Abstract
Background
Angiotensin-converting enzyme (ACE) 2 is known to be a functional receptor for SARS-CoV-2 in the current pandemic. Soluble ACE2 (sACE2) concentrations are elevated in patients with various cardiovascular disorders including heart failure.
Methods
In a total of 182 consecutive adult patients with complex congenital heart disease (CHD) and 63 healthy controls, sACE2 concentrations were measured in serum using the Human ACE2® assay by Cloud-Clone Corporation and associated with clinical, laboratory and echocardiographic parameters.
Results
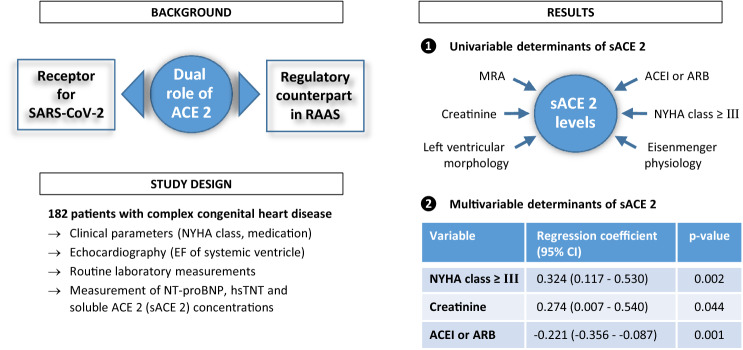
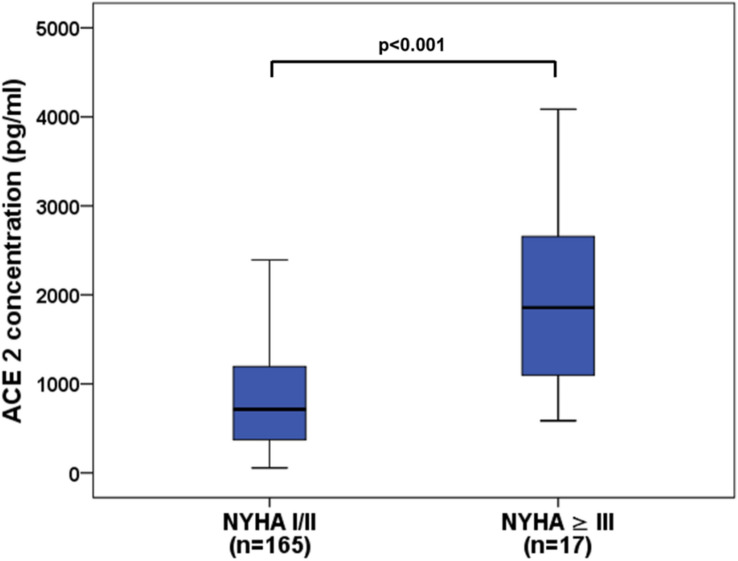
Median sACE2 levels were increased in patients with complex CHD as compared to healthy controls (761.9 pg/ml vs 365.2 pg/ml, p < 0.001). Moreover, sACE2 concentrations were significantly elevated in patients with a higher NYHA class ≥ III (1856.2 pg/ml vs 714.5 pg/ml in patients with NYHA class I/II, p < 0.001). Using linear regression analysis, higher sACE2 levels were associated with a higher NYHA class ≥ III, more severe CHD, a morphological left systemic ventricle, higher creatinine and the use of mineralocorticoid receptor antagonists (MRA) in the univariable model. The use of ACE inhibitors or angiotensin receptor blockers (ARB) was associated with lower sACE2 levels. In the multivariable model, higher sACE2 levels were independently associated with a higher NYHA class ≥ III (p = 0.002) and lower sACE2 levels with the use of ACE inhibitors or ARB (p = 0.001).
Conclusion
Soluble ACE2 concentrations were significantly increased in all types of complex CHD with highest levels found in patients with NYHA class ≥ III. Moreover, a higher NYHA class ≥ III was the most significant determinant that was independently associated with elevated sACE2 concentrations.
Graphic abstract
Keywords: Soluble ACE2, Complex congenital heart disease, Heart failure, COVID-19
Introduction
Angiotensin-converting enzyme 2 (ACE2) is a membrane-bound carboxymonopeptidase that converts Angiotensin I and Angiotensin II into Angiotensin 1–9 and Angiotensin 1–7, respectively, and is highly expressed on the surface of different organ cells in the heart, lungs, kidneys and testis [1–3]. The soluble form of ACE2 (by proteolytic shedding of membrane-bound ACE2) is also a key component of the counter-regulatory pathway within the renin–angiotensin–aldosterone system (RAAS) which plays a crucial role in the regulation of blood pressure, heart failure, diabetes and chronic kidney disease [4–6].
The current coronavirus SARS-CoV-2 uses ACE2 as a cellular receptor to invade target cells. In particular, the spike protein of SARS-CoV-2 is processed by transmembrane protease-serine 2 and favors the binding of the spike protein to ACE2 to enter the host cells [6]. To date, an increasing number of publications have confirmed the role of ACE2 receptor for the cell entry mechanism of SARS-CoV-2 in the current pandemic [2, 3, 6]. Several epidemiological studies have also shown the severity of the SARS-CoV-2 disease (COVID-19) in older patients and particularly those with cardiovascular rather than pulmonary predisposing risk factors [7–9]. Thus, sACE2 concentrations were found to be elevated in men and older patients with different cardiovascular morbidities, such as hypertension, diabetes, coronary heart disease and heart failure [8, 10–14]. These interactions of ACE2 may explain the higher vulnerability and predisposition of such patients to have a severe or fatal outcome after SARS-CoV-2 infection [15–17].
Adult patients with complex congenital heart disease (CHD) may also represent a vulnerable group of patients due to comorbidities, residual heart defects with longstanding pressure or volume overload as well as reduced intrinsic myocardial function finally resulting in symptomatic heart failure in early or mid-term adulthood [18]. Data on the SARS-CoV-2 infection rate in patients with CHD are still lacking. According to data from different countries and the Johns Hopkins University, children and young people seem to have a less severe time course and fatality rate with COVID-19 than older adults [19, 20] what may be due to lower sACE2 levels [21]. Moreover, an increased vulnerability of older and male patients with cardiovascular disease or heart failure can be seen that could also be related to increased sACE2 levels [10–12, 22]. In adult patients with complex CHD who often present with altered myocardial function or even symptomatic heart failure, data on sACE2 levels are lacking. Thus, in the light of the dual role of ACE2 during the current pandemic, the aim of our study was to assess sACE2 levels in this particular patient cohort and to evaluate determinants of sACE2 concentrations with special emphasis on factors that are independently associated with increased sACE2 concentrations.
Methods
Patients
In our outpatient clinic for adults with CHD, several prospective studies for the evaluation of different biomarkers in serum as well as micro-RNA signatures in whole blood had been performed in the past [23–25]. To assess sACE2 concentrations, existing serum samples of these patients frozen at -80° C representing the pre-COVID-19 era were used for analysis. Thus, serum samples of 182 consecutive patients with complex CHD ≥ 18 years of age who were regularly seen in our outpatient clinic between 22/01/2015 and 02/12/2019 were analyzed in the present study. Complex CHD was classified according to current ACC/AHA guidelines [26] and mainly related to severe congenital heart defects including all forms of pulmonary atresia, cyanotic CHD, Eisenmenger syndrome, transposition of the great arteries (TGA), common arterial trunk, all kinds of single ventricle physiology with or without Fontan palliation as well as CHD at increased risk for heart failure, such as tetralogy of Fallot [27]. Patients with mild or moderate CHD, pregnancy, severe renal dysfunction or dialysis and incapability to understand or sign informed consent were excluded. Of the 182 patients enrolled in the study, 60/182 (33.0%) patients had corrective surgery of congenital right heart disease (CRHD), such as pulmonary atresia, tetralogy of Fallot or common arterial trunk, 62/182 (34.1%) patients had TGA of whom 40/62 (64.5%) patients had a systemic morphological right ventricle (TGA-RV) and 22/62 (35.5%) patients a systemic morphological left ventricle (TGA-LV) after arterial switch operation, 37/182 (20.3%) patients showed a single ventricle physiology after complete Fontan palliation (FONT) and in 23/182 (12.6%) patients a non-corrected cyanotic heart defect or Eisenmenger syndrome classified as Eisenmenger physiology (EIS) was present. Mean age was 30.1 ± 10.5 years (range 18–62 years) and 103 patients were male (56.6%). The study protocol has been described in detail previously [23]. 63 healthy subjects with a mean age of 31.6 ± 11.7 years (range 18–61 years) served as controls. The control group was mainly recruited within our institution and consisted of medical students, medical staff or family members. To rule out a hemodynamically relevant heart abnormality, a physical examination and two-dimensional echocardiography were performed in all healthy participants. Clinical data of controls and patients are given in Table 1. All patients and healthy controls gave written informed consent before enrollment.
Table 1.
Comparison of healthy controls and patients
| Variables | Healthy controls (n = 63) | All patients (n = 182) | p value* |
|---|---|---|---|
| Age at enrollment (years) | 31.6 ± 11.7 | 30.1 ± 10.5 | 0.474 |
| Male sex | 39/63 (61.9%) | 103/182 (56.6%) | 0.462 |
| Body mass index (kg/m2) | 23.2 ± 3.3 | 23.9 ± 4.8 | 0.579 |
| NYHA class | 1.0 ± 0 | 1.5 ± 0.7 | < 0.001 |
| Systolic blood pressure (mmHg) | 120.8 ± 10.9 | 123.3 ± 13.9 | 0.304 |
| Diastolic blood pressure (mmHg) | 70.2 ± 7.7 | 70.8 ± 9.4 | 0.829 |
| Transcutaneous oxygen saturation at rest (%) | 98.2 ± 1.2 | 95.0 ± 5.9 | < 0.001 |
| Ejection fraction of SV (%) | 62.4 ± 4.4 | 54.9 ± 9.1 | < 0.001 |
| Enddiastolic volume of SV (ml) | 107.9 ± 26.9 | 131.9 ± 62.2 | 0.006 |
| Endsystolic volume of SV (ml) | 40.5 ± 12.6 | 61.9 ± 37.7 | < 0.001 |
| VTI above aortic valve (cm) | 27.4 ± 4.3 | 24.4 ± 4.5 | < 0.001 |
| sACE2 concentrations (pg/ml) | 365.2 (237.7–656.3) | 761.9 (419.2–1277.7) | < 0.001 |
Mean ± standard deviation or median (interquartile interval) are used
NYHA New York Heart Association, SV systemic ventricle, VTI velocity time integral, sACE2 soluble angiotensin-converting enzyme 2
*Mann–Whitney U test
Data and biochemical analyses
Clinical data of the patients were collected from medical records. Echocardiographic data sets were analysed on an Echopac server (Echopac Version 6, GE Healthcare, Horten, Norway) and assessed by investigators blinded to the laboratory results.
Venous blood samples were drawn into standard sampling tubes shortly after echocardiography, centrifuged at 3000g and serum removed, allocated and frozen at − 80 °C before analysis of ACE2 levels. In the patient group, routine laboratory tests, such as liver function tests and creatinine measurements, were performed using standard laboratory techniques. NT-proBNP and high sensitive troponin T levels were measured using an electrochemiluminescence sandwich immunoassay (Cobas® proBNP II and troponin T hs STAT, Roche Diagnostics, Basel, Switzerland) on the Elecsys® 2010 analyzer. Levels of ACE2 were measured using a commercially available sandwich enzyme immunoassay (Human ACE2®, Cloud-Clone Corporation, Houston, Texas, USA) on the FLUOstar® Omega Microplate Reader (BMG LABTECH Inc., Cary, North Carolina, USA) and has been described in detail previously [28]. All biochemical analyses were performed by investigators blinded to the clinical and echocardiographic data of the patients. Glomerular filtration rate (GFR) was estimated using the chronic kidney disease epidemiology collaboration (CKD-EPI) creatinine equation [29].
Statistical analysis
Data were analysed using standard statistical software (SPSS version 25; SPSS Inc., Chicago, Illinois). Continuous variables are expressed as mean ± standard deviation or median (interquartile interval) as appropriate. Differences between unpaired groups were analysed using a Mann–Whitney U or Kruskal–Wallis test for continuous variables and a chi-square test for nominal variables. Linear regression analysis was used in a univariable and multivariable way to identify determinants of sACE2 levels. For regression analysis, sACE2 concentrations were log10 transformed due to the skewed distribution. In the multivariable model, variables were entered that gave statistically significant values in the univariable model and did not show any multicollinearity. The multivariable model was based on forward stepwise regression. A two-tailed p value < 0.05 was considered statistically significant.
Results
Study population and sACE2 levels
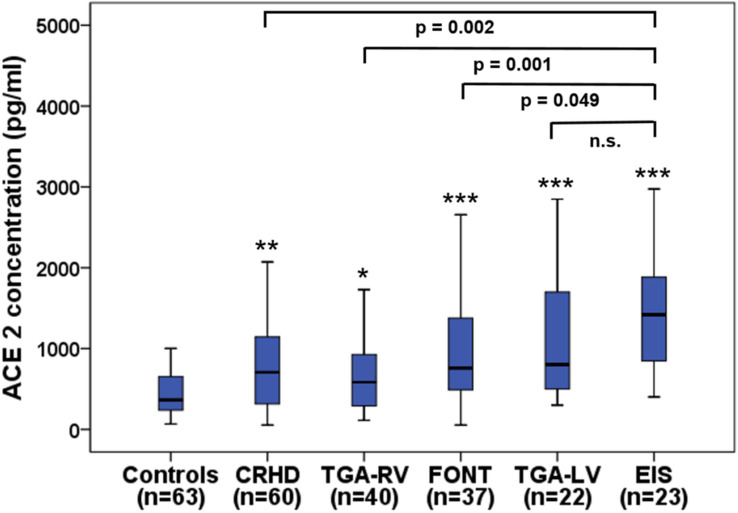
Clinical characteristics of patients based on quartile serum sACE2 concentrations are shown in Table 2. In patients with complex CHD, sACE2 levels ranged from 55.0 to 4526.1 pg/ml with a median of 761.9 pg/ml (419.2–1277.7 pg/ml) and were significantly elevated as compared to healthy controls showing a median sACE2 level of 365.2 pg/ml (237.7–656.3 pg/ml; p < 0.001). Significantly elevated sACE2 levels were found in all subgroups of CHD patients in comparison to the healthy control group with highest levels seen in patients with Eisenmenger physiology (median 1418.4 pg/ml) (Fig. 1). Moreover, patients with a systemic morphological left ventricle displayed higher sACE2 levels than those with a systemic morphological right ventricle (median 841.7 pg/ml vs 633.9 pg/ml, p = 0.026). Soluble ACE2 levels were also significantly increased in patients with a higher NYHA class ≥ III reflecting advanced heart failure as compared to patients with NYHA class I/II (median 1856.2 pg/ml vs 714.5 pg/ml, p < 0.001) (Fig. 2).
Table 2.
Baseline characteristics of patients according to sACE2 quartiles
| Variables | Quartile 1 (< 419.2) (n = 45) |
Quartile 2 (419.2–761.9) (n = 46) |
Quartile 3 (761.9–1277.7) (n = 46) |
Quartile 4 (> 1277.7) (n = 45) |
p value* |
|---|---|---|---|---|---|
| Age at enrollment (years) | 30.4 ± 9.8 | 29.5 ± 10.1 | 29.2 ± 10.2 | 31.4 ± 12.0 | 0.757 |
| Male sex | 25/45 (55.6%) | 25/46 (54.3%) | 28/46 (60.9%) | 25/45 (55.6%) | 0.925 |
| NYHA class | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.5 ± 0.7 | 1.8 ± 0.8 | 0.090 |
| Systolic blood pressure (mmHg) | 122.9 ± 12.0 | 125.1 ± 14.8 | 121.6 ± 13.9 | 123.6 ± 15.0 | 0.849 |
| Diastolic blood pressure (mmHg) | 69.6 ± 7.6 | 72.4 ± 9.8 | 69.7 ± 9.3 | 71.6 ± 10.7 | 0.489 |
| Transcutaneous oxygen saturation at rest (%) | 96.8 ± 2.8 | 96.3 ± 6.2 | 94.8 ± 5.8 | 92.2 ± 8.2 | 0.012 |
| Presence of atrial fibrillation | 0/45 (0%) | 1/46 (2.2%) | 2/46 (4.3%) | 3/45 (6.7%) | 0.326 |
| Left ventricular morphology of SV | 25/45 (55.6%) | 31/46 (67.4%) | 39/46 (84.8%) | 33/45 (73.3%) | 0.160 |
| Ejection fraction of SV (%) | 55.2 ± 8.5 | 56.5 ± 8.1 | 55.2 ± 9.4 | 53.0 ± 10.3 | 0.326 |
| VTI above aortic valve (cm) | 24.4 ± 3.9 | 25.8 ± 4.8 | 24.0 ± 4.7 | 23.6 ± 4.2 | 0.200 |
| Creatinine (mg/dl) | 0.81 (0.75–0.95) | 0.85 (0.71–0.96) | 0.85 (0.75–0.95) | 0.94 (0.81–1.09) | 0.013 |
| Estimated GFR (ml/min) | 106.9 (96.5–117.6) | 109.5 (96.3–121.8) | 109.5 (91.3–120.2) | 99.6 (71.7–118.5) | 0.313 |
| NT-proBNP (ng/l) | 151.0 (64.5–236.3) | 206.4 (87.8–314.9) | 125.5 (67.0–237.2) | 255.3 (89.6–627.7) | 0.022 |
| High sensitive troponin T (pg/ml) | 5.0 (3.0–6.0) | 4.0 (3.0–8.0) | 5.0 (3.0–7.0) | 5.0 (3.0–13.5) | 0.496 |
| Medication | |||||
| ACEI or ARB | 14/45 (31.1%) | 8/46 (17.4%) | 9/46 (19.6%) | 5/45 (11.1%) | 0.116 |
| β-blockers | 13/45 (28.9%) | 10/46 (21.7%) | 15/46 (32.6%) | 17/45 (37.8%) | 0.402 |
| MRA | 3/45 (6.7%) | 7/46 (15.2%) | 9/46 (19.6%) | 11/45 (24.4%) | 0.135 |
| Loop diuretics or thiazides | 10/45 (22.2%) | 9/46 (19.6%) | 11/46 (23.9%) | 18/45 (40.0%) | 0.116 |
| Antiarrhythmics | 2/45 (4.4%) | 0/46 (0%) | 3/46 (6.5%) | 7/45 (15.6%) | 0.072 |
Mean ± standard deviation or median (interquartile interval) are used
sACE2 soluble angiotensin-converting enzyme 2, NYHA New York Heart Association, SV systemic ventricle, VTI velocity time integral, GFR glomerular filtration rate, ACEI angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blockers, MRA mineralocorticoid receptor antagonists
*Kruskal–Wallis test
Fig. 1.
Boxplots displaying sACE2 levels in healthy controls and patients with various types of congenital heart defects. *p < 0.05, **p = 0.001, ***p < 0.001 as compared to controls. CRHD corrected congenital right heart disease, TGA transposition of the great arteries, RV systemic right ventricle, FONT Fontan palliation, LV systemic left ventricle, EIS Eisenmenger physiology
Fig. 2.
Boxplots illustrating sACE2 levels in patients with NYHA class I/II compared to NYHA class ≥ III
Association of sACE2 levels with different variables
Linear regression analysis revealed that higher sACE2 levels were significantly correlated with a higher NYHA class ≥ III, the presence of Eisenmenger physiology representing more severe CHD, a left ventricular morphology of the systemic ventricle, higher creatinine levels as well as the use of mineralocorticoid receptor antagonists (MRA) in the univariable model (Table 3). In contrast, the use of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARB) was associated with lower sACE2 levels. Interestingly, sACE2 concentrations were only weakly related to NT-proBNP (r = 0.127, p = 0.024) or high sensitive troponin T levels (r = 0.274, p = 0.010) in our study cohort.
Table 3.
Univariable and multivariable linear regression analysis for determinants of sACE2 levels
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Regression coefficient | p value | Regression coefficient (95% CI) | p value | |
| Age at enrollment | − 0.001 | 0.835 | – | – |
| Male sex | 0.018 | 0.756 | – | – |
| NYHA class ≥ III | 0.414 | < 0.001 | 0.324 (0.117–0.530) | 0.002 |
| Diagnosis of EIS | 0.313 | < 0.001 | – | – |
| Presence of atrial fibrillation | 0.301 | 0.065 | – | – |
| Left ventricular morphology of SV | 0.138 | 0.028 | – | – |
| Transcutaneous oxygen saturation at rest | − 0.019 | < 0.001 | – | – |
| Creatinine | 0.436 | 0.001 | 0.274 (0.007–0.540) | 0.044 |
| Glomerular filtration rate | − 0.003 | 0.027 | – | – |
| Medication | ||||
| ACEI or ARB | − 0.202 | 0.006 | − 0.221 (− 0.356–0.087) | 0.001 |
| β-blockers | 0.023 | 0.713 | – | – |
| MRA | 0.167 | 0.034 | – | – |
| Loop diuretics or thiazides | 0.121 | 0.067 | – | – |
| Antiarrhythmics | 0.219 | 0.053 | – | – |
sACE2 soluble angiotensin-converting enzyme 2, NYHA New York Heart Association, EIS Eisenmenger physiology, ACEI ACE inhibitors, ARB angiotensin receptor blockers, MRA mineralocorticoid receptor antagonists
In the multivariable model, however, the most significant factor independently associated with higher levels of sACE2 was found to be a higher NYHA class ≥ III (p = 0.002) whereas the use of ACEI or ARB was the most significant factor associated with lower levels of sACE2 (p = 0.001).
Discussion
In our study, significantly higher sACE2 serum levels were found in adult patients with complex CHD in comparison to healthy controls indicating the relationship of ACE2 expression to cardiovascular disease. Soluble ACE2 levels differed significantly between the studied CHD subgroups with highest concentrations found in the EIS subgroup, thus reflecting the severity of CHD (Fig. 1). Interestingly, patients with TGA-LV after the arterial switch procedure showed higher sACE2 levels than those with TGA-RV after the atrial switch operation what may be due to the morphology of the systemic ventricle since patients with a systemic morphological right ventricle showed lower sACE2 levels than those with a systemic morphological left ventricle. In addition, sACE2 concentrations were significantly increased in patients with NYHA class ≥ III reflecting advanced heart failure (Fig. 2).
Soluble ACE2 levels in other patient cohorts
Our results are consistent with a previous study illustrating increasing plasma sACE2 concentrations according to NYHA class in heart failure patients with highest levels found in the acute setting [30]. Moreover, in that study, sACE2 concentrations were found to be elevated in patients receiving MRA or loop diuretics whereas no difference was seen with the use of ACEI or ARB medication. Although brain natriuretic peptide levels were significantly related to sACE2 levels in that study, correlation of NT-proBNP with sACE2 was rather weak in our cohort of patients.
Elevated sACE2 levels were also detected in male and female patients with coronary heart disease using the same sACE2 assay by Cloud-Clone Corporation as in our study [31]. Surprisingly, the coronary heart disease patients displayed markedly higher sACE2 concentrations (about 5000–6000 pg/ml) than our CHD patients with NYHA class ≥ III (approximately 2000 pg/ml). These differences may be due to the different cardiovascular comorbidities (e.g. higher prevalence of cardiovascular risk factors, such as arterial hypertension or diabetes) and the ischemic pathophysiology of altered myocardial function in the cohort with coronary heart disease. Hence, sACE2 levels were also significantly higher in patients with multi-vessel than single-vessel disease in that study. In contrast, altered myocardial function in CHD patients is mainly due to longstanding pressure or volume overload.
Determinants of sACE2 levels
In the linear regression analysis, increased sACE2 levels were significantly related to a higher NYHA class ≥ III, more severe CHD, left ventricular morphology of the systemic ventricle, higher creatinine values and the use of MRA in the univariable model (Table 2). However, multivariable analysis revealed that a higher NYHA class ≥ III was the most significant determinant that was independently associated with higher sACE2 concentrations. In addition, the use of ACEI or ARB medication was independently associated with lower sACE2 concentrations. Our results are in accordance with a previous study investigating two independent cohorts of patients with left heart failure illustrating that ACEI or ARB uses were independent predictors of lower sACE2 concentrations whereas the use of MRA was an independent predictor of higher sACE2 concentrations [22]. In contrast to that study, however, sACE2 levels were not found to be higher in male or older patients in our study population what may be due to the different age of the studied patients. In our CHD cohort, patients were rather young with a mean age of about 30 years in contrast to the older age of the patients studied by Sama et al. with a median age of 70 years [22].
Study limitations
Our study had some limitations that need to be addressed. First, evaluation of the direct association of the measured sACE2 concentrations and the infection risk or severity of COVID-19 disease is currently not possible because data on the incidence of COVID-19 disease is not yet available for our study cohort. Moreover, sACE2 concentrations may not necessarily represent membrane-bound ACE2 expression because previous studies indicate that ACE2 shedding is mediated by a disintegrin and metalloproteinase (ADAM)-17 and may not be applied to the total extent of tissue-bound ACE2 activity [32–34]. Thus, the relation of sACE2 and the extent of ADAM-17 activity or membrane-bound ACE2 shedding is still unclear. Nevertheless, in the clinical setting, male sex, older age, the presence of cardiovascular risk factors or heart failure are known to be highly predictive for a severe course of COVID-19 disease [8, 16] and could be due to increased sACE2 concentrations [21, 22, 35]. However, further studies are warranted to evaluate the direct relationship of sACE2 concentrations and the susceptibility or course of COVID-19 disease in adult patients with complex CHD.
Conclusion
Soluble ACE2 concentrations were significantly elevated in adults with various types of complex CHD as compared to healthy controls with highest levels found in those with Eisenmenger physiology or a higher NYHA-class ≥ III reflecting advanced heart failure. However, a higher NYHA class ≥ III was the most significant determinant that was independently associated with increased sACE2 levels.
Acknowledgements
This work was supported by the German Heart Foundation, Frankfurt/Main, Germany, for the research on adults with congenital heart disease.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study has been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments, was approved by the local ethics committee (i.e. the Saarland Medical Association ethical board, approval no. 73/09) and all participants gave written and informed consent before enrollment.
References
- 1.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letko M, Marzi A. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/circresaha.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142:426–428. doi: 10.1161/circulationaha.120.047049. [DOI] [PubMed] [Google Scholar]
- 6.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/circresaha.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Molina-Molina M, Abdul-Hafez A, Uhal V, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L178–185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020 doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2:19. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culebras E, Hernández F. ACE2 is on the X chromosome: could this explain COVID-19 gender differences? Eur Heart J. 2020;41:3095. doi: 10.1093/eurheartj/ehaa521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Wang Z, Yang X, Hu B, Huang Y, Fan S. Association between circulating angiotensin-converting enzyme 2 and cardiac remodeling in hypertensive patients. Peptides. 2017;90:63–68. doi: 10.1016/j.peptides.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri L, Vanacore N, Donfrancesco C, Lo Noce C, Canevelli M, Punzo O, Raparelli V, Pezzotti P, Riccardo F, Bella A, Fabiani M, D'Ancona FP, Vaianella L, Tiple D, Colaizzo E, Palmer K, Rezza G, Piccioli A, Brusaferro S, Onder G. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020;75:1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stout KK, Broberg CS, Book WM, Cecchin F, Chen JM, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Law Y, Martin CM, Murphy AM, Ross HJ, Singh G, Spray TL. Chronic Heart Failure in Congenital Heart Disease: a Scientific Statement From the American Heart Association. Circulation. 2016;133:770–801. doi: 10.1161/cir.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 19.Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, Vidal E, Cogo P. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swärd P, Edsfeldt A, Reepalu A, Jehpsson L, Rosengren BE, Karlsson MK. Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit Care. 2020;24:221. doi: 10.1186/s13054-020-02942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laqqan M, Schwaighofer C, Graeber S, Raedle-Hurst T. Predictive value of soluble ST2 in adolescent and adult patients with complex congenital heart disease. PLoS ONE. 2018;13:e0202406. doi: 10.1371/journal.pone.0202406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Halima M, Meese E, Saleh MA, Keller A, Abdul-Khaliq H, Raedle-Hurst T. Micro-RNA 150–5p predicts overt heart failure in patients with univentricular hearts. PLoS ONE. 2019;14:e0223606. doi: 10.1371/journal.pone.0223606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Halima M, Meese E, Keller A, Abdul-Khaliq H, Radle-Hurst T. Analysis of circulating microRNAs in patients with repaired Tetralogy of Fallot with and without heart failure. J Transl Med. 2017;15:156. doi: 10.1186/s12967-017-1255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Page RL, Riegel B, Tarkington LG, Yancy CW. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Shaddy RE, Webb G. Applying heart failure guidelines to adult congenital heart disease patients. Expert Rev Cardiovasc Ther. 2008;6:165–174. doi: 10.1586/14779072.6.2.165. [DOI] [PubMed] [Google Scholar]
- 28.Chen YY, Zhang P, Zhou XM, Liu D, Zhong JC, Zhang CJ, Jin LJ, Yu HM. Relationship between genetic variants of ACE2 gene and circulating levels of ACE2 and its metabolites. J Clin Pharm Ther. 2018;43:189–195. doi: 10.1111/jcpt.12625. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol. 2008;52:750–754. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Zhang P, Liang T, Chen Y, Liu D, Yu H. Relationship between circulating levels of angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS axis and coronary heart disease. Heart Vessels. 2020;35:153–161. doi: 10.1007/s00380-019-01478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larouche-Lebel É, Loughran KA, Oyama MA. Plasma and tissue angiotensin-converting enzyme 2 activity and plasma equilibrium concentrations of angiotensin peptides in dogs with heart disease. J Vet Intern Med. 2019;33:1571–1584. doi: 10.1111/jvim.15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Oudit GY, Pfeffer MA. Plasma angiotensin-converting enzyme 2: novel biomarker in heart failure with implications for COVID-19. Eur Heart J. 2020;41:1818–1820. doi: 10.1093/eurheartj/ehaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]