Abstract
This scientific commentary refers to ‘Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease’, by Mattsson-Carlgren et al. (doi:10.1093/brain/awaa286).
This scientific commentary refers to ‘Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease’, by Mattsson-Carlgren et al. (doi:10.1093/brain/awaa286).
Alzheimer’s disease is characterized by a long presymptomatic phase during which neuropathology gradually accumulates. Detecting this pathology at the earliest possible stage offers the best chance of effective treatment. Clinical trials targeting the key pathological hallmarks of Alzheimer’s disease, such as cerebral amyloid-β aggregation and tangle formation, therefore focus on enrolling non-demented individuals with early-stage biomarker-defined disease. A reliable blood test for Alzheimer’s disease would have major implications for the selection of clinical trial participants by allowing non-demented individuals to be pre-screened in a minimally invasive and cost-effective manner, reducing the numbers who must undergo further testing with more invasive and expensive measures such as CSF analyses or PET scans.
Studies analysing plasma amyloid have shown that pre-screening with a blood test can significantly reduce further testing with more invasive measures (Verberk et al., 2018), while more recent evidence suggests that measures of plasma phosphorylated tau (p-tau) may have greater diagnostic power than plasma amyloid measures (Janelidze et al., 2020; Thijssen et al., 2020). Cross-sectional studies covering the complete clinical Alzheimer’s disease continuum have shown that plasma isoforms p-tau181 and p-tau217 can differentiate amyloid-PET or tau-PET positive cases from amyloid-PET or tau-PET negative cases (Janelidze et al., 2020; Karikari et al., 2020; Palmqvist et al., 2020; Thijssen et al., 2020). These cross-sectional studies have also shown that plasma p-tau measures can distinguish patients with Alzheimer’s disease dementia from those with frontotemporal lobar degeneration (Janelidze et al., 2020; Karikari et al., 2020; Palmqvist et al., 2020; Thijssen et al., 2020). In this issue of Brain, a timely longitudinal study by Mattsson-Carlgren and co-workers extends this work to the preclinical stages of Alzheimer’s disease, and shows the value of using p-tau217 for participant selection in clinical trials as well as for disease monitoring (Mattsson-Carlgren et al., 2020).
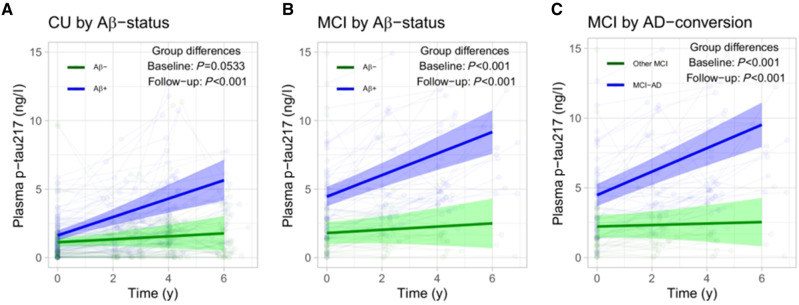
Mattsson-Carlgren et al. included 250 non-demented individuals from the Swedish BioFINDER study, and measured p-tau217 levels at baseline and during follow-up using the ‘Meso Scale Discovery’ (MSD) Eli Lilly immunoassay. The results showed that p-tau217 was increased in individuals in the preclinical and early clinical stages of Alzheimer’s disease when compared to cognitively healthy controls. In addition, higher p-tau217 levels were associated with a greater risk of converting to Alzheimer’s disease dementia and with steeper rates of cognitive decline and thinning of the temporal cortex and hippocampus. As well as showing that p-tau217 could be used to identify participants for inclusion in clinical trials, the results also suggest that p-tau217 could be used to monitor treatment responses over time. The authors showed that p-tau217 levels increased more steeply in non-demented individuals with Alzheimer’s disease pathology (amyloid positivity) than in those with no evidence of such pathology (Fig. 1). p-Tau217 levels also rose more steeply in non-demented individuals who developed Alzheimer’s disease dementia compared to those who remained non-demented during follow-up (Fig. 1). The logical next step would therefore be to analyse p-tau217 in blood samples obtained during trials that show reduction or even complete clearance of amyloid in the brain (Sevigny et al., 2016) to determine whether p-tau217 could help monitor treatment response.
Figure 1.
Longitudinal plasma p-tau217 data from Mattsson-Carlgren et al. (2020). Subject-specific biomarker data are shown together with main effects from linear mixed effects models, adjusted for age and sex, for p-tau217 in (A) amyloid-β-negative (Aβ−) cognitively unimpaired (CU) versus amyloid-β-positive (Aβ+) cognitively unimpaired; (B) amyloid-β-negative mild cognitive impairment (MCI) versus amyloid-β-positive MCI; and (C) MCI to Alzheimer’s disease (AD) dementia converters versus the remaining MCI population (i.e. stable MCI or ‘MCI to other dementia’ converters).
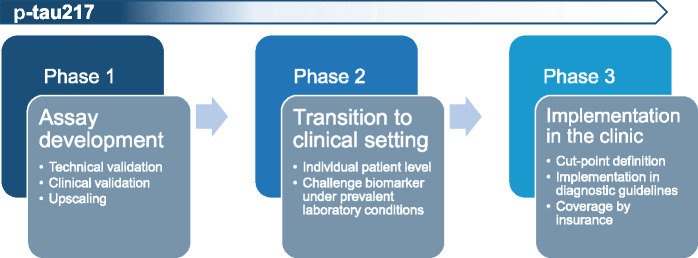
Results to date suggest that p-tau217 could also play a major role in a clinical diagnostic setting. For a biomarker to be used in the clinic, three phases of development must be completed (Fig. 2). The first phase, which focuses on assay development and validation plus initial clinical validation, has almost been completed for p-tau217. The Eli Lilly assay has shown robust outcomes (Palmqvist et al., 2020), although the results of assay validation studies have yet to be published. The second phase focuses on the transition from a research setting to a clinical setting, with the aim of ensuring that the results are consistent at the level of individual patients in different settings and to define the context of use. Here, clinical evaluation should focus on establishing large databases containing information on the plasma p-tau217 concentrations of individual patients alongside their clinical and biomarker characteristics. During this phase, work on the assay includes assessing its robustness, developing reference material, and testing biomarker stability under prevalent conditions. This may include testing the variation introduced by delays in processing samples or by the need to cool samples and transport them to the laboratory. The third phase focuses on the final definition of cut-points based on the data obtained in phase 2, and on evaluation of the assay results in real-life clinical settings in unselected patients. In parallel, insurance coverage of plasma testing for diagnostic purposes needs to be arranged where required, and the biomarker positioned in clinical diagnostic guidelines. Currently, we are close to reaching the end of phase one for p-tau217. It is thus time to transition to phases two and three, aiming for a swift implementation of the plasma p-tau217 biomarker in both trials and clinical practice, to accelerate drug development and improve clinical care for Alzheimer’s disease.
Figure 2.
Three-phase trajectory of biomarker development.
Competing interests
The authors report no competing interests.
References
- Janelidze S, Mattson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med 2020; 26: 379–86. [DOI] [PubMed] [Google Scholar]
- Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020; 19: 422–33. [DOI] [PubMed] [Google Scholar]
- Mattsson-Carlgren N, Janelidze S, Palmqvist S, Cullen N, Svenningsson AL, Strandberg O, et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer's disease. Brain 2020; 143: 3234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al.et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 2020; 324: 772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature 2016; 537: 50–6. [DOI] [PubMed] [Google Scholar]
- Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med 2020; 26: 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol 2018; 84: 648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]