See Federico and Wiebe (doi:10.1093/brain/awaa330) for a scientific commentary on this article.
Temporal lobe epilepsy is associated with progressive atrophy of the cortex at a rate more than double that of normal ageing. By measuring cortical thickness on serial structural MRI, Galovic et al. show that successful epilepsy surgery prevents further neurodegeneration and may be neuroprotective.
Keywords: epilepsy, seizures, MRI, surgery, neurodegeneration
Abstract
Focal epilepsy in adults is associated with progressive atrophy of the cortex at a rate more than double that of normal ageing. We aimed to determine whether successful epilepsy surgery interrupts progressive cortical thinning. In this longitudinal case-control neuroimaging study, we included subjects with unilateral temporal lobe epilepsy (TLE) before (n = 29) or after (n = 56) anterior temporal lobe resection and healthy volunteers (n = 124) comparable regarding age and sex. We measured cortical thickness on paired structural MRI scans in all participants and compared progressive thinning between groups using linear mixed effects models. Compared to ageing-related cortical thinning in healthy subjects, we found progressive cortical atrophy on vertex-wise analysis in TLE before surgery that was bilateral and localized beyond the ipsilateral temporal lobe. In these regions, we observed accelerated annualized thinning in left (left TLE 0.0192 ± 0.0014 versus healthy volunteers 0.0032 ± 0.0013 mm/year, P < 0.0001) and right (right TLE 0.0198 ± 0.0016 versus healthy volunteers 0.0037 ± 0.0016 mm/year, P < 0.0001) presurgical TLE cases. Cortical thinning in these areas was reduced after surgical resection of the left (0.0074 ± 0.0016 mm/year, P = 0.0006) or right (0.0052 ± 0.0020 mm/year, P = 0.0006) anterior temporal lobe. Directly comparing the post- versus presurgical TLE groups on vertex-wise analysis, the areas of postoperatively reduced thinning were in both hemispheres, particularly, but not exclusively, in regions that were affected preoperatively. Participants who remained completely seizure-free after surgery had no more progressive thinning than that observed during normal ageing. Those with postoperative seizures had small areas of continued accelerated thinning after surgery. Thus, successful epilepsy surgery prevents progressive cortical atrophy that is observed in TLE and may be potentially neuroprotective. This effect was more pronounced in those who remained seizure-free after temporal lobe resection, normalizing the rate of atrophy to that of normal ageing. These results provide evidence of epilepsy surgery preventing further cerebral damage and provide incentives for offering early surgery in refractory TLE.
See Federico and Wiebe (doi:10.1093/brain/awaa330) for a scientific commentary on this article.
Introduction
Emerging evidence from imaging (Liu et al., 2003; Bernhardt et al., 2009; Alvim et al., 2016; Caciagli et al., 2017; Govil-Dalela et al., 2018; Galovic et al., 2019b), psychometric (Jokeit and Ebner, 1999; Thompson and Duncan, 2005; Hermann et al., 2006), and EEG (Hughes, 1985; Gollwitzer et al., 2017) data suggest that epilepsy is a progressive rather than a static disease. In clinical terms, Sir William Gowers hypothesized that ‘seizures beget seizures’ more than 130 years ago (Gowers, 1881), but the notion of clinical progression remains controversial (Cole, 2000; Sutula et al., 2003).
Subjects with epilepsy showed a greater rate of cognitive decline compared to healthy individuals (Thompson and Duncan, 2005; Hermann et al., 2006). One-third of those with initially unilateral epileptic discharges on EEG progressed to bilateral discharges on subsequent recordings (Gollwitzer et al., 2017), with a progression rate of ∼1% per year (Hughes, 1985). Adults with epilepsy are well known to show widespread cortical thinning beyond the area typically considered as the epileptic focus (Whelan et al., 2018). Cortical thinning has not been shown in paediatric epilepsy patients and in siblings of patients with epilepsy, which may suggest that neurodegeneration is the consequence of a protracted disease process and, thus, may be preventable (Adler et al., 2018; Long et al., 2020).
We recently proposed serial structural MRI as a quantifiable, reproducible, and biologically valid (Cardinale et al., 2014) biomarker of progressive neurodegeneration in epilepsy (Fischl and Dale, 2000; Galovic et al., 2019b). We showed that cortical thinning in epilepsy advances at a rate more than double that of normal ageing, particularly during the first 5 years after the onset of seizures (Galovic et al., 2019b). Similar observations were made in a large number of longitudinal neuroimaging studies assessing changes in brain structure (Liu et al., 2003; Bernhardt et al., 2009; Alvim et al., 2016; Caciagli et al., 2017) or cerebral metabolism (Govil-Dalela et al., 2018). Several but not all studies observed that progressive changes were not related to seizure frequency, suggesting that neurodegeneration and loss of grey matter might be the manifestation of a widespread pathological process affecting neuronal networks in epilepsy that may continue even in the absence of overt seizures (Liu et al., 2003; Alvim et al., 2016; Galovic et al., 2019b).
Despite this mounting evidence it remains unknown how to prevent progressive neurodegeneration in epilepsy. Current anti-epileptic drugs (AEDs) are merely seizure suppressants and have not shown disease-modifying effects in humans (Marson et al., 2005). In contrast, successful epilepsy surgery can lead to disease-modification and cure, i.e. long-term freedom from seizures after withdrawing medication (de Tisi et al., 2011). Epilepsy surgery remains, however, under-utilized with a mean delay of 18 to 23 years between the onset of seizures and referral for surgery (Haneef et al., 2010). It is unknown whether epilepsy surgery affects progressive neurodegeneration in epilepsy. If so, there would be an additional incentive to identify surgical candidates early and to reduce delays in offering surgery.
Here, we aimed to determine whether epilepsy surgery prevents further progressive neurodegeneration in refractory focal epilepsy. We assessed pre- and postoperative progressive cortical thinning in patients with temporal lobe epilepsy (TLE) undergoing surgery and compared it with ageing-related thinning in a group of healthy volunteers.
Materials and methods
Participants
From an ongoing single-centre prospective cohort study of long-term outcome after epilepsy surgery (de Tisi et al., 2011), we identified consecutive individuals with medically refractory unilateral TLE who underwent standard anterior temporal lobe resections between 1 January 2004 and 31 December 2016. We included those who (i) had serial high-resolution T1-weighted MRI scans at least 6 months apart performed on the same scanner before (presurgical group) or after (postsurgical group) surgery; (ii) underwent surgery by the same neurosurgeon (A.W.M.); and (iii) had at least 1 year of postoperative follow-up. The subjects did not have a history of dementia, stroke, neurodegenerative conditions, white matter lesions, or other relevant active neurological disorders. All subjects with a pair of postoperative scans were additionally required to have had a presurgical scan to support MRI spatial preprocessing. Patients with MRI scans of insufficient quality (i.e. patient movement or technical artefacts) were excluded.
Diagnosis of TLE was made by a multidisciplinary epilepsy team evaluation based on clinical history, neurological examination, seizure semiology, long-term video-EEG telemetry, MRI, and neuropsychological and psychiatric assessments. Fluorodeoxyglucose PET, ictal single-PET, and intracranial EEG telemetry were used if needed to clarify the epileptogenic zone. Patients with concordant findings, including those with non-lesional MRI, were deemed potential surgical candidates and their anterior temporal lobe was resected as described below. Seizure outcome was prospectively assessed annually using a standard surgery outcome classification (Wieser et al., 2001). Patients were considered to be seizure-free only if they never experienced seizures or auras throughout follow-up (class Ia outcome), not considering seizures within the first week after surgery.
Repeat preoperative imaging is frequently performed at our centre for presurgical planning (mean 43 ± 22 and 15 ± 12 months before surgery in included subjects, mean interscan interval 28 ± 16 months). Serial postoperative imaging is routinely performed at our centre ≥3 months and ≥12 months after surgery (mean 4 ± 1 and 18 ± 11 months in included subjects, mean interscan interval 14 ± 11 months). MRIs in patients were acquired on a 3 T GE Signa HDx scanner using a coronal T1-weighted 3D inversion-recovery fast spoiled gradient echo sequence and 0.9 × 0.9 × 1.1 mm voxel dimensions (for detailed MRI acquisition protocols see Supplementary material).
The study was classified by the Institutional Review Board as a service evaluation involving further anonymized analysis of previously acquired data that did not require individual participant consent.
As described previously (Galovic et al., 2019b), we compared the epilepsy data to an age- and sex-matched comparison group of healthy volunteers from three publicly available anonymized longitudinal MRI datasets (Supplementary material) (Parkinson Progression Marker Initiative, 2011; Kogan et al., 2016; Liu et al., 2017). Healthy volunteers were aged between 20 and 70 years, each having two high-resolution T1-weighted scans at least 6 months apart (mean interval 20 ± 8 months).
Neurosurgical procedure
The standard neurosurgical procedure consisted of identifying the temporal horn entering from the collateral sulcus to minimize damage to the optic radiation and removing the temporal pole en bloc. This was followed by debulking of the amygdala, resection of the piriform cortex and en bloc resection of the hippocampus with a posterior resection margin at the mid-brainstem level. The resection of the parahippocampal gyrus is also taken to the same level as the hippocampus.
Typically, the anterior-posterior extent of the temporal lobe resection as measured from the temporal pole to the posterior margin of resection is 30% and 35% of the distance from the temporal pole to the occipital pole after left and right anterior temporal lobe resection, respectively. As surgery was performed by the same operator there was little variation of the temporal neocortical extent of the resection.
MRI preprocessing
The MRI preprocessing procedure is described in detail in the Supplementary material. In brief, we first extracted surgical resection masks with an automated procedure, as described previously (Galovic et al., 2019a). The masks were manually checked by an investigator (M.G.) for segmentation errors and adjusted if necessary.
Second, preprocessing of postsurgical scans can be problematic due to the lack of appropriate normalization templates and brain shift caused by surgery. We aimed to minimize the impact of the resection by patching the resected area with the corresponding region from the presurgical scan. This shares the concept with enantiomorphic normalization (i.e. patching a unilateral lesion with the unaffected contralateral side) that was shown to effectively prevent bias in scans with large lesions (Nachev et al., 2008). In this manner, we effectively mimicked the behaviour of a presurgical scan during spatial preprocessing. A detailed description and example are shown in the Supplementary material.
Third, cortical thickness was estimated using the fully automated (Dahnke et al., 2013), validated (Dahnke et al., 2013; Righart et al., 2017; Seiger et al., 2018), and reliable (Dahnke et al., 2013; Seiger et al., 2018) Computational Anatomy Toolbox (CAT12) running in SPM12 (Wellcome Centre for Human Neuroimaging), as previously described (Galovic et al., 2019b) (Supplementary material). All data were quality controlled according to procedures implemented in CAT12 and scans with misalignment, misregistration, or inaccurate thickness estimation were excluded.
Lastly, all postsurgical scans were masked with the resection mask dilated by 3 mm to remove the patched areas and to apply a safety margin for potential structural alterations in the regions immediately surrounding the resection (Supplementary material). Similarly, all presurgical and healthy volunteer scans were masked with the respective left or right temporal mean resection mask with a safety margin of 3 mm, when comparisons with postsurgical scans were performed. Cortical thickness maps were smoothed with a 15-mm surface-based kernel.
To validate the performance of the preprocessing procedure, we determined the correspondence of the processed images with the normalization template using Dice coefficients (Dice, 1945). No differences were noted in the normalization accuracy between pre- and postsurgical scans and healthy control subjects (0.96 ± 0.02 versus 0.96 ± 0.01 versus 0.95 ± 0.03, P = 0.18).
Statistical analysis
Categorical variables are displayed as n (%) and were analysed with Fisher’s exact test. Continuous variables are displayed as mean ± standard deviation (SD) and were analysed with one-way ANOVA. Calculations were carried out in SPSS (IBM Corp, Version 24.0).
Cortical thinning within regions of interest was determined in areas that showed significant vertex-wise cortical thinning in left or right TLE before surgery (Fig. 1A, different regions of interest were defined for left and right TLE). The rationale was to determine whether progressive cortical thinning changed after surgery in the areas that showed significant thinning before surgery.
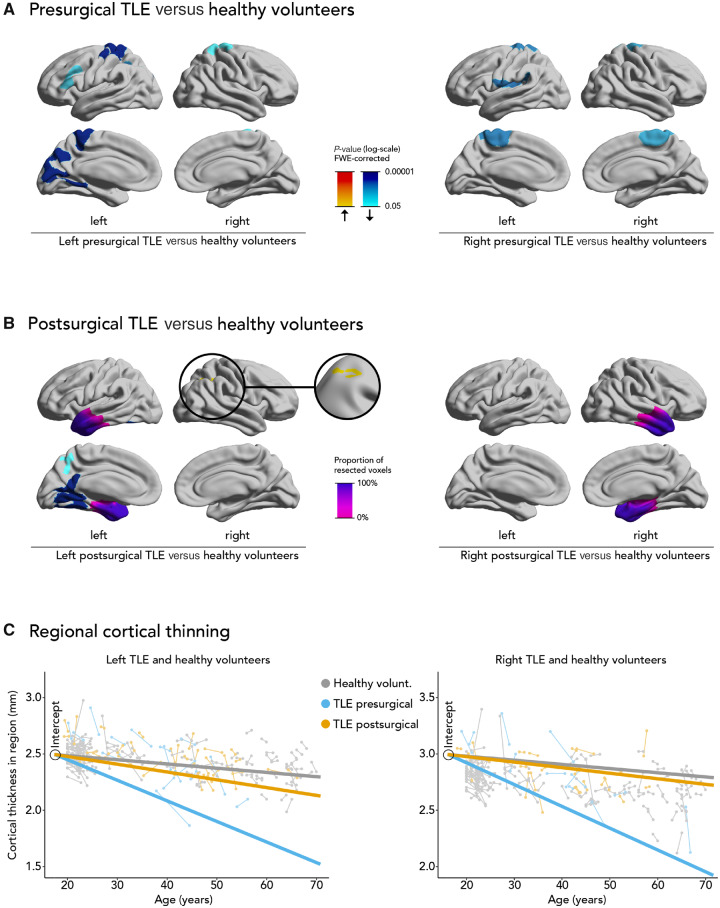
Figure 1.
Progressive cortical thinning in pre- and postsurgical epilepsy and healthy volunteers. Comparison of progressive cortical thinning in pre- (A) and postsurgical (B) epilepsy cohorts with healthy volunteers. Significant clusters (P < 0.05, correction for multiple comparisons using random field theory) and the mean resection extent are superimposed on hemispheric surface templates. Blue clusters indicate progressive atrophy, red colours indicate progressive hypertrophy, resection extent is displayed in shades of purple. FWE = familywise error. (C) Regional cortical thickness estimates and the predicted rate of regional cortical thinning in healthy control subjects (grey), pre- (blue) and postoperative (orange) TLE patients. Each scan is represented by a dot and scans corresponding to the same patient are connected by a thin line. The three thick lines are linear regression lines of mixed effects models and their slopes represent the estimated rate of cortical thinning in each group. Because mixed effects models were fitted with a variable intercept, all linear regression lines were adjusted to have the same intercept. The graph shows an accelerated rate of cortical thinning in preoperative TLE patients compared to healthy controls. This rate is largely normalized in postoperative TLE patients. The analysed regions were defined as those areas that showed significant cortical thinning before surgery in A.
Cortical thickness measurements were analysed with SurfStat within MATLAB (http://www.math.mcgill.ca/keith/surfstat) using vertex-wise and region of interest-wise approaches. We fitted linear mixed-effects models, a flexible framework for longitudinal analysis of multiple repeated measurements per subject with irregular measurement intervals. In order to test for differences between groups (e.g. pre- versus postsurgical patients) on change of cortical thickness over time, we tested for a main effect of an interaction between the group allocation and age at scan, correcting for a random effect of subject and fixed effects of age at scan, sex, and group. With this approach, we were able to test for within-subject thickness changes over time while correcting for baseline demographic differences and for different inter-scan intervals. We report findings at P < 0.05 corrected for multiple comparisons using random field theory for non-isotropic images on a cluster level (Worsley et al., 1999) or using Bonferroni correction for region of interest values of annualized cortical thinning. Annualized cortical thinning in regions of interest was estimated as the predicted slope of the linear regression lines from the mixed effects models.
Data availability
The scripts used for data preprocessing and anonymized data are available upon reasonable request.
Results
The studied cohorts involved 418 scans in 209 subjects. We included 29 TLE patients [16 (55%) left TLE] with paired presurgical scans and 56 TLE patients [31 (55%) left TLE] with paired postsurgical scans, all of whom underwent unilateral anterior temporal lobe removal. Eight patients (all left TLE) were included in both groups because they had paired pre- and postsurgical scans. We compared the pre- and postsurgical patient groups with 124 healthy control subjects that were comparable for age (38 ± 11 versus 39 ± 12 versus 38 ± 17 years, P = 0.88) and sex (52% versus 64% versus 61% female, P = 0.51). Except for differences in interscan interval, there were no differences between the pre- and postsurgical groups in baseline characteristics (Table 1). There were no differences in baseline characteristics between patients with left and right TLE (Supplementary material).
Table 1.
Baseline characteristics
| TLE presurgical (n = 29) | TLE postsurgical (n = 56) | Healthy volunteers (n = 124) | P-value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 15 (52%) | 36 (64%) | 76 (61%) | 0.51 |
| Male | 14 (48%) | 20 (36%) | 48 (39%) | |
| Age | ||||
| Age mid-scan, years | 38 ± 11 | 39 ± 12 | 38 ± 17 | 0.88 |
| Age at seizure onset, years | 16 ± 12 | 13 ± 10 | – | 0.27 |
| Age at surgery, years | 41 ± 11 | 39 ± 12 | – | 0.64 |
| Duration of epilepsy at surgery, years | 25 ± 12 | 26 ± 14 | – | 0.66 |
| Presurgical seizures | ||||
| Focal aware | 12 (41%) | 30 (54%) | – | 0.36 |
| Focal impaired awareness | 28 (97%) | 54 (96%) | – | 1.00 |
| Focal to bilateral tonic-clonic | 21 (72%) | 97 (82%) | – | 0.59 |
| Focal impaired awareness frequency, per month | 8 ± 7 | 26 ± 130 | – | 0.47 |
| Focal to bilateral tonic-clonic frequency, per month | 0.8 ± 1.8 | 0.6 ± 1.8 | – | 0.68 |
| Side of surgery | ||||
| Right | 12 (41%) | 24 (43%) | – | 1.00 |
| Left | 17 (59%) | 32 (57%) | – | |
| Pathology | ||||
| Hippocampal sclerosis | 20 (69%) | 44 (79%) | – | 0.43 |
| Dysembryoplastic neuroepithelial tumour | 5 (17%) | 4 (7%) | – | 0.26 |
| Cavernoma | 1 (3%) | 2 (4%) | – | 1.00 |
| Other | 7 (24%) | 9 (16%) | – | 0.39 |
| Surgical outcome | ||||
| Seizure free after surgery, ILAE Class Ia | 12 (41%) | 23 (41%) | – | 1.00 |
| Other | ||||
| Number of AEDs at surgery | 3 ± 1 | 3 ± 1 | – | 0.49 |
| History of a precipitating injurya | 3 (10%) | 4 (7%) | – | 0.69 |
| History of childhood febrile convulsions | 4 (14%) | 9 (16%) | – | 1.00 |
| History of depression | 9 (32%) | 19 (34%) | – | 1.00 |
| History of psychosis | 3 (11%) | 4 (7%) | – | 0.68 |
| History of anxiety disorder | 3 (11%) | 9 (16%) | – | 0.74 |
| Preprocessing accuracy (Dice coefficient) | 0.96 ± 0.02 | 0.96 ± 0.01 | 0.95 ± 0.03 | 0.18 |
Data displayed as n (%) or mean ± standard deviation. Data analysed with Fisher’s exact test for nominal variables or with one-way ANOVA for scalar variables.
Most commonly reported precipitating injuries were a history of meningitis or traumatic brain injury.
Temporal lobe epilepsy versus normal ageing
Compared to ageing-related cortical thinning in healthy volunteers, left presurgical TLE (Fig. 1A) showed accelerated thinning in the left superior pre- and postcentral gyri (1575 vertices, P < 0.0001), left cuneus, precuneus and lingual gyrus (1087 vertices, P < 0.0001), left inferior parietal lobule (548 vertices, P = 0.0005), left rostral medial and inferior frontal gyri (397 vertices, P = 0.007) and right superior pre- and postcentral gyri (511 vertices, P = 0.03). Right presurgical TLE (Fig. 1A) showed more thinning in the left inferior pre- and postcentral gyri (918 vertices, P = 0.0002), right superior pre- and postcentral gyri (822 vertices, P = 0.0004), and left superior pre- and postcentral gyri (691 vertices, P = 0.002). Presurgical cortical thinning in left or right TLE was not associated with seizure frequency.
Left postsurgical TLE (Fig. 1B) showed greater thinning than control subjects in the left posterior fusiform, lingual and cingulate gyri (1705 vertices, P < 0.0001) and left precuneus (290 vertices, P = 0.05), but these areas were smaller than those observed in the presurgical group. Conversely, progressive cortical thickening was observed in the right supramarginal gyrus and superior parietal lobule (512 vertices, P = 0.02). Progressive atrophy in right postsurgical TLE did not differ from healthy control subjects (no significant clusters, Fig. 1B). Postsurgical cortical thinning in left or right TLE was not associated with resection volume.
Presurgical left TLE patients had more cortical thinning per year (0.0192 ± 0.0014 mm/year) compared to healthy volunteers (0.0032 ± 0.0013 mm/year, P < 0.0001) and to postsurgical left TLE patients (0.0074 ± 0.0016 mm/year, P = 0.0006, Fig. 1C) in the regions that were significantly affected in left TLE before surgery (Fig. 1A). Similarly, presurgical right TLE patients had more annualized cortical thinning (0.0198 ± 0.0016 mm/year) compared to healthy control subjects (0.0037 ± 0.0016 mm/year, P < 0.0001) and to postsurgical right TLE patients (0.0052 ± 0.0020 mm/year, P = 0.0006, Fig. 1C) in the regions that were significantly affected in right TLE before surgery (Fig. 1A).
Post- versus presurgical temporal lobe epilepsy
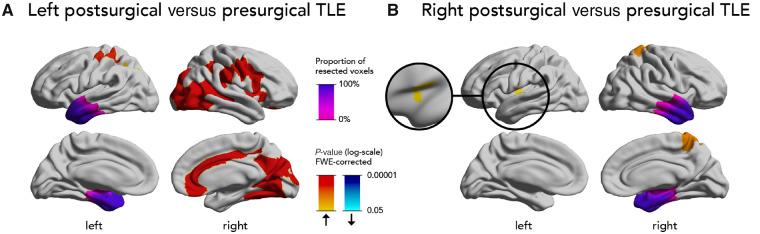
Directly comparing progressive structural changes before and after left temporal surgery (Fig. 2A), the postsurgical group showed less progressive atrophy in large clusters involving the right posterior temporal, parietal and occipital cortices (6196 vertices, P < 0.0001), right inferior and opercular frontal and parietal cortices (3715 vertices, P < 0.0001), right anterior and middle cingulate gyri (1412 vertices, P < 0.0001), left superior pre- and postcentral gyri (842 vertices, P < 0.0001), and left superior parietal lobule (415 vertices, P = 0.007).
Figure 2.
Direct comparison of progressive cortical thinning after versus before surgery. Comparison of progressive cortical thinning in postsurgical versus presurgical patients with left (A) or right (B) TLE. Significant clusters (P < 0.05, correction for multiple comparisons using random field theory) and the mean resection extent are superimposed on hemispheric surface templates. Blue clusters indicate accelerated atrophy after surgery, red colours indicate reduced atrophy after surgery, resection extent is displayed in shades of purple.
In right TLE (Fig. 2B), the postsurgical group showed less progressive cortical thinning in the right paracentral lobule and postcentral gyrus (665 vertices, P = 0.0007) and left anterior insular cortex (550 vertices, P = 0.01) compared to the presurgical group.
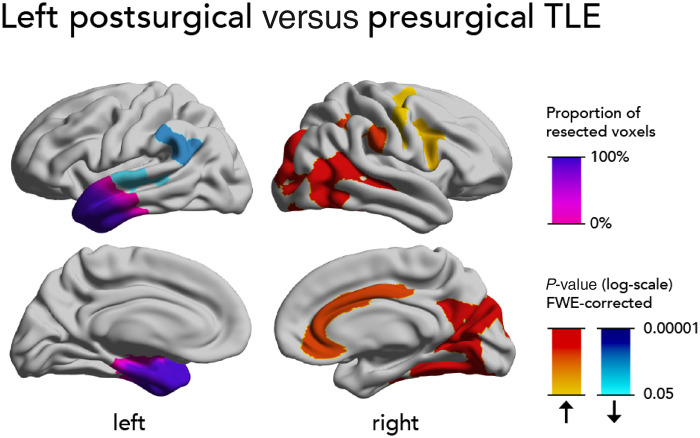
We performed a secondary analysis in eight patients with left TLE, who had both pre- and postsurgical scans (Fig. 3). Such a within-subject analysis eliminates the influence of between group differences and other confounders on the results because the pre- and postsurgical epochs are compared within the same individuals. After surgery, there was less progressive cortical thinning in large clusters involving the right posterior temporal, parietal and occipital cortices (5458 vertices, P < 0.0001), right supramarginal gyrus (1103 vertices, P < 0.0001), right cingulate gyrus (1023 vertices, P < 0.0001), right frontal opercular cortex (631 vertices, P = 0.004), and right precentral and superior frontal gyri (394 vertices, P = 0.008). We also found more progressive cortical thinning after surgery in the left supramarginal gyrus (547 vertices, P = 0.0003) and superior temporal gyrus (404 vertices, P = 0.009).
Figure 3.
Direct within-subject comparison of progressive cortical thinning after versus before surgery. Comparison of within-subject changes to progressive cortical thinning in a subgroup of eight patients with left TLE who had both pre- and postsurgical paired scans. Significant clusters (P < 0.05, correction for multiple comparisons using random field theory) and the mean resection extent are superimposed on hemispheric surface templates. Blue clusters indicate accelerated atrophy after surgery, red colours indicate reduced atrophy after surgery, resection extent is displayed in shades of purple.
Influence of surgical outcome
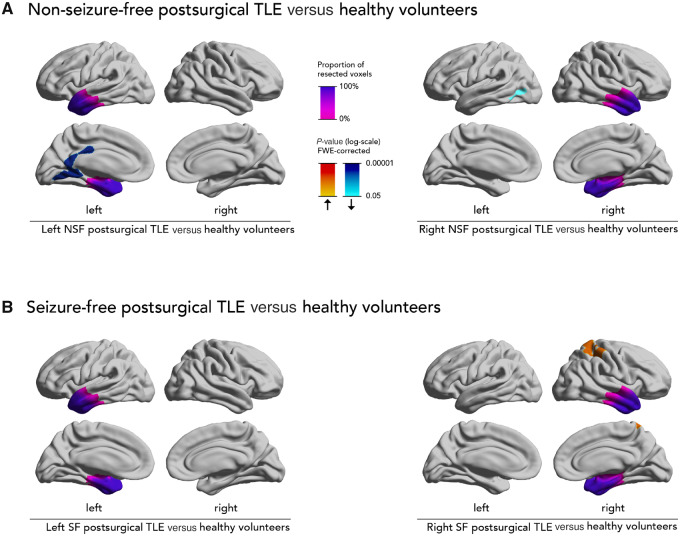
Compared to normal ageing in healthy volunteers, non-seizure-free postsurgical left TLE patients (Fig. 4A) had more progressive cortical thinning in the left lingual and posterior cingulate gyri (1037 vertices, P < 0.0001). Non-seizure-free postsurgical right TLE patients (Fig. 4A) had accelerated cortical thinning in the left lateral occipital cortex (202 vertices, P = 0.05).
Figure 4.
Progressive cortical thinning in non-seizure-free and seizure-free postsurgical epilepsy patients versus healthy volunteers. Comparison of progressive cortical thinning in non-seizure-free and seizure-free postsurgical epilepsy patients with healthy control subjects. Significant clusters (P < 0.05, correction for multiple comparisons using random field theory) and the mean resection extent are superimposed on hemispheric surface templates. Blue clusters indicate accelerated atrophy after surgery, red colours indicate reduced atrophy after surgery, resection extent is displayed in shades of purple.
Conversely, seizure-free postsurgical left TLE patients (Fig. 4B) did not differ from normal ageing in healthy volunteers (no significant clusters). Seizure-free postsurgical right TLE patients (Fig. 4B) did not show any accelerated cortical thinning compared to healthy volunteers, but they additionally showed focal cortical thickening in the right postcentral gyrus (809 vertices, P = 0.0003).
Sensitivity analyses
We performed several sensitivity analyses. We measured the coefficient of variation (CoV) of cortical thickness measurements in all groups (Supplementary material). The CoV was lower in healthy control subjects (4.0%) and in the postsurgical patient group (5.1%) compared to the presurgical patient group (5.8%).
To address the influence of AEDs on progressive thinning, we assessed the association of number of AEDs with progressive thinning and did not find any association before or after surgery (Supplementary material).
Because the preoperative group had longer interscan intervals compared to the postoperative group, we performed subgroup analyses (Supplementary material) in a presurgical subgroup with short interscan intervals (15 ± 5 months) that were comparable to the postsurgical group (14 ± 11 months). In this subgroup we confirmed accelerated cortical thinning in presurgical patients with epilepsy compared to healthy volunteers. We also confirmed that cortical thinning was significantly lessened postoperatively.
Discussion
We assessed progressive cortical thinning before and after anterior temporal lobe resection in a well-characterized cohort of unilateral TLE patients. We used a robust and reliable longitudinal neuroimaging pipeline to compare progressive changes in TLE before versus after surgery and to ageing-related thinning in matched healthy volunteers. We confirmed that individuals with TLE had accelerated cortical thinning prior to surgery in areas extending beyond the epileptic focus. We demonstrated that the rate of progressive thinning is significantly reduced during the first year after surgical removal of the anterior temporal lobe. The effect of surgery correlated with postoperative outcome and was more pronounced in those who remained completely seizure-free. Postoperatively seizure-free subjects had no more annualized thinning than that observed during normal ageing. Our results suggest that successful epilepsy surgery in the temporal lobe could have a neuroprotective effect and might prevent ongoing neurodegeneration in TLE, providing further support for the early utilization of surgery in refractory epilepsy.
Cortical thinning is a morphometric marker of neuronal loss and neurodegeneration that can be reliably and non-invasively assessed using structural MRI (Fischl and Dale, 2000; Cardinale et al., 2014). Longitudinal neuroimaging is a statistically powerful framework (Steen et al., 2007) to assess progressive neurodegeneration. Here, we showed that refractory TLE is associated with accelerated cortical thinning with a rate more than twice that of normal ageing in the affected areas (Fig. 1C), as has been characterized by us and others in previous studies (Liu et al., 2003; Bernhardt et al., 2009; Alvim et al., 2016; Caciagli et al., 2017; Govil-Dalela et al., 2018; Galovic et al., 2019b). This rate is particularly striking considering the mean 25-year duration of epilepsy in our cohort (Table 1), suggesting that even chronic refractory focal epilepsy can lead to ongoing neuronal damage. The affected areas were bilateral and spread beyond what is traditionally considered the epileptic focus (Fig. 1A), reinforcing the concept of focal epilepsy as a network disorder. These regions may be particularly vulnerable because of their high interconnection with the epileptogenic zone in the temporal lobe (Galovic et al., 2019b).
Proposed pathophysiological mechanisms underlying progressive cortical thinning in epilepsy are either the direct or indirect effects of seizures or the consequences of spreading neuronal and synaptic abnormalities in pathological epileptic networks (Galovic et al., 2019b). Seizures and synaptic alterations might lead to excitotoxicity, metabolic stress, inflammation (Vezzani et al., 2011), and cell death (Sutula et al., 2003). We hypothesized that successful surgical removal of a sufficient portion of the epileptic network leading to postoperative seizure freedom would, thus, reduce these pathological processes and would prevent further progressive cortical thinning, as has been confirmed by our results.
The key result and novel finding of the current study is that cortical thinning was significantly lessened after epilepsy surgery compared to before the surgery (Figs 1C and 2). The overall thinning rate was normalized postoperatively to a level comparable with the control subjects (Fig. 1C). After right temporal lobe resection, there were no focal areas of progressive thinning that exceeded the effects of normal ageing (Fig. 1B). After left anterior temporal lobe resections, the continuation of progressive atrophy was reduced but there still remained foci of progressive thinning in the ipsilateral posterior temporal lobe, cingulate gyrus and precuneus (Figs 1B and 3). This could be the consequence of ongoing Wallerian degeneration in nerve bundles disconnected during surgery. Alternatively, it could be explained by surgical failure in a portion (59%) of these subjects, who had continued epileptic activity and seizures that may be associated with ongoing neurodegeneration. This explanation is supported by the observation that postsurgically accelerated atrophy was not observed in patients who became completely seizure-free after surgery (Fig. 4B). Thus, accelerated cortical thinning observed in patients with ongoing seizures after surgery may be driven by continuing epileptic activity due to the incomplete removal of the epileptic focus. Whereas those with continued postoperative seizures had small areas of accelerated thinning (Fig. 4A), seizure-free subjects did not have any detectable thinning beyond normal ageing (Fig. 4B). In other words, successful surgery leading to long-term seizure-freedom might be required to completely normalize the rate of cortical thinning, whereas failed surgery with ongoing postoperative seizures might only achieve a partial reduction in cortical thinning.
The finding of reduced cortical thinning after surgery was largely consistent between the two independent groups with left and right TLE, adding support to the robustness of this result. However, there were also relevant differences between left and right temporal surgery. We detected more pronounced effects on cortical structure after removal of the left compared to the right temporal lobe (Fig. 2). This could be due to the larger sample size and statistical power of the left (n = 47) versus right (n = 38) TLE groups. In addition, TLE in the left, usually language-dominant, hemisphere is associated with more extensive presurgical structural alterations (Fig. 1A) (Whelan et al., 2018; Galovic et al., 2019b). Left TLE shows more widespread cortical thinning (Whelan et al., 2018) and abnormal microstructural integrity (Focke et al., 2008) compared to right TLE. Left TLE has an earlier onset of seizures (Blümcke et al., 2017), suggesting a more severe disease with more widespread network affection on the left. Thus, removal of the epileptic focus on the left may have more marked postoperative effects. In contrast, removal of the right anterior temporal lobe may have a smaller impact, because progressive cortical thinning is less pronounced preoperatively.
Interestingly, most of the effects after left temporal lobe resection were observed in the right hemisphere (Figs 2A and 3), potentially suggesting restitution of normal cortical structure and function in the contralateral hemisphere, that might have been presurgically affected by the spread of epileptic activity. On the other hand, these changes could reflect structural compensation as a physiological adaptation after removal of the left temporal lobe. Likewise, increases in fractional anisotropy of white matter networks after left-sided resections were thought to be linked to plasticity relevant to language function (Yogarajah et al., 2010). In a similar manner, areas of focal hypertrophy after left- (right supramarginal gyrus and superior parietal lobule, Fig. 1B) and successful right-sided (right postcentral gyrus, Fig. 4B) resections could also reflect structural compensation. The underlying mechanisms could represent reversal of a functional (Dahal et al., 2019) or metabolic (Spanaki et al., 2000) disruption leading to secondary synaptogenesis.
Consistent with our findings, one previous study found relative postsurgical increases in grey matter concentration, particularly in seizure-free cases and in the hemisphere contralateral to the resection (Yasuda et al., 2010). Postsurgically normalized cortical thinning in the ipsilateral pericentral areas observed in our study (Fig. 2) is paralleled by previously described postoperative increases in fractional anisotropy in the ipsilateral internal and external capsules and corona radiata after anterior temporal lobe removal, interpreted as postoperative plasticity after the insult of surgery (Yogarajah et al., 2010; Winston et al., 2014). Compensatory functional reorganization of language and memory networks has been observed after anterior temporal lobe resection (Bonelli et al., 2013; Sidhu et al., 2016). Efficient reorganization was associated with plasticity in the contralateral hippocampus, insular, and anterior cingulate cortex (Sidhu et al., 2016).
This study has limitations. First, our findings only apply to the first year after surgery. It is unclear whether the beneficial effects of surgery on brain structure extend beyond this timeframe, warranting further long-term studies. Studies of individuals 5–10 years after temporal resection are needed to determine if progressive atrophy is halted in the longer-term after surgery. Our results only apply to standard anterior temporal lobe resections and future research will be needed to determine whether they can be extended to other types of surgery.
Second, data from patients with epilepsy and healthy control subjects were acquired on different 3 T MRI scanners. As has been discussed in our previous study (Galovic et al., 2019b), the statistical analyses focused on within-individual changes and all individuals were rescanned on the same equipment, minimizing the effect of between-cohort differences. Moreover, all groups were comparable for baseline characteristics and the statistical analyses were additionally adjusted for relevant covariates. Post hoc analyses (Supplementary material) showed that our results cannot be explained by a reduced sensitivity to detect cortical thinning in healthy volunteers or postsurgical patients compared with presurgical patients. A significantly reduced cortical thinning after successful surgery (Fig. 2), which is the main result of the study, was not affected by scanner differences.
Third, pre- and postsurgical cortical thinning was analysed in two independent groups and the results would be more robust if all participants had paired pre- and postsurgical scans. However, a sensitivity analysis in eight patients with both pre- and postsurgical scans replicated similar results to the overall analysis. Both the main (Fig. 2A) and the sensitivity (Fig. 3) analyses showed a highly concordant reduction of progressive cortical thinning in the right hemisphere, particularly in the right posterior temporal, parietal, and occipital cortices, right supramarginal gyrus, right cingulate cortex, right frontal opercular cortex, and right precentral and superior frontal gyri. The only area of reduced atrophy in the main analysis, that was not replicated in the sensitivity analysis, was in the left superior pre- and postcentral gyri. On the other hand, the sensitivity analysis also found more progressive cortical thinning in the left supramarginal and superior temporal gyrus, possibly pointing to ongoing Wallerian degeneration after surgery.
Fourth, a limitation inherent in most epilepsy studies is the possible influence of AED intake in patients compared with healthy control subjects. There was no difference in the pre- and postsurgical number of AEDs, because medication withdrawal is not usually commenced during the first postoperative year at our centre. Additionally, it is unlikely that our results could be explained by differences in medication only because we did not find any association of AED load with progressive atrophy before or after surgery (Supplementary material).
Fifth, our epilepsy cohort was single-centre. Nevertheless, it is likely that the results are generalizable to other centres performing standard anterior temporal lobe resections, as established recommendations for this surgical procedure were followed.
Sixth, some of the secondary findings were anatomically not immediately intuitive. Focal left temporo-occipital cortical thinning after unsuccessful right temporal lobe resection (Fig. 4A) may reflect ongoing neurodegeneration due to incomplete removal of the epileptic focus. Focal right postcentral cortical thickening after successful right temporal lobe resection (Fig. 4B) may in turn reflect normalization and consequent structural hypertrophy in this area after removal of the epileptic focus. These observations may be related to the small sample size of subgroup analyses and the findings may need to be replicated in larger studies.
Lastly, immediate effects of surgery such as brain shift or Wallerian degeneration may interfere with image preprocessing, particularly during the first postoperative weeks. By studying the interval from 3 to 12 months after surgery we reduced the effect of these immediate changes on our findings. In addition, we applied a dedicated preprocessing pipeline and masked the resulting images with a safety margin to eliminate any residual effect. The spatial preprocessing accuracy was comparable between all groups, making it unlikely that the results could be explained by image misregistration.
To conclude, we provide evidence that epilepsy surgery in TLE, especially if the individuals become seizure-free, abrogates the accelerated cerebral atrophy associated with refractory TLE. Successful resective surgery is—to date—the first procedure that may prevent further neurodegeneration in epilepsy. This consideration provides a further incentive to consider surgical treatment earlier in individuals with refractory TLE, ideally after two appropriate AEDs have been tried (Haneef et al., 2010).
Funding
This study was not funded by an external agency.
Competing interests
M.G. and M.J.K. reported receiving grant MR/L013215/1 from the Medical Research Council (MRC) outside the submitted work. S.B.V. reported receiving grants from University College London/University College London Hospital (UCLH) National Institute for Health Research (NIHR) Biomedical Research Centres (BRC) during the conduct of the study. P.N. is funded by the Wellcome Trust, the Department of Health, and the UCLH NIHR BRC. S.B.V. is funded by the UCLH NIHR BRC. J.S.D. reported receiving grants from National Institute for Health Research during the conduct of the study and grants from Wellcome Trust and MRC outside the submitted work. The views expressed in this publication are those of the authors and not necessarily those of the Wellcome Trust. Data used in preparation of this article were obtained from the Neuromorphometry by Computer Algorithm Chicago (NMorphCH), Parkinson Progression Marker Initiative, and Southwest University Longitudinal Imaging Multimodal study data sets. Data collection and sharing for the NMorphCH project was funded by grant R01 MH056584 from the National Institute for Mental Health. The investigators within the Neuromorphometry by Computer Algorithm Chicago, Parkinson Progression Marker Initiative, and Southwest University Longitudinal Imaging Multimodal study contributed to the design and implementation of the respective datasets and/or provided data but did not participate in the analysis or writing of this report. No other disclosures were reported.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
- AED =
anti-epileptic drug
- TLE =
temporal lobe epilepsy
References
- Adler S, Blackwood M, Northam GB, Gunny R, Hong S-J, Bernhardt BC, et al. Multimodal computational neocortical anatomy in pediatric hippocampal sclerosis. Ann Clin Transl Neurol 2018; 5: 1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvim MKM, Coan AC, Campos BM, Yasuda CL, Oliveira MC, Morita ME, et al. Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy. Epilepsia 2016; 57: 621–9. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N.. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology 2009; 72: 1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med 2017; 377: 1648–56. [DOI] [PubMed] [Google Scholar]
- Bonelli SB, Thompson PJ, Yogarajah M, Powell RHW, Samson RS, McEvoy AW, et al. Memory reorganization following anterior temporal lobe resection: a longitudinal functional MRI study. Brain 2013; 136: 1889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caciagli L, Bernasconi A, Wiebe S, Koepp MJ, Bernasconi N, Bernhardt BC.. A meta-analysis on progressive atrophy in intractable temporal lobe epilepsy: time is brain? Neurology 2017; 89: 506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale F, Chinnici G, Bramerio M, Mai R, Sartori I, Cossu M, et al. Validation of FreeSurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinform 2014; 12: 535–42. [DOI] [PubMed] [Google Scholar]
- Cole AJ. Is epilepsy a progressive disease? The neurobiological consequences of epilepsy. Epilepsia 2000; 41 (Suppl 2): S13–22. [DOI] [PubMed] [Google Scholar]
- Dahal P, Ghani N, Flinker A, Dugan P, Friedman D, Doyle W, et al. Interictal epileptiform discharges shape large-scale intercortical communication. Brain 2019; 142: 3502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahnke R, Yotter RA, Gaser C.. Cortical thickness and central surface estimation. Neuroimage 2013; 65: 336–48. [DOI] [PubMed] [Google Scholar]
- de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WFJ, Sander JW, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 2011; 378: 1388–95. [DOI] [PubMed] [Google Scholar]
- Dice LR. Measures of the amount of ecologic association between species. Ecology 1945; 26: 297–302. [Google Scholar]
- Fischl B, Dale AM.. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97: 11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS.. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 2008; 40: 728–37. [DOI] [PubMed] [Google Scholar]
- Galovic M, Baudracco I, Wright-Goff E, Pillajo G, Nachev P, Wandschneider B, et al. Association of piriform cortex resection with surgical outcomes in patients with temporal lobe epilepsy. JAMA Neurol 2019. a; 76: 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galovic M, van Dooren VQH, Postma T, Vos SB, Caciagli L, Borzì G, et al. Progressive cortical thinning in patients with focal epilepsy. JAMA Neurol 2019. b; 76: 1230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer S, Scott CA, Farrell F, Bell GS, de Tisi J, Walker MC, et al. The long-term course of temporal lobe epilepsy: from unilateral to bilateral interictal epileptiform discharges in repeated video-EEG monitorings. Epilepsy Behav 2017; 68: 17–21. [DOI] [PubMed] [Google Scholar]
- Govil-Dalela T, Kumar A, Behen ME, Chugani HT, Juhász C.. Evolution of lobar abnormalities of cerebral glucose metabolism in 41 children with drug-resistant epilepsy. Epilepsia 2018; 59: 1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers WR, Epilepsy and other chronic convulsive disorders. London: J. & A. Churchill; 1881. [Google Scholar]
- Haneef Z, Stern J, Dewar S, Engel J.. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010; 75: 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol 2006; 60: 80–7. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Long-term clinical and EEG changes in patients with epilepsy. Arch Neurol 1985; 42: 213–23. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Ebner A.. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatr 1999; 67: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan A, Alpert K, Ambite JL, Marcus DS, Wang L.. Northwestern University schizophrenia data sharing for SchizConnect: a longitudinal dataset for large-scale integration. Neuroimage 2016; 124: 1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RSN, Lemieux L, Bell GS, Hammers A, Sisodiya SM, Bartlett PA, et al. Progressive neocortical damage in epilepsy. Ann Neurol 2003; 53: 312–24. [DOI] [PubMed] [Google Scholar]
- Liu W, Wei D, Chen Q, Yang W, Meng J, Wu G, et al. Longitudinal test-retest neuroimaging data from healthy young adults in southwest China. Sci Data 2017; 4: 170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L, Galovic M, Chen Y, Postma T, Vos SB, Xiao F, et al. Shared hippocampal abnormalities in sporadic temporal lobe epilepsy patients and their siblings. Epilepsia 2020; 61: 735–46. [DOI] [PubMed] [Google Scholar]
- Marson A, Jacoby A, Johnson A, Kim L, Gamble C, Chadwick D, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet 2005; 365: 2007–13. [DOI] [PubMed] [Google Scholar]
- Nachev P, Coulthard E, Jäger HR, Kennard C, Husain M.. Enantiomorphic normalization of focally lesioned brains. Neuroimage 2008; 39: 1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011; 95: 629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righart R, Schmidt P, Dahnke R, Biberacher V, Beer A, Buck D, et al. Volume versus surface-based cortical thickness measurements: a comparative study with healthy controls and multiple sclerosis patients. PLoS One 2017; 12: e0179590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiger R, Ganger S, Kranz GS, Hahn A, Lanzenberger R.. Cortical thickness estimations of FreeSurfer and the CAT12 Toolbox in patients with Alzheimer's disease and healthy controls. J Neuroimaging 2018; 28: 515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu MK, Stretton J, Winston GP, McEvoy AW, Symms M, Thompson PJ, et al. Memory network plasticity after temporal lobe resection: a longitudinal functional imaging study. Brain 2016; 139: 415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanaki MV, Kopylev L, Decarli C, Gaillard WD, Liow K, Fazilat S, et al. Postoperative changes in cerebral metabolism in temporal lobe epilepsy. Arch Neurol 2000; 57: 1447–52. [DOI] [PubMed] [Google Scholar]
- Steen RG, Hamer RM, Lieberman JA.. Measuring brain volume by MR imaging: impact of measurement precision and natural variation on sample size requirements. AJNR Am J Neuroradiol 2007; 28: 1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula TP, Hagen J, Pitkänen A.. Do epileptic seizures damage the brain? Curr Opin Neurol 2003; 16: 189–95. [DOI] [PubMed] [Google Scholar]
- Thompson PJ, Duncan JS.. Cognitive decline in severe intractable epilepsy. Epilepsia 2005; 46: 1780–7. [DOI] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ.. The role of inflammation in epilepsy. Nat Rev Neurol 2011; 7: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan CD, Altmann A, Botía JA, Jahanshad N, Hibar DP, Absil J, et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 2018; 141: 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 2001; 42: 282–6. [PubMed] [Google Scholar]
- Winston GP, Stretton J, Sidhu MK, Symms MR, Duncan JS.. Progressive white matter changes following anterior temporal lobe resection for epilepsy. Neuroimage Clin 2014; 4: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC.. Detecting changes in nonisotropic images. Hum Brain Mapp 1999; 8: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda CL, Valise C, Saúde AV, Pereira AR, Pereira FR, Ferreira Costa AL, et al. Dynamic changes in white and gray matter volume are associated with outcome of surgical treatment in temporal lobe epilepsy. Neuroimage 2010; 49: 71–9. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain 2010; 133: 2348–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The scripts used for data preprocessing and anonymized data are available upon reasonable request.