern about pregnancy protection after menopause and an under appreciation of risk for sexually transmitted infections. Additionally, menopausal changes may alter the biological risk by increasing target cell expression, vaginal pH, epithelial function, and innate immunity [16–18]. Given that current dosing for oral PrEP achieves concentrations in the female genital tract (FGT) that may be close to the threshold for efficacy [10, 19], we sought to evaluate the metabolite concentrations of tenofovir (TFV) and emtricitabine (FTC) in cervical tissues from postmenopausal (postM) women using a cervical explant model [19].
Methods
Tissue procurement
Cervical specimens were procured by the University of Minnesota Biological Materials Procurement Network (BioNet) from HIV negative women undergoing gynecologic surgery. All women consented to tissue collection for research prior to their surgeries. Clinical information including age, race, co-medications, and menopause status, defined as 12 months amenorrhea, were recorded from medical records. Specimens were collected fresh and transferred in culture medium prepared with: Iscove’s Modified Dulbecco’s Medium (Gibco, USA), 10% fetal bovine serum (Gibco), 240 units/mL nystatin suspension (Sigma, St. Louis, MO, USA), 100 units/mL penicillin/streptomycin (Gibco), and MEM Vitamin Solution (Sigma). Tissues were typically brought to laboratory for processing within 30 minutes of surgery. Tissues were cleaned and dissected leaving only the epithelial layer and underlying submucosa for explant cultures. Endocervix and ectocervix were separated. Biopsy punches (Integra Miltex, Plainsboro, NJ, USA) were used to create 3mm2 explants. Hematoxylin and eosin (H&E) staining was used on explants fixed in Safefix II (Fisher Scientific, USA) to confirm correct identification of ectocervical vs endocervical portions.
Ex- Vivo Metabolism of Nucleotide Reverse Transcriptase Inhibitors.
Drug stocks of 1 mg/mL TFV and FTC (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, Germantown, MD) were reconstituted in sterile water. Drug was then diluted in culture media in dilutions of 10, 30, 100, or 300 μg/mL. Explants were incubated in 500 μL in a 48 well tissue culture plate for 24 hours. Tissues were weighed and then snap frozen in liquid nitrogen and stored at −80°C. Tenofovir-diphosphate (TFVdp) and emtricitabine- triphosphate (FTCtp) were measured in tissue explant homogenates by LC-MS/MS over a concentration range of 0.02–20ng/mL. To prevent degradation of intracellular metabolites, acetonitrile was added to frozen tissues before homogenization. Samples were extracted by protein precipitation with isotopically labeled 13C5-TFVdp as the internal standard. Calibration standards and QC samples were prepared in human vaginal tissue homogenate. Calibration standards and QC samples met acceptance criteria of +/−15% of nominal concentrations for all analyses.
Statistical Analysis
When applicable, values from replicate samples were averaged. Measured TFVdp and FTCtp concentrations were normalized for tissue weight. To normalize for incubation dose, dose-adjusted concentrations were calculated (TFVdp or FTCtp expressed as a molar percent of TFV or FTC incubation concentrations.) Differences in metabolite concentrations were tested for significance using the Wilcoxon Rank Sum Test. Differences in the proportions of unquantifiable samples were tested using the Chi-square test. Statistics were performed using SAS 9.4 (Cary, NC). Data are reported as median (range) unless otherwise noted.
Results
Demographic information of tissue donors is shown in Table 1. No subjects were on hormonal therapy at the time of surgery with the exception of one premenopausal (preM) subject who had been receiving testosterone injections prior to a sex reassignment procedure. A second preM subject was diagnosed with Turner Syndrome (45, X karyotype), a condition that commonly presents with ovarian insufficiency resulting in reduced estrogen production [20]. Since metabolite concentrations in these subjects were similar to median values, they were not excluded from analysis.
Table 1:
Demographics of tissue donors
| Premenopausal (n=16) | Postmenopausal (n=11 ) | ||
|---|---|---|---|
| Age | median (range) | 38.5 (21–56) | 56 (52–68) |
| Race | Caucasian | 13 | 9 |
| African American | 1 | 1 | |
| Asian | 2 | 0 | |
| unknown | 0 | 1 |
Tenofovir:
Conversion of TFV to TFVdp in explants is shown in Figure 1A and Supplemental Table 1. Measurements from 28% of explants were below the limit of assay quantification (BLQ) for TFVdp (21% of preM vs 38% of postM, p=0.18). BLQ samples were distributed across the doses and frequency of proportion unquantifiable did not decrease with increasing dose. TFVdp conversion in postM explants was >9-fold lower than in preM explants in ectocervix (p=0.007). This trend was also observed in endocervix although it was not statistically significant due to fewer samples available (p=0.7) (Figure 1A). An overall correlation was noted between age and TFVdp phosphorylation in ectocervical tissue (r= −0.5, p=0.001) but there was no correlation within preM (r= −0.3, p=0.2) or postM (r= −0.14, p=0.7) groups. There was no correlation between age and TFV phosphorylation in endocervical tissue. Sensitivity analyses confirmed higher TFV phosphorylation in preM tissues even when BLQ samples were removed from analysis (p=0.04) and when the subjects receiving hormonal treatment were excluded (p=0.03). Similar findings were also obtained when comparing TFVdp concentrations rather than dose-normalized values (p=0.01).
Figure 1:
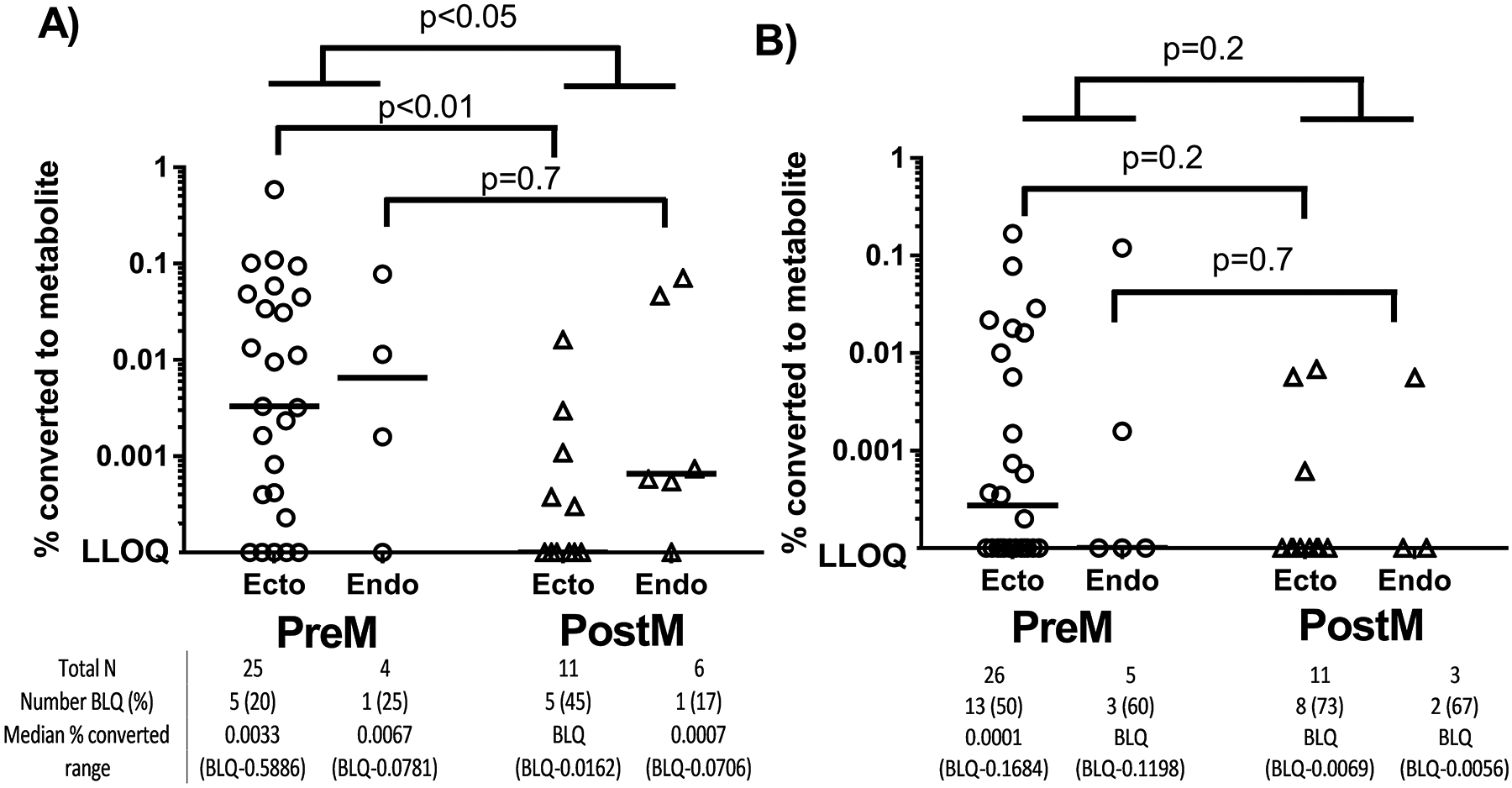
Reduced nucleotide metabolite concentrations in postM explants. The measured TFVdp (A) or FTCtp (B) following a 24 hour incubation in TFV or FTC 10–300 μg/mL is shown as a percentage of the parent incubation concentration. Circles (preM) and triangles (postm) represent individual explant concentrations and the lines represent median vales. The uppermost p-values represent comparisons between preM and postM when ectocervix and endocervix were pooled together while the middle and lower p-values comparison between ectocervix and endocervix respectively.
Emtricitabine:
Conversion of FTC to FTCtp in explants is shown in Figure 1B and in Supplemental Table 2. Consistent across all doses, 56% of all FTCtp concentrations were BLQ. Although the difference was not statistically significant, unquantifiable FTCtp concentrations were more frequent in the postM group compared to the preM group (71% vs 52%, p=0.2). Unlike TFVdp, there was no correlation between age and FTCtp concentrations (r = −0.1, p=0.5).
Discussion
As the rollout of nucleoside/tide-based PrEP continues to expand, it becomes increasingly important to understand the factors modulating efficacy and identify populations at risk for drug-failure. In this study we found reduced TFVdp concentrations in cervical tissues from postM women. Since the majority FTCtp concentrations were below the assay limit of quantification, we were not able to adequately assess differences of this metabolite between preM and postM tissue, although the trend for a greater percentage of unquantifiable concentrations in the postM group was consistent with the lower TFVdp exposures.
Our findings of decreased TFVdp in the estrogen-deficient state of menopause are consistent with Shen et al who found phosphorylation of TFVdp increased in epithelial cells isolated from the FGT when treated with estradiol [21]. The authors also found differential phosphorylation in different FGT cell types with the greatest TFVdp/cell quantified in epithelial cells followed by fibroblasts, CD14+ cells, and then CD4+ cells. Thinning of the vaginal epithelium has been well documented in women past the age of menopause [17]. In our study we were not able to assess the relative distribution of TFVdp in different cell types and therefore it is possible that, although we normalized for tissue weight, a smaller proportion of epithelial cells in our postM explants contributed to the overall lower TFVdp exposure. The role epithelial cells play in providing a TFV reservoir for HIV target cells is not clear [21] and further studies are required.
Hormonal modulation of nucleotide-based PrEP could also have implications outside of the postM population. Depo-Provera® (medroxyprogesterone acetate) is a progestin-only injectable contraceptive that, similar to menopause, significantly reduces plasma estradiol exposures [22]. Whether Depo-Provera users on TDF have reduced cervical TFVdp. Following multiple oral doses of TDF, trough TFVdp concentrations in PBMCs are 240% lower in women taking oral or injectable hormonal contraception compared to a non-hormonal group following multiple oral doses of TDF [23]. This raises implications for both PrEP and treatment of HIV as Depo-Provera is widely used in sub-Saharan Africa, where the highest rates of HIV infection occur and young women of reproductive age are particularly vulnerable [24]. Notably, a post-hoc (not powered) analysis of the Partners PrEP study found Depo-Provera users still received protective benefit from oral TDF/FTC or TDF alone; however there was an observed 10% increase in HIV incidence in Depo-Provera users assigned to active PrEP arms compared to female PrEP users with no hormonal contraception, suggesting more data is needed in this area [25].
We cannot rule out that the observed differences are due to other processes of aging rather than hormonal regulation. Although overall correlations with age were significant, we did not observe any correlation between age and concentration within either preM or postM groups, making it difficult to separate menopausal effects from overall aging. A pilot study assessing antiretroviral pharmacokinetics in HIV+ adults over the age of 55 reported higher TFVdp and lower FTCtp in PBMC compared to younger historic controls [26]. However, only one-half of the 12 subjects in this pilot study were female and sex differences were not reported. This differential effect of aging on TFVdp compared to FTCtp was hypothesized to be due to TFV phosphorylation being more influenced by cellular activation [27,28]. Decreased innate immunity in mucosal tissues of postM women may be one explanation for the lack of similar findings in mucosal tissues [29]. Further analysis is needed to separate the effects of aging from the hormonal changes due to menopause, and to determine whether effects are tissue specific.
There are limitations to this study. First, hormone concentrations were not directly measured. It is possible some of our preM subjects were peri-menopausal with declining estrogen levels. In addition, we could not control for hormonal variability across the menstrual cycle. Second, other factors not accounted for, such as microbial populations, cell populations, and inflammatory state, may also contribute to variability in phosphorylation or activity.
In conclusion, in this ex-vivo analysis, we found a decrease in TFVdp concentrations in postM tissues. If validated in vivo, these findings could have implications on the use of tenofovir-based PrEP regimens in older women.
Supplementary Material
Acknowledgements.
This work was financially supported by the Department of Experimental and Clinical Pharmacology, College of Pharmacy, University of Minnesota and the Deborah E Powell Women’s Center, University of Minnesota. Tissue specimens were collected under the University of Minnesota BioNet Tissue Procurement Facility. Tenofovir was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH.
Support: Funding for this research was provided by the University of Minnesota College of Pharmacy and the University of Minnesota Deborah Powell Center for Women’s Health. Drugs were provided by the NIH AIDS Reagent Program.
References
- [1].Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segologi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. [DOI] [PubMed] [Google Scholar]
- [4].Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–90. [DOI] [PubMed] [Google Scholar]
- [5].“PrEP Watch: Country Updates”. AVAC. Found at http://www.prepwatch.org/scaling-up/country-updates/. Accessed 14Dec2016.
- [6].Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. World Health Organization; September 2015. URL: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. Accessed 14Dec2016. [PubMed] [Google Scholar]
- [7].Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2015;372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis. 2016;214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012; 4(151): 151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Center for Disease Control. HIV Surveillance- Epidemiology of HIV Infection (through 2013). Available at http://www.cdc.gov/hiv/pdf/g-l/cdc-hiv-genepislideseries-2013.pdf. Updated 23 February 2015. Accessed 10 March 2015.
- [13].Taylor TN, Weedon J, Golub ET, Karpiak SE, Gandhi M, Cohen MH, et al. Longitudinal Trends in Sexual Behaviors with Advancing Age and Menopause Among Women With and Without HIV-1 Infection. AIDS Behav. 2015;19:931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Massad LS, Evans CT, Wilson TE, Golub ET, Goparaju L, Howard A, et al. Impact of menopause on condom use by HIV-seropositive and comparison seronegative women. J Acquir Immune Defic Syndr. 2008;47:401–2. [DOI] [PubMed] [Google Scholar]
- [15].Rosenberg MS, Gomez-Olive FX, Rohr JK, Houle BC, Kabudula CW, Wagner RG, et al. Sexual Behaviors and HIV Status: A Population-Based Study Among Older Adults in Rural South Africa. J Acquir Immune Defic Syndr. 2017;74:e9–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Meditz AL, Moreau KL, MaWhinney S, Gozansky WS, Melander K, Kohrt WM, et al. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Farage MA and Maibach HI. Morphology and physiological changes of genital skin and mucosa. Curr Probl Dermatol. 2011; 40: 9–19. [DOI] [PubMed] [Google Scholar]
- [18].Chappell CA, Isaacs CE, Xu W, Meyn LA, Uranker K, Dezzutti CS. The effect of menopause on the innate anti-viral activity of cervicovaginal lavage. Am J Obstet Gynecol. 2015; 213(2): 204 e201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nicol MR, Emerson CW, Prince HM, Nelson JA, Fedoriw Y, Sykes C, et al. Models for Predicting Effective HIV Chemoprevention in Women. J Acquir Immune Defic Syndr. 2015;68:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morgan T Turner syndrome: diagnosis and management. Am Fam Physician. 2007; 76(3): 405–410. [PubMed] [Google Scholar]
- [21].Shen Z, Fahey JV, Bodwell JE, Rodriguez-Garcia M, Kashuba AD, Wira CR. Sex hormones regulate tenofovir-diphosphate in female reproductive tract cells in culture. PLoS One. 2014;9:e100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Briggs M and Briggs M Plasma hormone concentrations in women receiving steroid contraceptives. J Obstet Gynaecol Br Commonw. 1972; 79(10): 946–950. [DOI] [PubMed] [Google Scholar]
- [23].Coleman JS, Chaturvedula A, Hendrix C. Method of hormonal contraception is associated with lower tenofovir concentration in healthy women (MTN-001): implications for pre-exposure prophylaxis. [Abstract FRLBC03]. Journal of the International AIDS Society 2012, 15 (Suppl 3) 10.7448/IAS.15.5.18440. [DOI] [Google Scholar]
- [24].Joint United Nations Programme on HIV/AIDS. Global AIDS Update 2016. Published 31May2016. Available at http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. Accessed 7Aug2016.
- [25].Heffron R, Mugo N, Were E, Kiarie J, Bukusi E, Mujugira A, et al. (2014). Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS. 2014; 28(18): 2771–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dumond JB, Adams JL, Prince HM, Kendrick RL, Wang R, Jennings SH, et al. Pharmacokinetics of two common antiretroviral regimens in older HIV-infected patients: a pilot study. HIV Med. 2013;14:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gao WY, Agbaria R, Driscoll JS, Mitsuya H. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2’,3’-dideoxynucleoside analogs in resting and activated human cells. J Biol Chem. 1994; 269:12633–8. [PubMed] [Google Scholar]
- [28].Shirasaka T, Chokekijchai S, Yamada A, Gosselin G, Imbach JL, Mitsuya H. Comparative analysis of anti-human immunodeficiency virus type 1 activities of dideoxynucleoside analogs in resting and activated peripheral blood mononuclear cells. Antimicrob Agents Chemother. 1995; 39(11): 2555–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chappell CA, Isaacs CE, Xu W, Meyn LA, Uranker K, Dezzutti CS et al. The Effect of Menopause on the Innate Anti-Viral Activity of Cervicovaginal Lavage. Am J Obstet Gynecol 2015; 213(2): 204 e201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


