Abstract
Treat-to-target strategies have changed the approach to management of many chronic conditions, with improvements in patient outcomes. The key to success of treat to target is the availability of validated treatment endpoints, which have been difficult to derive for SLE, a condition notorious for its heterogeneity. This review will focus on the development and validation of the definitions of remission in SLE framework and the lupus low disease activity state. Lupus low disease activity state is more attainable than remission, with a stepwise concentric relationship between the target states indicating increasing stringency. Both lupus low disease activity state and definitions of remission in SLE remission have been proven to be associated with reduction in disease flares, reduced risk of accrual of irreversible end organ damage, and improvement in patient reported outcomes. These endpoints have therefore provided the key for the development of a treat-to-target approach in clinical practice in SLE and for the design of future clinical trials.
Keywords: SLE, treat to target, treatment endpoints, remission, lupus low disease activity state
Rheumatology key messages
Treat to target (T2T) is needed to improve outcomes for patients with systemic lupus erythematosus.
T2T endpoints in the form of low disease activity and remission have been recently developed.
Attainment of either of these is associated with reduced disease flares and damage accrual.
Introduction
SLE is a chronic multisystem autoimmune disease resulting in significant morbidity and loss of life expectancy. Compared with other rheumatic conditions where new targeted therapies have resulted in high rates of remission or low disease activity, the effect sizes of therapies for SLE have been relatively small [1, 2]. The majority of SLE patients are still treated with chronic glucocorticoids and non-specific immunosuppressants. Despite overall improvement, ten-year mortality from SLE is estimated at up to 1 in 8 for patients with renal involvement [3]; and thus premature death remains a risk for the young women who comprise the majority of patients affected. Those patients that do survive are often burdened with problems of chronic disease, which include not only activity of the disease itself, adverse effects of treatment and complications such as irreversible end organ damage, but also impacts on outcomes such as quality of life, employment and disability.
The adoption of treat-to-target (T2T) strategies have improved patient outcomes in other chronic conditions that follow the paradigm that poor control of the disease process leads to irreversible organ damage. This has been achieved without necessarily the need for new therapeutic agents, such as is the case of tighter control of hypertension or diabetes to prevent ischaemic cardiovascular events [4–7]. This, coupled with the successful implementation of low disease activity and remission as target states in RA, prompted a push for the development of equivalent treatment target states for SLE [8].
The process of developing clinical treatment targets for a complex, multisystem, heterogeneous disease such as SLE has been challenging, and is arguably not entirely complete. Nonetheless, work over the past several years has seen the emergence of key target states in the form of the lupus low disease activity state (LLDAS) and the definitions of remission in SLE (DORIS) framework; which may, for the first time, allow the adoption of a T2T approach in SLE.
Impact of T2T in other disease
In the clinical context, ‘T2T’ implies a process of initiating and adjusting therapy to achieve and maintain a predefined treatment goal (clinical state, laboratory marker or combination of both). Conceptually these treatment targets or endpoints must have utility (be attainable and sustainable by the majority of patients) and validity (empirical evidence of their association with desired patient outcomes).
T2T approaches have had profound impact in the management of many chronic diseases, especially those in which treatment endpoints are measurable in single organ systems, with resultant substantial reduction in the frequency of complications as well as overall mortality [4–7]. No inflammatory rheumatic condition, perhaps with the exception of uric acid in gout, has a stand-alone biomarker that accurately corresponds to treatment effect or can be undisputedly linked to improved outcomes. Correspondingly, clinical improvement may not necessarily rule out underlying inflammatory activity. As such, composite instruments using both clinical and laboratory measures are relied on to quantify a target state in rheumatology. In RA, T2T approaches, based on the attainment of low disease activity or remission defined by number of inflamed joints, physician and/or patient global assessment and measurement of inflammatory markers, have resulted in dramatically improved outcomes even prior to the introduction of biological therapies [9], and have been adopted in treatment guidelines and the assessment of novel therapies [10]. Moreover, there is evidence that attainment of a target state, rather than measuring treatment response as a change in disease activity from baseline, confers greater protection from accrual of joint damage [11], and as such there is a move to change the primary outcome endpoints in clinical trials of RA to ‘time to’ and ‘time in’ low disease activity and/or remission [10].
Targetable risk factors predicting poor outcomes in SLE
High morbidity in SLE is driven predominantly by poorly controlled disease activity and accrual of irreversible organ damage, both of which impact on health-related quality of life (HR-QoL); thus, making damage and HR-QoL the most frequent outcomes studied in SLE [12]. Damage in SLE refers to the diagnosis of irreversible end organ manifestations such as stroke, end stage renal failure or osteoporosis; it is therefore not surprising that damage accrual increases the likelihood of early mortality [13]. While some predictors of damage are not modifiable, such as older age and non-Caucasian ethnicity [14, 15], there are strong associations of high disease activity levels and glucocorticoid use as independent and modifiable risk factors for damage accrual [16–18].
SLE has a fluctuating nature with periods of relative inactivity contrasted by disease flares, although some patients have persistently active disease despite best efforts at management [19]. There is evidence that both persistent disease activity and disease flares can contribute to irreversible damage [16, 17]; therefore, reduction of overall activity levels and prevention of disease flares are valuable conceptual treatment targets. Disease activity in SLE can be measured as clinical activity (reflecting inflammation in end organs) or serological activity (elevation of antibodies to dsDNA levels or lowering of complement component 3 and/or 4 levels). While there is no doubt that untreated end organ inflammation leads to damage accrual, the role of serological activity in contributing to outcomes is less clear. ‘Serologically active clinically quiescent’ disease is a well-described entity in SLE [20], with some literature suggesting a proportion of serologically active clinically quiescent patients can spend years without emergence of new disease features [21], while others may flare [22, 23]. Certain clinical manifestations, such as lupus nephritis, are more frequent in patients with elevated anti-dsDNA levels. Patients with serologically active disease, particularly the classic markers described above, are more likely to respond to some targeted therapy [24]. The same group of patients were also found to be more likely to flare [25]. Given this potential link between serological activity and disease outcomes, the most stringent target states in SLE, such as DORIS ‘complete’ remission, require the absence of both clinical and serological disease activity (Table 1). In contrast, the more lenient target states such as LLDAS or clinical remission on treatment (CROT) allow for the presence of serological activity, but not together with clinical activity (Table 1).
Table 1.
Definition of LLDAS, DORIS clinical remission on treatment and DORIS complete remission
| LLDAS | DORIS clinical remission on treatmenta | DORIS complete remissionb |
|---|---|---|
| SLEDAI-2K ≤4, with no activity in major organ systems and no new features of activity compared to previous assessment | Clinical SLEDAI=0 | Clinical SLEDAI=0 |
| Serological activity allowed (as long as total SLEDAI-2K ≤4) | Serological activity allowed | No serological activity |
| SELENA-SLEDAI PGA ≤1 (scale 0–3) | SELENA-SLEDAI PGA ≤0.5 (scale 0–3) | SELENA-SLEDAI PGA ≤0.5 (scale 0–3) |
| Current prednisolone (or equivalent) dose ≤7.5 mg | Low-dose glucocorticoids (e.g. prednisone ≤5 mg/ day) allowed | No glucocorticoids |
| Standard maintenance doses of immunosuppressive drugs and approved biological agents, excluding investigational drugs | Maintenance antimalarials, immunosuppressives and/or stable (maintenance) biologics allowed | Maintenance antimalarials allowed, but no immunosuppresives and/or biologics |
Serological activity – elevation of antibodies to dsDNA levels above the upper limit of laboratory normal or lowering of complement component 3 and/or 4 levels below the lower limit of laboratory normal. aMost attainable of the eight possible definitions of remission. bLeast attainable of the eight possible definitions of remission. DORIS: definitions of remission in systemic lupus erythematosus; LLDAS: lupus low disease activity state; PGA: physician global assessment; SELENA-SLEDAI: Safety of Estrogen in Lupus National Assessment-SLEDAI; SLEDAI-2K: SLEDAI 2000.
Despite evidence that prednisolone doses of ≥7.5 mg are associated with adverse outcomes and independently predict damage accrual [18], glucocorticoids continue to be relied upon in the absence of effective alternate therapies. More recently, glucocorticoids have also been shown to independently contribute to damage not traditionally associated with steroid use in domains other than osteoporosis, avascular necrosis, diabetes mellitus or cataracts [26]. Therefore, use and dosing of glucocorticoids must be considered when thinking about target clinical states in SLE. Perhaps most importantly, it is now known that once damage is established it propagates further damage, irrespective of disease activity control [27], further highlighting the need to control the disease and reduce activity levels early in the treatment course to minimize the risk of damage accrual in the first place.
Challenges of developing and adopting T2T in SLE
The success of the T2T approach in the treatment of hypertension and diabetes is underpinned by the availability of easily measurable treatment targets (blood pressure and HbA1c, respectively). Such is not the case for SLE, which poses some unique challenges, mainly due to the heterogeneous nature of the disease and incomplete understanding of its pathophysiology.
Because of this clinical heterogeneity, composite measures are used to assess the extent and levels of activity within different organ systems. Several instruments have been developed, of which the SLEDAI 2000 [28], BILAG [29] and ECLAM [30] are amongst the most commonly used. These indices include clinical manifestations and in some cases serological markers of disease activity. The lack of one accepted disease activity score probably reflects the inability of the available options to optimally express disease activity. One of the biggest differences between the indices is their ability to capture incremental changes in disease activity. For example, in SLEDAI 2000, the extent of arthritis does not alter the score (i.e. a patient with two swollen joints has the same score as a patient with 12 swollen joints). While BILAG does account for incremental differences to some extent, training and time to complete BILAG makes this measure less feasible for routine clinical practice; in addition, the requirement in BILAG for the current state to be compared with the previous assessment limits its suitability for use in defining T2T states. Thus, assessing the ceiling of disease activity in SLE is a challenge. However, the absence of disease activity is more attainably measured, as with diminishing activity, patients become more homogeneous and easier to group together and the lack of sensitivity of cutoffs is less impactful. Therefore, defining a state based on low levels or absence of disease activity may be intrinsically more achievable than attempting to quantify active disease across multiple systems. This allows for outcome measurement in a binary fashion – a patient is either in the desired low or absent activity state or not, and ensures that the endpoint or outcome achieved is consistent across all patients.
Development of remission and LLDAS definitions and their effects on damage and flares
In the treatment of any disease, cure is the ultimate goal. As a cure for SLE does not seem possible in the foreseeable future, attaining remission is the next best disease state that can be envisaged. Remission is usually defined as the absence of disease activity as measured by a chosen disease activity index. Subjective symptoms such as fatigue and pain are not considered in these indices. Previous studies have shown that attaining prolonged drug-free remission is rare. In a Canadian study, in which prolonged remission was defined as a 5-year consecutive period of no disease activity (SLEDAI = 0) and no treatment (corticosteroids, antimalarials or immunosuppressants) allowed, only 1.7% of patients fulfilled the criteria [31]. Thus, even though this stringent form of remission is desirable, it does not seem feasible for the majority of patients.
Since 2012, an international group of SLE experts has developed remission criteria to be used in a T2T approach in SLE. The DORIS taskforce proposed eight potential definitions of remission. All require the absence of any clinical activity as measured by a clinical SLEDAI of 0 and a physician global assessment ≤0.5 on a scale of 0–3, but vary in allowing for serological activity, use of immunosuppression and prednisolone of up to 5 mg. Out of the eight possible definitions of remission according to the DORIS framework, the least stringent and therefore the one with the most practical use is the definition based on the clinical SLEDAI=0, irrespective of serology and allowing for certain treatments (antimalarials, low-dose glucocorticoids, and immunosuppressives including biologicals), sometimes also referred to as CROT (Table 1). Observational studies from Northern and Southern America, Europe and Asia applying these criteria showed an association with reduced damage accrual for patients in DORIS remission consistently (Table 2) [32, 34–36, 40, 41, 47]. Prolonged remission on treatment according to DORIS criteria is more attainable than strict remission (off treatment) and was seen in between 25% and 37% of patients. Furthermore, there was an association between the duration of remission and the reduction in damage accrual [38, 41, 48]. The variables associated with likelihood of attaining remission are those indicative of less severe disease, such as lower disease activity at diagnosis, lower damage index at the start of observation or absence of lupus nephritis [34, 35, 49].
Table 2.
Effect of remission and LLDAS on damage accrual in observational cohorts
| Author | Year of publication | Number of patients | Clinical remission on/off therapy | LLDAS ≥50% | Association with damage accrual | |
|---|---|---|---|---|---|---|
| Remission | LLDAS ≥50% | |||||
| Zen et al. [32] | 2015 | 224 | 37.4% of patients had ≥5 consecutive years of remission | Unremitted disease had higher odds of damage accrual (OR 2.53; 95% CI 1.28, 4.99) | ||
| Franklyn et al. [33] | 2016 | 191 | 33.0% of visits in LLDAS | Patients with LLDAS ≥50% lower risk of damage accrual (RR 0.47; 95% CI 0.28, 0.79) | ||
| Tsang-A-Sjoe et al. [34] | 2017 | 183 | 32.5% of patients had ≥5 consecutive years of remission | 64.5% of patients had ≥50% of visits in LLDAS | Reduced risk of damage accrual for patients ≥5 consecutive years of remission (OR 0.20; 95% CI 0.07, 0.052) | Reduced risk of damage accrual (OR 0.52; 95% CI 0.28, 0.99) |
| Mok et al. [35] | 2017 | 769 | 25.1% of patients had ≥5 consecutive years of remission | Reduced risk of damage accrual for patients ≥5 consecutive years of remission (OR 0.17; s.d. ±0.53, P<0.001) | ||
| Ugarte-Gil et al. [36] | 2017 | 1350 | 11.6% of visits in remission | 10% of visits in LDAS | Patients in remission had a lower hazard of new damage (HR 0.53; 95% CI 0.38, 0.75). No effect on mortality. | Patients in LDAS had a lower hazard of new damage (HR 0.61; 95% CI 0.44, 0.85). No effect on mortality. |
| Zen et al. [37] | 2017 | 293 | 37.2% of patients in LLDAS ≥5 years | Reduced damage accrual for patients in ≥2 years of LLDAS | ||
| Petri et al. [38] | 2018 | 1356 | 40% of follow-up time was spent in any form of remission | 50% of follow-up time was spent in LLDAS |
|
|
| Tani et al. [39] | 2018 | 115 | 45% of visits in remission on therapy | 70% of visits in LLDAS | Reduced damage accrual for patients in remission at all visits compared to patients who were not (0.12 vs 0.48 points, P=0.018) | Reduced damage accrual for patients in LLDAS at all visits compared to patients who were not (0.11 vs 0.63 points, P<0.001) |
| Fasano et al. [40] | 2019 | 294 | 44.5% of patients had ≥5 consecutive years of remission | Patients in remission for 5 consecutive years had a greater overall cardiovascular event-free rate. HR 0.11 (95% CI 0.02, 0.47) | ||
| Alarcón et al. [41]a | 2019 | 558 | 1.8% of visits in remission | 15.1% of visits in LDAS | Time spent in combined remission/LDAS was associated with reduced damage accrual. Rate ratio 0.18 (95% CI 0.12, 0.26) | |
| Golder et al. [42] | 2019 | 1707 | 47.9% of visits in LLDAS | Attainment of LLDAS at any timepoint was associated with reduction in damage accrual (hazard ratio 0.59, 95% CI 0.45, 0.76). Patients in LLDAS ≥50% reduced risk of damage accrual (HR 0.54; 95% CI 0.42, 0.70) and flare (HR 0.41; 95% CI 0.35, 0.48) | ||
| Golder et al. [43] | 2019 | 1707 | 35.8% of visits in remission (definition 3) | Patients in ≥50% visits in remission (definition 3) reduced risk of damage accrual (HR 0.49; 95% CI 0.38, 0.65) | LLDAS more attainable (47.9% of visits) with similar risk reduction (HR 0.54; 95% CI 0.42, 0.70) | |
| Floris et al. [44] | 2019 | 116 | 21.6% clinical remission 6 months after diagnosis | 42.2% LLDAS 6 months after diagnosis | Reduced damage accrual for patients in clinical remission after 6 months (OR 0.10; 95% CI 0.01, 0.77) | Reduced damage accrual for patients in LLDAS after 6 months (OR 0.20; 95% CI 0.05, 0.50) |
| Sharma et al. [45] | 2020 | 69 | 33.5% of patients LLDAS ≥50% of visits | Patients in LLDAS ≥50% reduced risk of damage accrual (HR 0.37; 95% CI 0.19, 0.73) and mortality (HR 0.31; 95% CI 0.16, 0.62) | ||
Disease activity measured with SLAM. HR: hazard ratio; LDAS: low disease activity score according to individual study criteria; LLDAS: lupus low disease activity score; OR: odds ratio; RR: relatice risk as defined by Franklyn et al. [46]
Whilst remission should remain the key target state in any inflammatory disease, in SLE the stricter forms of remission are seldom attained or sustained with currently available therapies. As such, the need for a more attainable target that is still associated with protection from adverse outcomes became apparent [8, 46]. In response to this, the Asia Pacific Lupus Collaboration (APLC) embarked on a series of studies to define and validate the LLDAS. Like DORIS remission, LLDAS is a composite outcome measure that includes activity and treatment-related domains, derived using Delphi consensus methods [33]. Intuitively, the cut-offs for these are more lenient than remission, such as a SLEDAI ≤ 4 and physician global assessment ≤1, as well as prednisolone ≤7.5 mg/day; but with the additional criteria of no new activity (clinical or serological) since the previous patient assessment (Table 1) [33]. Over the course of 6 years, the APLC has completed face, content, construct and criterion validity studies of LLDAS, with the overall conclusion that LLDAS is an attainable treatment target that is associated with reduced disease flares and damage accrual, as well as improved patient-reported outcomes (Table 2) [33, 42, 50–52].
In a prospective APLC study that followed 1707 patients for a mean of 2 years, LLDAS was attained in just under half of all visits, with almost 80% of the cohort being able to attain LLDAS on at least one occasion, demonstrating the utility of LLDAS as a feasible target state. In the same study, even a single visit in LLDAS resulted in a 30–40% reduction in subsequent disease flare and damage accrual [42]. Furthermore, the magnitude of the protective effect increased incrementally with increasing durations of time spent in LLDAS, with almost a 90% reduction in risk of damage in patients who sustained LLDAS for 12 months or more. Similar associations of attainment of LLDAS with significant reduction in damage accrual have been found in other cohorts, with a ‘dose-dependent’ relationship between time spent in LLDAS and reduction in risk of damage. In particular, studies of three separate cohorts have shown that 50% of observed time in LLDAS corresponds to a ∼50% reduction in damage accrual [34, 38, 45]. In a longer follow-up study of 200 Norwegian patients with SLE, not only was ≥50% observed time spent in LLDAS protective against damage accrual, it was also associated with an almost 70% reduction in mortality [45].
Several studies have compared the effects of DORIS remission and LLDAS on flares and damage accrual (Table 2) [34, 37–39, 43, 44]. While both LLDAS and remission were associated with reduced flares and damage [34], less time was needed in remission to see significant associations (<25% observed time for remission vs 25–50% observed time for LLDAS), suggesting a stronger protective effect of the deeper remission states [38]. On the other hand, the stricter definitions of remission were difficult to achieve [47] and to maintain for more than a single visit (7.1% of patients in complete remission for ≥2 consecutive visits) [43]. In all but one study, LLDAS was more attainable and sustainable than any remission definition; the exception was described in a monocentric prevalent cohort of Caucasian patients with unusually high rates of remission [37]. In contrast, in an inception cohort of newly diagnosed SLE, LLDAS was attained in twice as many patients compared with clinical remission within 6 months of treatment (42.2% vs 21.6%) [44]. In the APLC cohort, all eight DORIS definitions of remission and LLDAS were compared side by side. LLDAS and remission were shown to be concentric stepwise target states, such that patients who fulfil the criteria for the stricter forms of remission also fulfilled criteria for LLDAS, but not vice versa (Fig. 1) [43]. Interestingly, a small proportion of patients fulfilled the criteria for CROT but not LLDAS, based on new serological activity from previous assessment (new elevation of antibodies to dsDNA or lowering of complement levels), which is not allowed in LLDAS and not accounted for in CROT (Fig. 1). Likewise, each increase in the stringency of a target state resulted in a reduction in attainability, with sufficient separation between LLDAS and remission (tested by assessing only those visits meeting criteria for LLDAS but not for remission) to demonstrate the usefulness of LLDAS and remission as individual stepwise targets.
Fig. 1.
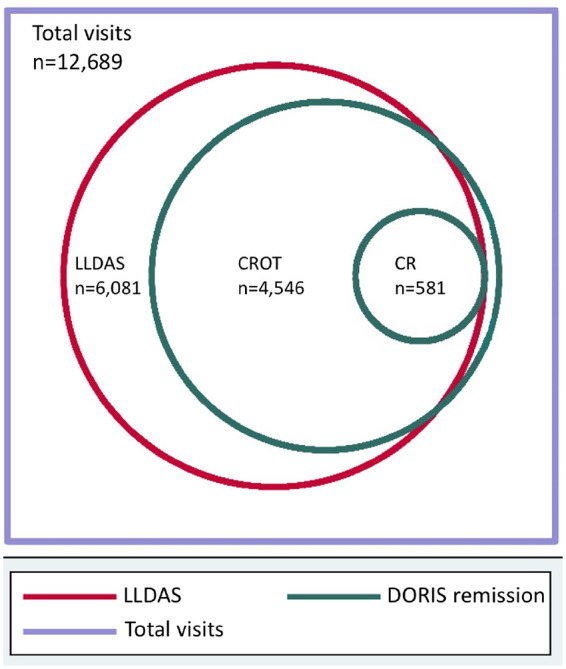
Stepwise concentric attainment of LLDAS and DORIS remission
Adapted with permission from Golder et al. [49], 1707 SLE patients were followed for a mean of 2.2 years, totalling 12 689 observed visits. Of these, 6081 visits (47.9%) fulfilled criteria for LLDAS, 4546 visits (35.8%) fulfilled criteria for DORIS CROT and 581 visits (4.6%) fulfilled criteria for DORIS complete remission. CROT: clinical remission on treatment; DORIS: definitions of remission in systemic lupus erythematosus; LLDAS: lupus low disease activity state.
Effect of LLDAS and remission on patient-reported outcomes
The negative impact of SLE on HR-QoL is comparable to other chronic diseases such as chronic heart failure, coronary artery disease, end-stage airways disease, human immunodeficiency virus and RA [53–55]. From a patient’s perspective, HR-QoL is an important outcome parameter, as it reflects aspects of the burden of disease not captured in physician measures.
In two studies of different cohorts, prolonged remission (≥5 years) was associated with higher HR-QoL as measured by both SF-36 and LupusPRO [35, 56]. No association was found with the mental component, which was also shown in a separate Italian study [57]. Likewise, two large cohort studies assessing the relationship between LLDAS and HR-QoL have demonstrated that LLDAS is associated with improved HR-QoL, as measured with both a generic (SF-36) and an SLE-specific (SLEQOL) instrument [51, 58]. These observations further support the use of clinical remission or LLDAS as a target of SLE. While the more lenient definitions of remission and the LLDAS definition of low disease activity may perform similarly from a measurement standpoint, it is important to note the conceptual difference between a disease state that is defined as the complete absence of clinical symptoms and a state that allows for a certain low level of disease activity.
The role of T2T endpoints in clinical trials
When compared with other rheumatic diseases, there has been a considerable lag in the development of effective targeted therapies for treatment of SLE. Of the multiple potential therapeutic agents in the clinical trial pipeline, only two have managed to hit Phase III trial primary endpoints in the last 8 years. Belimumab, an antibody blocking B-lymphocyte stimulation, reached statistical significance in two Phase III trials, particularly in serologically active patients with musculoskeletal and mucocutaneous disease [1, 2]. However, the absolute effect size of belimumab over placebo as measured by the SLE responder index (SRI) appears to be small, suggesting both the need for a more robust endpoint to better discriminate responders from non-responders and the need for therapies with different mechanisms of action. Anifrolumab, an antibody to the type I interferon receptor, achieved the primary endpoint of the British Isles Composite Lupus Assessment (BICLA) in a second pivotal Phase III trial [59].
The reasons for the lacklustre results in SLE trials are multifactorial, including complex immunopathogenesis, clinical and biological disease heterogeneity, debate about optimal trial design with criticisms regarding the dose of concomitant glucocorticoids and immunosuppression allowed, and, most importantly, endpoint measures that may have hindered the ability to differentiate responders from non-responders [49, 60]. The use of historical endpoints that lack sufficient validation for use as primary outcome measures for clinical trials in SLE is a state of affairs recently described as a ‘crisis’ [61]. And yet, in the absence of other options, these historical endpoints have continued to be used as a priori primary outcomes.
In this setting, LLDAS is now being tested as an outcome measure in clinical trials of existing and novel therapies. In a head-to-head superiority comparison of mycophenolate and azathioprine in patients with active SLE, LLDAS was assessed as a secondary discriminant outcome measure, with more patients in the mycophenolate treatment group attaining and sustaining LLDAS compared with patients treated with azathioprine (79% vs 57% at 12 months, respectively) [62]. In studies of novel therapies including belimumab, atacicept, baricitinib and anifrolumab, LLDAS was able to discriminate responders to active drug from placebo [63–66]. Moreover, LLDAS was a more stringent discriminator compared with currently used responder indices such as SRI and BICLA [63–65]. In the post-hoc analysis of the anifrolumab Phase IIb trial, 74–87% of patients in LLDAS at week 52 were also SRI/BICLA responders; on the other hand, only 47–51% of SRI/BICLA responders reached LLDAS [64]. Compared with placebo, patients treated with anifrolumab were two to three times more likely to attain LLDAS at 52 weeks. The implications of this for design of future Phase III trials is enormous, as a more discriminatory endpoint may enable trials with more robust findings.
In contrast, DORIS remission has been harder to achieve in the clinical trial setting, thus far only studied in relation to belimumab, with the lack of discrimination between placebo and active treatment using remission as an endpoint potentially reflecting the result of combining moderate efficacy of drug with a higher stringency outcome measure [67].
Conclusions and future directions
The body of work giving rise to and validating DORIS remission and LLDAS has, for the first time, provided feasible and validated treatment targets for the adoption of T2T strategies in SLE. Several key steps need to occur to build on existing studies prior to the approval of these target states by regulatory agencies, and hence use as primary endpoints in clinical trials; or the adoption into clinical guidelines, and hence use in routine patient care.
As with any study arising from an observational cohort, there are inherent limitations to the conclusions on the causal relationship between remission or LLDAS and improved disease outcomes. In order to test this, an interventional trial is needed using a T2T approach with non-attainment of LLDAS or remission as an inflection point for treatment escalation, compared with conventional management, as has been done for RA [9]. Not only will such a study address causal impact on patient outcomes, it may also address the deployability of LLDAS or remission with assessment of the resources required for use in clinical practice. DORIS remission requires refinement of criteria, particularly pertaining to immunosuppression and glucocorticoids, using data from longer follow-up prospective cohorts, allowing narrowing from eight framework definitions to one or two, in order to have utility in routine practice or clinical trials.
In summary, LLDAS and DORIS remission represent tangible and concentric clinical target states, shown to be associated with a reduction in adverse outcomes including disease flares and damage accrual, as well as improvement in patient-reported measures such as HR-QoL. With some further work, these endpoints have the potential to allow the adoption of a T2T approach in routine patient care, and provide robust and discriminative outcome measures for use in clinical trials.
Acknowledgements
Prof Eric F. Morand and Prof Ronald F. van Vollenhoven contributed to the concept design of the review.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript. This paper was published as part of a supplement supported by an educational grant from GSK.
Disclosure statement: the authors have declared no conflicts of interest.
References
- 1. Furie R, Petri M, Zamani O et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Navarra SV, Guzman RM, Gallacher AE et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 3. Cervera R, Khamashta MA, Font J et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine 2003;82:299–308. [DOI] [PubMed] [Google Scholar]
- 4. Verdecchia P, Staessen JA, Angeli F et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009;374:525–33. [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Cleary PA, Backlund JY et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson SC, Jones WN, Evanko TM. Dosage of beta-adrenergic blockers after myocardial infarction. Am J Health Syst Pharm 2003;60:2471–4. [DOI] [PubMed] [Google Scholar]
- 7. New JP, Mason JM, Freemantle N et al. Specialist nurse-led intervention to treat and control hypertension and hyperlipidemia in diabetes (SPLINT): a randomized controlled trial. Diabetes Care 2003;26:2250–5. [DOI] [PubMed] [Google Scholar]
- 8. van Vollenhoven RF, Mosca M, Bertsias G et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014;73:958–67. [DOI] [PubMed] [Google Scholar]
- 9. Grigor C, Capell H, Stirling A et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. [DOI] [PubMed] [Google Scholar]
- 10. Smolen JS, Landewe R, Bijlsma J et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 11. Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 2005;23:S93–9. [PubMed] [Google Scholar]
- 12. Strand V, Chu AD. Measuring outcomes in systemic lupus erythematosus clinical trials. Expert Rev Pharmacoecon Outcomes Res 2011;11:455–68. [DOI] [PubMed] [Google Scholar]
- 13. Chambers SA, Allen E, Rahman A, Isenberg D. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology 2009;48:673–5. [DOI] [PubMed] [Google Scholar]
- 14. Alarcón GS, McGwin G, Bartolucci AA et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum 2001;44:2797–806. [DOI] [PubMed] [Google Scholar]
- 15. Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum 2012;64:4021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stoll T, Sutcliffe N, Mach J, Klaghofer R, Isenberg DA. Analysis of the relationship between disease activity and damage in patients with systemic lupus erythematosus–a 5-yr prospective study. Rheumatology 2004;43:1039–44. [DOI] [PubMed] [Google Scholar]
- 17. Ugarte-Gil MF, Acevedo-Vasquez E, Alarcon GS et al. The number of flares patients experience impacts on damage accrual in systemic lupus erythematosus: data from a multiethnic Latin American cohort. Ann Rheum Dis 2015;74:1019–23. [DOI] [PubMed] [Google Scholar]
- 18. Ruiz-Arruza I, Ugarte A, Cabezas-Rodriguez I et al. Glucocorticoids and irreversible damage in patients with systemic lupus erythematosus. Rheumatology 2014;53:1470–6. [DOI] [PubMed] [Google Scholar]
- 19. Nikpour M, Urowitz MB, Ibanez D, Gladman DD. Frequency and determinants of flare and persistently active disease in systemic lupus erythematosus. Arthritis Rheumat 2009;61:1152–8. [DOI] [PubMed] [Google Scholar]
- 20. Gladman DD, Urowitz MB, Keystone EC. Serologically active clinically quiescent systemic lupus erythematosus. Am J Med 1979;66:210–5. [DOI] [PubMed] [Google Scholar]
- 21. Steiman AJ, Gladman DD, Ibanez D, Urowitz MB. Prolonged serologically active clinically quiescent systemic lupus erythematosus: frequency and outcome. J Rheumatol 2010;37:1822–7. [DOI] [PubMed] [Google Scholar]
- 22. Ng KP, Manson JJ, Rahman A, Isenberg DA. Association of antinucleosome antibodies with disease flare in serologically active clinically quiescent patients with systemic lupus erythematosus. Arthritis Rheumat 2006;55:900–4. [DOI] [PubMed] [Google Scholar]
- 23. Floris A, Piga M, Cauli A, Mathieu A. Predictors of flares in systemic lupus erythematosus: preventive therapeutic intervention based on serial anti-dsDNA antibodies assessment. Analysis of a monocentric cohort and literature review. Autoimmun Rev 2016;15:656–63. [DOI] [PubMed] [Google Scholar]
- 24. Furie R, Petri MA, Strand V et al. Clinical, laboratory and health-related quality of life correlates of Systemic Lupus Erythematosus Responder Index response: a post hoc analysis of the phase 3 belimumab trials. Lupus Sci Med 2014;1:e000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petri MA, van Vollenhoven RF, Buyon J et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum 2013;65:2143–53. [DOI] [PubMed] [Google Scholar]
- 26. Apostolopoulos D, Kandane-Rathnayake R, Raghunath S et al. Independent association of glucocorticoids with damage accrual in SLE. Lupus Sci Med 2016;3:e000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bruce IN, O’Keeffe AG, Farewell V et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Touma Z, Urowitz MB, Gladman DD. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2013;40:733–91. [DOI] [PubMed] [Google Scholar]
- 29. Isenberg DA, Rahman A, Allen E et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology 2005;44:902–6. [DOI] [PubMed] [Google Scholar]
- 30. Bencivelli W, Vitali C, Isenberg DA et al. Disease activity in systemic lupus erythematosus: report of the Consensus Study Group of the European Workshop for Rheumatology Research. III. Development of a computerised clinical chart and its application to the comparison of different indices of disease activity. The European Consensus Study Group for Disease Activity in SLE. Clin Exp Rheumatol 1992;10:549–54. [PubMed] [Google Scholar]
- 31. Steiman AJ, Urowitz MB, Ibañez D, Papneja A, Gladman DD. Prolonged remission in systemic lupus erythematosus. J Rheumatol 2014;41:1808–72. [DOI] [PubMed] [Google Scholar]
- 32. Zen M, Iaccarino L, Gatto M et al. Prolonged remission in Caucasian patients with SLE: prevalence and outcomes. Ann Rheum Dis 2015;74:2117–22. [DOI] [PubMed] [Google Scholar]
- 33. Franklyn K, Lau CS, Navarra SV et al. Definition and initial validation of a lupus low disease activity state (LLDAS). Ann Rheum Dis 2016;75:1615–21. [DOI] [PubMed] [Google Scholar]
- 34.>Tsang-A-Sjoe MW, Bultink IE, Heslinga M, Voskuyl AE. Both prolonged remission and lupus low disease activity state are associated with reduced damage accrual in systemic lupus erythematosus. Rheumatology 2017;56:121–8. [DOI] [PubMed] [Google Scholar]
- 35. Mok CC, Ho LY, Tse SM, Chan KL. Prevalence of remission and its effect on damage and quality of life in Chinese patients with systemic lupus erythematosus. Ann Rheum Dis 2017;76:1420–5. [DOI] [PubMed] [Google Scholar]
- 36. Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ et al. Remission and Low Disease Activity Status (LDAS) protect lupus patients from damage occurrence: data from a multiethnic, multinational Latin American Lupus Cohort (GLADEL). Ann Rheum Dis 2017;76:2071–4. [DOI] [PubMed] [Google Scholar]
- 37. Zen M, Iaccarino L, Gatto M et al. Lupus low disease activity state is associated with a decrease in damage progression in Caucasian patients with SLE, but overlaps with remission. Ann Rheum Dis 2018;77:104–10. [DOI] [PubMed] [Google Scholar]
- 38. Petri M, Magder LS. Comparison of remission and lupus low disease activity state in damage prevention in a United States systemic lupus erythematosus cohort. Arthritis Rheumatol 2018;70:1790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tani C, Vagelli R, Stagnaro C, Carli L, Mosca M. Remission and low disease activity in systemic lupus erythematosus: an achievable goal even with fewer steroids? Real-life data from a monocentric cohort. Lupus Sci Med 2018;5:e000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fasano S, Margiotta DPE, Pierro L et al. Prolonged remission is associated with a reduced risk of cardiovascular disease in patients with systemic lupus erythematosus: a GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Clin Rheumatol 2019;38:457–63. [DOI] [PubMed] [Google Scholar]
- 41. Alarcon GS, Ugarte-Gil MF, Pons-Estel G et al. Remission and low disease activity state (LDAS) are protective of intermediate and long-term outcomes in SLE patients. Results from LUMINA (LXXVIII), a multiethnic, multicenter US cohort. Lupus 2019;28:423–6. [DOI] [PubMed] [Google Scholar]
- 42. Golder V, Kandane-Rathnayake R, Huq M et al. Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: a prospective validation study. Lancet Rheumatology 2019;1:E95–102. [DOI] [PubMed] [Google Scholar]
- 43. Golder V, Kandane-Rathnayake R, Huq M et al. Evaluation of remission definitions for systemic lupus erythematosus: a prospective cohort study. Lancet Rheumatol 2019;1:e103–10. [DOI] [PubMed] [Google Scholar]
- 44. Floris A, Piga M, Perra D et al. Treatment target in newly diagnosed systemic lupus erythematosus. Arthritis Care Res 2019;doi: 10.1002/acr.24086. [DOI] [PubMed] [Google Scholar]
- 45. Sharma C, Raymond W, Eilertsen G, Nossent J. Achieving Lupus Low Disease Activity State (LLDAS-50) is associated with both reduced damage accrual and mortality in patients with systemic lupus erythematosus. Arthritis Care Res 2020;72:447–51. [DOI] [PubMed] [Google Scholar]
- 46. Franklyn K, Hoi A, Nikpour M, Morand EF. The need to define treatment goals for systemic lupus erythematosus. Nat Rev Rheumatol 2014;10:567–71. [DOI] [PubMed] [Google Scholar]
- 47. Wilhelm TR, Magder LS, Petri M. Remission in systemic lupus erythematosus: durable remission is rare. Ann Rheum Dis 2017;76:547–53. [DOI] [PubMed] [Google Scholar]
- 48. Zen M, Iaccarino L, Gatto M et al. The effect of different durations of remission on damage accrual: results from a prospective monocentric cohort of Caucasian patients. Ann Rheum Dis 2017;76:562–5. [DOI] [PubMed] [Google Scholar]
- 49. Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ et al. Predictors of remission and low disease activity state in systemic lupus erythematosus: data from a Multiethnic, Multinational Latin American Cohort. J Rheumatol 2019;46:1299–308. [DOI] [PubMed] [Google Scholar]
- 50. Golder V, Kandane-Rathnayake R, Hoi AY et al. Frequency and predictors of the lupus low disease activity state in a multi-national and multi-ethnic cohort. Arthritis Res Ther 2016;18:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Golder V, Kandane-Rathnayake R, Hoi AY et al. Association of the lupus low disease activity state (LLDAS) with health-related quality of life in a multinational prospective study. Arthritis Res Ther 2017;19:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Golder V, Huq M, Franklyn K et al. Does expert opinion match the operational definition of the lupus low disease activity state (LLDAS)? A case-based construct validity study. Semin Arthritis Rheum 2017;46:798–803. [DOI] [PubMed] [Google Scholar]
- 53. Kiani A, Petri M. Quality-of-life measurements versus disease activity in systemic lupus erythematosus. Curr Rheumatol Rep 2010;12:250–8. [DOI] [PubMed] [Google Scholar]
- 54. Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005;32:1706–8. [PubMed] [Google Scholar]
- 55. Wolfe F, Michaud K, Li T, Katz RS. EQ-5D and SF-36 quality of life measures in systemic lupus erythematosus: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, and fibromyalgia. J Rheumatol 2010;37:296–304. [DOI] [PubMed] [Google Scholar]
- 56. Tsang A, Bultink IEM, Heslinga M et al. The relationship between remission and health-related quality of life in a cohort of SLE patients. Rheumatology 2019;58:628–35. [DOI] [PubMed] [Google Scholar]
- 57. Margiotta DPE, Fasano S, Basta F et al. The association between duration of remission, fatigue, depression and health-related quality of life in Italian patients with systemic lupus erythematosus. Lupus 2019;28:1705–11. [DOI] [PubMed] [Google Scholar]
- 58. Louthrenoo W, Kasitanon N, Morand E, Kandane-Rathnayake R. Comparison of performance of specific (SLEQOL) and generic (SF36) health-related quality of life questionnaires and their associations with disease status of systemic lupus erythematosus: a longitudinal study. Arthritis Res Ther 2020;22:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morand EF, Furie R, Tanaka Y et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- 60. Durcan L, Petri M. Why targeted therapies are necessary for systemic lupus erythematosus. Lupus 2016;25:1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dolgin E. Lupus in crisis: as failures pile up, clinicians call for new tools. Nat Biotechnol 2019;37:7–8. [DOI] [PubMed] [Google Scholar]
- 62. Ordi-Ros J, Saez-Comet L, Perez-Conesa M et al. Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Ann Rheum Dis 2017;76:1575–82. [DOI] [PubMed] [Google Scholar]
- 63. Morand E, Merrill J, Kao A et al. Attainment of low disease activity by patients with systemic lupus erythematosus starting with high disease activity in a 24-week, randomized, placebo-controlled, phase IIb study of atacicept (ADDRESS II). Rheumatology 2017;69(Suppl 10): Abstract number 889. [Google Scholar]
- 64. Morand EF, Trasieva T, Berglind A, Illei GG, Tummala R. Lupus low disease activity state (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the phase IIb MUSE trial of anifrolumab. Ann Rheuma Dis 2018;77:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oon S, Huq M, Golder V et al. Lupus low disease activity state (LLDAS) discriminates responders in the BLISS-52 and BLISS-76 phase III trials of belimumab in systemic lupus erythematosus. Ann Rheum Dis 2019;78:629–33. [DOI] [PubMed] [Google Scholar]
- 66. Wallace DJ, Furie RA, Tanaka Y et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:222–31. [DOI] [PubMed] [Google Scholar]
- 67. Parodis I, Emamikia S, Gomez A et al. Definitions of remission in systemic lupus erythematosus: a post-hoc analysis of two randomised clinical trials. Lancet Rheumatol 2019;1:e163. [DOI] [PubMed] [Google Scholar]


