Abstract
SLE is a chronic autoimmune rheumatic disorder of high heterogeneity in clinical presentation, treatment response and prognosis. Long-term outcomes in SLE have been dramatically improved over the past decades, however, increased morbidity and mortality, especially among young individuals, still exists. Unmet needs include residual disease activity and frequent flares, glucocorticoid treatment dependency and toxicity, comorbidity burden, reduced health-related quality of life, health disparities and damage. The main determinants of long-term outcomes in SLE are age, sex, race/ethnicity, genetic profile, environmental factors including smoking, disease activity, major organ involvement such as lupus nephritis and CNS involvement, comorbidities including cardiovascular disease and serious infections, coexistence with APS, treatment adherence, socio-economic factors and access to care. In this review we discuss trends in long-term outcomes in SLE over the years and major contributors such as genetic, disease-related, treatment, comorbidity, socio-economic and other factors.
Keywords: SLE, long-term outcomes, lupus nephritis, damage, mortality, comorbidities, glucocorticoids, treatment adherence, socio-economic factors, smoking
Rheumatology key messages
Despite advances over decades, long-term outcomes, especially damage and mortality, remain a challenge in SLE.
An interplay between genetic, disease-related, comorbidity and socio-economic factors contributes to long-term prognosis in SLE.
Introduction
SLE is a chronic systemic autoimmune disorder of multiple and heterogeneous clinical phenotypes with variations in disease severity and damage accrual. Earlier diagnosis and treatment advances have resulted in improved outcomes over the past decades. However, increased morbidity and mortality risks persist in SLE [1–4], indicating that several unmet needs are still present among SLE patients [5, 6].
Unmet needs in SLE include persistent disease activity and flares, glucocorticoid (GC) treatment dependency, comorbidity burden, reduced health-related quality of life, access to high-quality care, damage accrual and long-term survival [1, 2, 5, 6] (Table 1). Better understanding of SLE pathogenesis, optimization of prevention, a treat-to-target approach and introduction of safe and effective treatments based on better-designed trials and clinical research tools, taking the heterogeneity of the disease into account as well, is crucial for improving outcomes.
Table 1.
Unmet needs related with long-term outcomes in SLE
| Persistent disease activity, frequent flares |
| Glucocorticoid treatment dependency |
| Glucocorticoid toxicity: infections, cardiovascular disease, diabetes, myopathy, cataract, osteoporosis, osteonecrosis |
| High comorbidity burden: cardiovascular disease, infections, neoplasms, osteoporosis |
| Reduced health-related quality of life: fatigue, pain and depression |
| Health and healthcare disparities |
| Implementation of a treat-to-target strategy |
| Adherence to treatment |
| Damage accrual |
| Long-term survival |
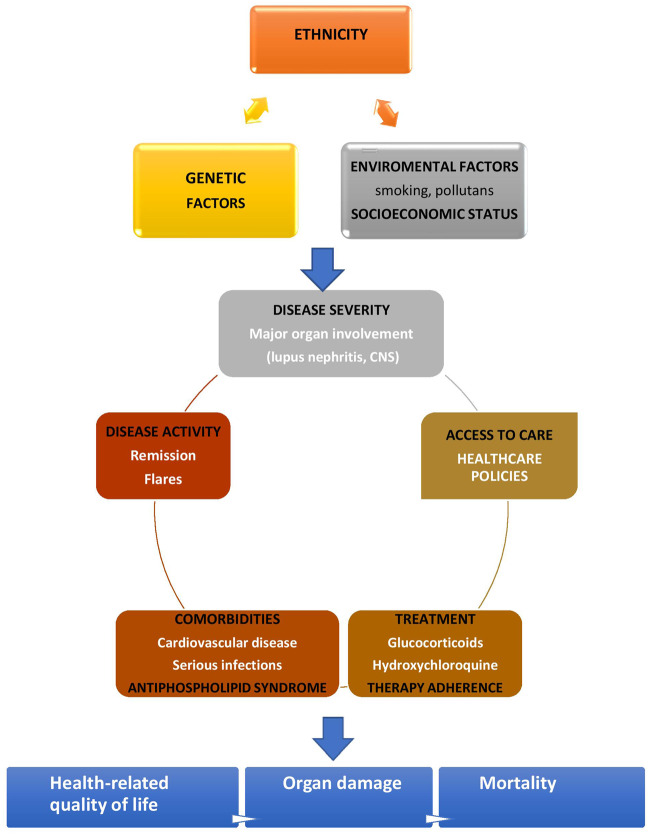
In this review we discuss the main outcomes in SLE, such as long-term remission or recurrent flares, end-stage renal disease, cardiovascular and infections burden and whether, and how, each of these has changed over time, as well as the impact of genetic, disease-related, treatment, comorbidity, socio-economic and other factors (e.g. adherence to treatment, access to care or smoking exposure) (Fig. 1).
Fig. 1.
Determinants of long-term outcomes in SLE
Long-term outcomes in SLE: trends over time and major contributors
Demographics and genetics
Age, sex and race/ethnicity
Age at disease onset has a significant impact on both disease presentation and prognosis. Juvenile-onset SLE patients have more frequently severe clinical manifestations such as lupus nephritis (LN) or serositis and a higher risk of flares, organ damage and treatment side effects and higher mortality rates compared with adult SLE patients [7, 8]. Late-onset disease (onset at age ≥50 years) is described in 3–18% of SLE patients with more insidious clinical presentation, but often with worse outcomes and higher mortality rates than adult- and juvenile-onset SLE, due to the increased comorbidity burden [9]. SLE is a sexually dimorphic autoimmune disorder, with female predominance in both paediatric and adult populations, but usually with more severe disease presentation and worse outcomes among male patients [10].
Regarding race/ethnicity, non-white (Black, Hispanics and Asian) patients with SLE tend to develop more severe clinical phenotypes and more damage accrual than whites [11], explained by increased genetic risk burden in the former populations. Socio-economic factors may confound the above associations, however, after adjustment for these factors, race/ethnicity remains a major determinant of poor outcomes in SLE, including end-stage renal disease (ESRD) risk and mortality [12]. In a recent population-based study using U.S. nationwide death certificates between 2000 and 2015, SLE was among the leading causes of death in young women, especially among those ages 15–24 years and those of African American and Hispanic origin [13]. Increased awareness of the above high-risk populations can help to improve long-term outcomes in SLE.
Genetic risk profile
An interplay between genetic, epigenetic and environmental factors is involved in SLE pathogenesis and disease presentation [14]. The role of genetic profiling to predict disease outcomes has been investigated in several chronic diseases [15]. Some studies have shown an association between variants of signal transducer and activator of transcription 4 and renal impairment, cardiovascular events and organ damage in SLE [16]. A few studies have also examined the relationship between the cumulative genetic risk score (defined as the weighted sum of the number of high-risk alleles) and lupus phenotypes [17, 18]. A recent large study of 1001 SLE patients has shown an association between a high genetic risk score and severe disease presentation and outcomes involving earlier disease onset, increased risk of first organ damage, cardiovascular events, ESRD and all-cause mortality, introducing the genetic risk score as a potential tool for prediction of disease severity and organ damage [18]. The above findings emphasize a potential role of genetic profiling, and the genetic risk score of single genes, for predicting clinical phenotypes and outcomes in this complex disease.
Major organ involvement: renal and CNS
LN and CNS lupus represent two of the most severe clinical manifestations of SLE, often associated with organ damage accrual.
Lupus nephritis
Renal involvement in SLE has been associated with an increased risk of ESRD [19] and a 5- to 8-fold increased risk of death compared with the general population [20, 21]. Several baseline characteristics have been associated with long-term prognosis in LN patients, including male gender, ethnicity, arterial hypertension, renal insufficiency and high activity and chronicity index scores [22]. Use of the histological classification for LN management and prognosis, better understanding of the pathogenetic basis of LN and advances in immunosuppressive treatments have been largely responsible for the substantial improvement of renal survival in SLE over the past 5 decades. However, LN remains a major cause of morbidity and mortality among patients with SLE. A systematic literature review and Bayesian meta-analysis showed that ESRD risks in LN improved gradually between the 1970s and the mid-1990s but then plateaued, with a slight increase in the late 2000s. In addition, despite absolute decreases of 10% in 10 year and 15 year ESRD risks in developed countries, the 15 year risk during the 2000s was still high (22% of patients overall), especially in class IV LN (44% of patients) and in developing vs developed countries [23].
A recent multicentre study from Italy including 499 LN patients showed much lower ESRD risk rates in all examined time periods that corresponded to the time of LN diagnosis (1970–1985, 1986–2001 and 2002–2016) [24]. This may be explained by the predominant Caucasian population, but also by the homogeneous renal biopsies and treatments used in these centres vs the heterogeneity in the protocols and access to care among the studies from developed and developing countries included in the meta-analysis. Prompt recognition of renal involvement and early renal biopsy is essential for earlier diagnosis and a histological-based therapeutic approach and prognosis assessment. In addition, HCQ use, adjunctive treatments such as angiotensin-converting enzyme or angiotensin receptor inhibitors based on their antiproteinuric effect, thrombosis prevention in nephrotic syndrome and a regular assessment of renal involvement signs for prompt diagnosis of flares are also protective against damage accrual [25].
Risk stratification based on clinical, histological, urine and/or serum biomarkers or genetic profiles can help to identify groups at high risk and optimize prevention strategies and individualization of treatment. In addition, given the failure of several previous LN trials to fulfil their primary endpoints, a long discussion among experts supports the need for re-evaluation of treatment response tools and primary outcomes as well as patient selection according to clinical phenotypes and biologic markers [26–28].
CNS involvement
NPSLE is among the most challenging manifestations of SLE and can affect the peripheral nervous system or CNS [29]. Involvement of the CNS is observed in >90% of NPSLE events [30] and remains an important cause of morbidity and mortality in SLE [31]. NPSLE manifestations with the highest incidence include cerebrovascular disease and seizures. Conversely, severe cognitive dysfunction, acute confusional state, psychosis and peripheral nervous disorders are less common [32]. The exact incidence of NPSLE manifestations varies greatly across studies [33] but appears to remain stable over time [34, 35]. NPSLE remains essentially a diagnosis of presumption and exclusion and is often confirmed retrospectively based on the response to treatment. In a recent paper by the SLICC group [30], neuropsychiatric events occurred in 955/1827 (52.3%) patients and 18–31% unique events were attributed to SLE, depending on the attribution model. The risk for NPSLE events was strongly increased during the first 2 years of follow-up [relative risk 6.16 (95% CI 4.96, 7.66)] in this inception cohort. Multistate modelling showed that patients without NPSLE events at initial assessment had a 74% probability of being NPSLE event-free after 10 years. While the majority of NP events resolved over 10 years, the mortality was higher in patients with NPSLE (16%) compared with those with no NPSLE events (6%) or non-SLE neuropsychiatric events (7%). Importantly, NPSLE continues to present a significant diagnostic and therapeutic challenge, especially because more recent clinical trials in SLE have excluded patients with severe NPSLE manifestations. Overall, the management of NPSLE remains an important unmet need in SLE.
Comorbidities
Patients with SLE have an increased risk of multiple comorbidities affecting long-term prognosis and all-cause mortality in SLE [36]. Cardiovascular complications and infections have the highest impact on long-term and hard outcomes in SLE, such as damage accrual and mortality.
Cardiovascular disease (CVD)
CVD is one of the leading causes of morbidity and mortality in SLE. Patients with SLE have 2- to 10-fold increased risk of clinical CVD compared with the general population [37] and approximately a 2.5-fold higher risk of subclinical atherosclerosis vs matched healthy individuals and comparable to that in RA and diabetes mellitus [38]. An interplay between the traditional CVD risk factors and disease-related factors such as disease activity and damage, GCs and aPLs has been involved in CVD pathogenesis in SLE [34]. Despite a growing awareness of CVD risk among patients with SLE, CVD burden remains high [20, 39, 40]. Data from a US population-based study using nationwide data showed increased age-adjusted rates of hospitalizations for myocardial infarction and stroke between 1996 and 2012 in both younger (age 18–49 years) and older (age ≥50 years) women and in men with SLE, while, in contrast, the hospitalization rates for both cardiovascular events were decreased in those without SLE. For myocardial infarction, the rate was 9.6/1000 patients in 1996 and 14.5/1000 patients in 2012, while for stroke it was 9.0/1000 patients and 14.2/1000 patients, respectively [41].
Rigorous assessment and modification of traditional and disease-related CVD risk factors in SLE patients is warranted, especially in high-risk populations such as those with baseline disease severity [42], renal involvement [43], high cumulative GC dose and positive aPLs [37]. Good control of disease activity, minimization of GC exposure and lifestyle optimization are of great importance. In addition, implementation strategies for risk factor prevention are also needed. Studies have shown that monitoring of traditional risk factors is suboptimal in SLE [44, 45]. The assessment of CVD risk is currently based on generic CVD risk prediction models, however, accumulating evidence shows that the majority of the currently used clinical risk scores in the general population do not accurately assess CVD risk in patients with SLE [46, 47]. A recent study showed underperformance of four generic and three SLE-modified scores to identify high CVD risk as defined by the presence of carotid and/or femoral plaques [48], underlying the importance of the development of validated disease-specific risk prediction tools [49].
Serious infections
Serious infections, defined as those requiring hospitalization or resulting in death, constitute one of the leading causes of morbidity and mortality in SLE along with CVD, accounting for approximately one-third of deaths. The most common sites for serious infections are the lower respiratory system, skin and urinary system, while bacteraemia and sepsis have been recognized as the main causes of infection-related mortality in several single-centre, multicentre and nationwide studies [50, 51]. A population-based study using data from the Nationwide Inpatient Sample between 1996 and 2011 showed that the rates of hospitalization for serious infections in SLE steadily increased over the study period, reaching 12 times higher than the non-SLE population in 2011. Opportunistic infections and pneumonia or severe sepsis requiring mechanical ventilation were associated with a higher risk of inpatient mortality among SLE patients [52]. In a Dutch population study, pneumococcal infections were 13 times higher compared with the general population [53].
Advanced age, disease activity, renal disease, dose of prednisone >7.5 mg/day, immunosuppressive/biologic therapy, damage accrual, comorbidities and low socio-economic status are recognized as major predictors for infections in SLE, whereas antimalarial use has been shown to have a protective effect [54–56], supporting the importance of low disease activity or remission achievement, glucocorticoid use minimization and consistent use of HCQ. Early diagnosis, validation of clinical scores to predict the risk of severe infection in SLE [57] and appropriate treatment of serious infections, which depends on timely access to quality care, is also important to reduce infection complications. The significance of the access to centres with clinical expertise in SLE has also been addressed by some studies. In a study of all-cause SLE readmissions using hospital discharge databases (2008–2009) from five geographically dispersed US states, one in six SLE patients were readmitted within 30 days and infections were associated with higher readmission rates. Interestingly, lower risk-adjusted readmission rates were observed in a state with a high concentration of dedicated SLE centres [58]. A recent study using national population-based data on outcomes for adults with SLE admitted with sepsis (2002–2011) showed a wide variation in mortality rates between hospitals, with lower rates in hospitals treating more SLE patients [59].
Vaccination strategies specifically for SLE are lacking. The EULAR has recently provided recommendations for vaccines in adults with autoimmune inflammatory rheumatic diseases [60]. Inactivated vaccines can be administered to patients on immunosuppressive treatment, but preferably prior to planned immunosuppression, especially in the case of B cell depletion therapy [60]. Live attenuated vaccines may be considered with caution in patients with autoimmune rheumatic diseases, with a time window of 4 weeks prior to treatment initiation. Satisfactory immunogenicity of influenza and PPSV23 vaccination has been demonstrated in SLE patients [61, 62] and no disease flares have been documented in the majority of studies [60]. Low vaccination rates are reported in SLE [63], emphasizing the need for broader adoption of a vaccination programme for autoimmune inflammatory rheumatic diseases but also development and validation of SLE-specific vaccine strategies.
Coexistence with APS
Approximately one-third of SLE patients with persistently positive aPL develop arterial or venous thrombotic events, the so-called SLE-associated APS [64]. In addition to classic thrombotic events, a number of severe manifestations of SLE, such as alveolar haemorrhage, renal microangiopathy, myelitis, adrenal insufficiency and cognitive impairment, are also more common among SLE patients with positive aPL and/or APS [65]. APS has been consistently recognized as an independent risk factor for organ damage and mortality in SLE [66, 67]. Long-term follow-up studies of SLE patients have shown more severe damage in patients with coexisting APS vs those without APS (median SLICC/ACR Damage Index score 2 vs 0 at 5 years, P < 0.001; 4 vs 1 at 15 years, P < 0.001) and a significantly lower cumulative survival at 15 years in SLE-APS than in non-APS patients (65% vs 90%, P = 0.03).
Risk stratification and appropriate management can prevent APS-related damage in several organs, such as the brain, heart, lungs and kidneys, or the development of its catastrophic form (catastrophic APS), characterized by a fatal outcome in more than half of cases [64]. The recently published EULAR recommendations for the management of APS in adults emphasize the importance of the identification of high-risk groups among aPL-positive individuals, including those with a high-risk aPL profile, defined as the presence of lupus anticoagulant or multiple aPL positivity or high aPL titres, and those with coexistent SLE and with traditional risk factors [63]. Preventive measures include lifestyle changes, prophylactic use of heparin in high-risk situations, daily use of HCQ (as in every patient with SLE) and low-dose aspirin in SLE patients with a high-risk aPL profile after bleeding/thrombosis risk evaluation [68].
The antithrombotic role of HCQ in SLE [69], especially among patients with positive aPL, has long been recognized, with growing evidence about the effect of treatment duration and dosage [70, 71]. In addition, a recent pilot, 3-year follow-up, open-label, randomized controlled study confirmed previous observations from retrospective studies about the effect of HCQ on aPL level reduction [72].
Impact of disease activity, treatment adherence, socio-economic and other factors on long-term outcomes
Remission and low disease activity
One major objective in the treatment of SLE is to reduce long-term organ damage. This may be achieved through remission and low-disease activity. While a single main definition of low disease activity [the lupus low disease activity state (LLDAS)] has emerged [73], various definitions for remission have been proposed by different groups [74, 75] and the optimal one remains to be determined. Several studies have shown that the risk of damage decreases with the length of time in either LLDAS or remission. While low disease activity intuitively appears to be a less desirable outcome than remission, patients in the LLDAS nevertheless achieve significantly better scores on both the SLE Quality of Life questionnaire and the 36-item Short Form Health Survey [76], reduced direct healthcare costs [77], damage and mortality than those with active disease [78]. Importantly, patients who spent even a short time in clinical remission (even <25% of visits) had a significant decrease in the rate of damage compared with never achieving remission. Notably, patients had to achieve the LLDAS in at least 50% of the visits to benefit from a similar decrease in the rates of damage as when achieving remission. Of note, 52.5% of patients achieved the LLDAS in at least 50% of the visits in the Baltimore cohort [79], and this was even less in African Americans, in whom time to reach the LLDAS was longer [80]. By comparison, clinical remission with or without treatment was achieved in only 27% and 13% of the follow-up visits, respectively. Overall, these data underline that remission is a more desirable outcome than the LLDAS, but that the latter is a more achievable target.
Therapeutic adherence
Poor adherence to therapeutic regimens is a common problem in chronic diseases such as SLE, in which non-adherence rates are as high as 76% in some studies, depending upon the assessment method [81]. Importantly, adherence measures used in interventional studies are very heterogeneous and consensus on the most relevant outcomes is currently lacking [82]. Non-adherence is multifactorial for most patients and typically varies according to unintentional and intentional patterns. Several studies have shown an association between non-adherence and a higher risk for flares, morbidity, hospitalizations, renal failure and death. Conversely, adherence to HCQ has been associated with a reduced risk of type 2 diabetes in SLE [83] as well as with being in the LLDAS [80]. The accurate identification of non-adherence is crucial, as it may help avoid unnecessary treatment escalation. Black ethnicity is also associated with lower therapeutic adherence in population-based studies [84].
Socio-economic factors
Health disparities continue to exist among socially disadvantaged populations [85], including African Americans, Hispanics and patients with lower education levels [86], and the overall burden of rheumatic diseases has been shown to correlate with a country’s economic level [87]. SLE is a costly disease that disproportionately affects disadvantaged populations [86]. However, it is difficult to distinguish the role of increased genetic susceptibility to SLE from contributing socio-economic factors in minority populations [86]. In many publications originating from outside of Europe, low socio-economic status at SLE diagnosis is associated with significantly greater direct medical costs for the management of SLE and associated complications [88], including during pregnancy [89]. In a multicentre Canadian SLE cohort, lower education level was associated with higher disease activity and work disability [90]. Falasinnu et al. [91] recently reviewed 24 773 SLE deaths in the USA (2003–2014), showing that the annual mortality rate is highest among blacks, including average-income blacks, southern low-income blacks and high-risk urban blacks. Conversely, mortality was lowest among non-blacks living in average-income settings. However, it has not been formally shown that socio-economic factors remain significant predictors of long-term SLE outcomes in European countries.
Smoking
In addition to its usual adverse effects, cigarette smoking is a risk factor for SLE and negatively influences the course of the disease and its treatment. A meta-analysis showed an increased risk of SLE in current smokers compared with never-smokers [92]. Also, there is a higher frequency of tobacco use among SLE patients than in the general population [92]. The impact of smoking in established SLE is strongly modulated by the ethnic background, with African Americans who smoke more likely to have increased damage compared with Caucasians who smoke. Caucasian smokers have been shown to accrue more cardiovascular damage, while African American smokers have more skin damage [93]. Importantly, tobacco smoking significantly reduces the therapeutic effectiveness of HCQ for cutaneous lesions [94] and belimumab in systemic manifestations [95]. This contrasts with the fact that SLE patients report rarely receiving cessation counselling and having limited awareness that smoking can worsen disease status or reduce treatment efficacy [96].
GCs
Since their first use in inflammatory diseases in 1948, GCs have remained a cornerstone of SLE treatment [6]. In a recent study by the SLICC group, 81.3% of patients received oral GCs and 26.3% received parenteral GCs. In a multicentre study performed in five European countries, 93% of SLE patients with active disease received GCs. Importantly, the initial GC dose is a strong predictor of overall GC exposure, independent of initial disease activity [97]. In this context, the use of methylprednisolone infusions has been shown to favour non-genomic effects of GCs [98] and subsequently allow the use of reduced doses of GCs [99]. However, there has been no direct comparative trial to demonstrate the validity of this strategy. Numerous studies have emphasized the risk of damage accrual in SLE patients treated with GCs, including the chronic use of ∼5 mg/day prednisone-equivalent doses [100, 101]. In this context, treat-to-target recommendations in SLE advocate the use of the lowest GC dosage needed to control disease activity for the shortest duration and, if possible, complete withdrawal of GCs. Surprisingly, the recent EULAR guidelines for SLE [55] recommend the use of long-term doses ≤7.5 mg/day prednisone equivalent. This is the same threshold as what was believed to be a ‘low dose’ of GC almost 15 years ago [102]. A GC-sparing effect has been demonstrated for several immunosuppressive agents and biologics [103]. Unfortunately, several studies have shown that despite the availability of immunosuppressive agents, up to one-third of patients never discontinue GCs [104]. Longitudinal studies show that the use of GCs may have decreased with time in SLE [34], which is an encouraging sign.
Conclusion
Despite a substantial improvement in SLE diagnosis and treatment over the years, some long-term outcomes are still not adequately improved. Several unmet needs remain, including a variety of disease-related, treatment, comorbidity and access-to-care factors. Ongoing research on lupus pathogenesis and novel treatments, as well as a re-evaluation of older agents, will help to improve outcomes in SLE.
Acknowledgements
LA wishes to thank Sylvie Thuong for her invaluable assistance in the preparation of his parts of the manuscript.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article. This paper was published as part of a supplement supported by an educational grant from GSK.
Disclosure statement: LA has received consulting fees from Alexion, Amgen, AstraZeneca, GlaxoSmithKline, Janssen-Cilag, LFB, Eli Lilly, Menarini France, Medac, MSD, Novartis, Pfizer, Roche-Chugai and UCB. MGT has received consultant fees and unrestricted grants from AbbVie, GlaxoSmithKline, MSD, Novartis, Pfizer and UCB deposited to the Special Account for Research Funding (ELKE) of the National and Kapodistrian University of Athens Medical School.
References
- 1. Durcan L, O’Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019;393:2332–43. [DOI] [PubMed] [Google Scholar]
- 2. Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet 2019;393:2344–58. [DOI] [PubMed] [Google Scholar]
- 3. Tektonidou MG, Lewandowski LB, Hu J, Dasgupta A, Ward MM. Survival in adults and children with systemic lupus erythematosus: a systematic review and Bayesian meta-analysis of studies from 1950 to 2016. Ann Rheum Dis 2017;76:2009–16. [DOI] [PubMed] [Google Scholar]
- 4. Jorge AM, Lu N, Zhang Y, Rai SK, Choi HK. Unchanging premature mortality trends in systemic lupus erythematosus: a general population-based study (1999–2014). Rheumatology (Oxford) 2018;57:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Felten R, Sagez F, Gavand P-E et al. 10 most important contemporary challenges in the management of SLE. Lupus Sci Med 2019;6:e000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamirou F, Arnaud L, Talarico R et al. Systemic lupus erythematosus: state of the art on clinical practice guidelines. RMD Open 2018;4:e000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hersh AO, von Scheven E, Yazdany J et al. Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum 2009;61:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato VA, Marques ID, Goldenstein PT et al. Lupus nephritis is more severe in children and adolescents than in older adults. Lupus 2012;21:978–83. [DOI] [PubMed] [Google Scholar]
- 9. Pons-Estel GJ, Ugarte-Gil MF, Alarcón GS. Epidemiology of systemic lupus erythematosus. Expert Rev Clin Immunol 2017;13:799–814. [DOI] [PubMed] [Google Scholar]
- 10. Christou EAA, Banos A, Kosmara D, Bertsias GK, Boumpas DT. Sexual dimorphism in SLE: above and beyond sex hormones. Lupus 2019;28: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kabani N, Ginzler EM. Is ethnicity linked to the severity of SLE manifestations? Nat Rev Rheumatol 2019;15:515–6. [DOI] [PubMed] [Google Scholar]
- 12. Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology (Oxford) 2017;56(Suppl 1):i67–77. [DOI] [PubMed] [Google Scholar]
- 13. Yen EY, Singh RR. Lupus-an unrecognized leading cause of death in young females: a population-based study using nationwide death certificates, 2000-2015. Arthritis Rheumatol 2018;70:1251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng Y, Tsao BP. Advances in lupus genetics and epigenetics. Curr Opin Rheumatol 2014;26:482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krarup NT, Borglykke A, Allin KH et al. A genetic risk score of 45 coronary artery disease risk variants associates with increased risk of myocardial infarction in 6041 Danish individuals. Atherosclerosis 2015;240:305–10. [DOI] [PubMed] [Google Scholar]
- 16. Svenungsson E, Gustafsson J, Leonard D et al. A STAT4 risk allele is associated with ischaemic cerebrovascular events and anti-phospholipid antibodies in systemic lupus erythematosus. Ann Rheum Dis 2010;69:834–40. [DOI] [PubMed] [Google Scholar]
- 17. Gianfrancesco MA, Balzer L, Taylor KE et al. Genetic risk and longitudinal disease activity in systemic lupus erythematosus using targeted maximum likelihood estimation. Genes Immun 2016;17:358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reid S, Alexsson A, Frodlund M et al. High genetic risk score is associated with early disease onset, damage accrual and decreased survival in systemic lupus erythematosus. Ann Rheum Dis 2020;79:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanly JG, O’Keeffe AG, Su L et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yurkovich M, Vostretsova K, Chen W, Aviña-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res (Hoboken) 2014;66:608–16. [DOI] [PubMed] [Google Scholar]
- 21. Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus 2016;25:727–34. [DOI] [PubMed] [Google Scholar]
- 22. Parodis I, Tamirou F, Houssiau FA. Prediction of prognosis and renal outcome in lupus nephritis. Lupus Sci Med 2020;7:e000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol 2016;68:1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moroni G, Vercelloni PG, Quaglini S et al. Changing patterns in clinical-histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis 2018;77:1318–25. [DOI] [PubMed] [Google Scholar]
- 25. Bertsias GK, Tektonidou M, Amoura Z et al. Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 2012;71:1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dall’Era M, Bruce IN, Gordon C et al. Current challenges in the development of new treatments for lupus. Ann Rheum Dis 2019;78:729–35. [DOI] [PubMed] [Google Scholar]
- 27. Houssiau FA. Time to change the primary outcome of lupus trials. Ann Rheum Dis 2019;78:581–2. [DOI] [PubMed] [Google Scholar]
- 28. van Vollenhoven R. String of successful trials in SLE: have we cracked the code? Lupus Sci Med 2020;7:e000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 30. Hanly JG, Urowitz MB, Gordon C et al. Neuropsychiatric events in systemic lupus erythematosus: a longitudinal analysis of outcomes in an international inception cohort using a multistate model approach. Ann Rheum Dis 2020;79:356–62. [DOI] [PubMed] [Google Scholar]
- 31. Kampylafka EI, Alexopoulos H, Kosmidis ML et al. Incidence and prevalence of major central nervous system involvement in systemic lupus erythematosus: a 3-year prospective study of 370 patients. PLoS One 2013;8:e55843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bertsias GK, Ioannidis JP, Aringer M et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 2010;69:2074–82. [DOI] [PubMed] [Google Scholar]
- 33. Ainiala H, Hietaharju A, Loukkola J et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum 2001;45:419–23. [DOI] [PubMed] [Google Scholar]
- 34. Uramoto KM, Michet CJ Jr, Thumboo J et al. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum 1999;42:46–50. [DOI] [PubMed] [Google Scholar]
- 35. Rosa GPD, Ortega MF, Teixeira A, Espinosa G, Cervera R. Causes and factors related to hospitalizations in patients with systemic lupus erythematosus: analysis of a 20-year period (1995–2015) from a single referral centre in Catalonia. Lupus 2019;28:1158–66. [DOI] [PubMed] [Google Scholar]
- 36. Kuo CF, Chou IJ, Rees F et al. Temporal relationships between systemic lupus erythematosus and comorbidities. Rheumatology (Oxford) 2019;58:840–8. [DOI] [PubMed] [Google Scholar]
- 37. Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum 2013;43:77–95. [DOI] [PubMed] [Google Scholar]
- 38. Tselios K, Gladman DD, Sheane BJ, Su J, Urowitz M. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971–2013). Ann Rheum Dis 2019;78:802–6. [DOI] [PubMed] [Google Scholar]
- 39. Arkema EV, Svenungsson E, Von Euler M, Sjöwall C, Simard JF. Stroke in systemic lupus erythematosus: a Swedish population-based cohort study. Ann Rheum Dis 2017;76:1544–9. [DOI] [PubMed] [Google Scholar]
- 40. Li D, Yoshida K, Feldman CH et al. Initial disease severity, cardiovascular events and all-cause mortality among patients with systemic lupus erythematosus. Rheumatology (Oxford) 2020;59:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford) 2017;56:709–15. [DOI] [PubMed] [Google Scholar]
- 42. Tektonidou MG, Wang Z, Ward MM., Li D., Yoshida K., Feldma BH. et al. Trends in hospitalizations due to acute coronary syndromes and stroke in patients with systemic lupus erythematosus, 1996 to 2012. Arthritis Rheumatol 2016;68:2680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol 2003;30:493–6. [PubMed] [Google Scholar]
- 44. Esmaeilbeigi F, Pope JE. Appropriate cardiovascular disease risk assessment in systemic lupus erythematosus may be lacking in rheumatology practice. Clin Exp Rheumatol 2018;36:526–32. [PubMed] [Google Scholar]
- 45. Boulos D, Koelmeyer RL, Morand EF, Hoi AY. Cardiovascular risk profiles in a lupus cohort: what do different calculators tell us? Lupus Sci Med 2017;4:e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Di Battista M, Tani C, Elefante E et al. Framingham, ACC/AHA or QRISK3: which is the best in systemic lupus erythematosus cardiovascular risk estimation? Clin Exp Rheumatol 2019; PMID: 31694741. [PubMed] [Google Scholar]
- 47. Drosos GC, Konstantonis G, Sfikakis PP, Tektonidou MG. Underperformance of clinical risk scores in identifying vascular ultrasound-based high cardiovascular risk in systemic lupus erythematosus. Eur J Prev Cardiol 2020; doi: 10.1177/2047487320906650. [DOI] [PubMed] [Google Scholar]
- 48. Petri MA, Barr E, Magder LS. Development of a systemic lupus erythematosus cardiovascular risk equation. Lupus Sci Med 2019;6:e000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldblatt F, Chambers S, Rahman A, Isenberg DA. Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus 2009;18:682–9. [DOI] [PubMed] [Google Scholar]
- 50. Rúa-Figueroa I, López-Longo J, Galindo-Izquierdo M et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum 2017;47:38–45. [DOI] [PubMed] [Google Scholar]
- 51. Tektonidou MG, Wang Z, Dasgupta A, Ward MM. Burden of serious infections in adults with systemic lupus erythematosus: a national population-based study, 1996–2011. Arthritis Care Res (Hoboken) 2015;67:1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luijten RK, Cuppen BV, Bijlsma JW, Derksen RH. Serious infections in systemic lupus erythematosus with a focus on pneumococcal infections. Lupus 2014;23:1512–6. [DOI] [PubMed] [Google Scholar]
- 53. González-Echavarri C, Capdevila O, Espinosa G et al. Infections in newly diagnosed Spanish patients with systemic lupus erythematosus: data from the RELES cohort. Lupus 2018;27:2253–61. [DOI] [PubMed] [Google Scholar]
- 54. Pimentel-Quiroz VR, Ugarte-Gil MF, Harvey GB et al. Factors predictive of serious infections over time in systemic lupus erythematosus patients: data from a multi-ethnic, multi-national, Latin American lupus cohort. Lupus 2019;28:1101–10. [DOI] [PubMed] [Google Scholar]
- 55. Fanouriakis A, Kostopoulou M, Alunno A et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 56. Tejera Segura B, Rua-Figueroa I, Pego-Reigosa JM et al. Can we validate a clinical score to predict the risk of severe infection in patients with systemic lupus erythematosus? A longitudinal retrospective study in a British cohort. BMJ Open 2019;9:e028697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yazdany J, Marafino BJ, Dean ML et al. Thirty-day hospital readmissions in systemic lupus erythematosus: predictors and hospital and state-level variation. Arthritis Rheumatol 2014;66:2828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tektonidou MG, Dasgupta A, Ward MM. Interhospital variation in mortality among patients with systemic lupus erythematosus and sepsis in the USA. Rheumatology (Oxford) 2019;58:1794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Furer V, Rondaan C, Heijstek MW et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:39–52. [DOI] [PubMed] [Google Scholar]
- 60. Rezende RPV, Ribeiro FM, Albuquerque EMN et al. Immunogenicity of pneumococcal polysaccharide vaccine in adult systemic lupus erythematosus patients undergoing immunosuppressive treatment. Lupus 2016;25:1254–9. [DOI] [PubMed] [Google Scholar]
- 61. Pugès M, Biscay P, Barnetche T et al. Immunogenicity and impact on disease activity of influenza and pneumococcal vaccines in systemic lupus erythematosus: a systematic literature review and meta-analysis. Rheumatology (Oxford) 2016;55:1664–72. [DOI] [PubMed] [Google Scholar]
- 62. Chehab G, Richter JG, Brinks R et al. Vaccination coverage in systemic lupus erythematosus-a cross-sectional analysis of the German long-term study (LuLa cohort). Rheumatology (Oxford) 2018;57: 1439–47. [DOI] [PubMed] [Google Scholar]
- 63. Pons-Estel GJ, Andreoli L, Scanzi F, Cervera R, Tincani A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J Autoimmun 2017;76:10–20. [DOI] [PubMed] [Google Scholar]
- 64. Cervera R, Serrano R, Pons-Estel GJ et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2015;74:1011–8. [DOI] [PubMed] [Google Scholar]
- 65. Ruiz-Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med 2004;164:77–82. [DOI] [PubMed] [Google Scholar]
- 66. Pericleous C, D’Souza A, McDonnell T et al. Antiphospholipid antibody levels in early systemic lupus erythematosus: are they associated with subsequent mortality and vascular events? Rheumatology (Oxford) 2020;59:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tektonidou MG, Andreoli L, Limper M et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019;78:1296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ugarte A, Danza A, Ruiz-Irastorza G. Glucocorticoids and antimalarials in systemic lupus erythematosus: an update and future directions. Curr Opin Rheumatol 2018;30:482–9. [DOI] [PubMed] [Google Scholar]
- 69. Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum 2009;61:29–36. [DOI] [PubMed] [Google Scholar]
- 70. Konig M, Li J, Petri M. Hydroxychloroquine blood levels and risk of thrombotic events in systemic lupus erythematous. Abstract 2783. 2019 ACR/ARP Annual Meeting, 8–13 November 2019, Atlanta, GA, USA. [Google Scholar]
- 71. Kravvariti E, Koutsogianni A, Samoli E, Sfikakis PP, Tektonidou MG. The effect of hydroxychloroquine on thrombosis prevention and antiphospholipid antibody levels in primary antiphospholipid syndrome: a pilot open label randomized prospective study. Autoimmun Rev 2020;19:102491. [DOI] [PubMed] [Google Scholar]
- 72. Morand EF, Trasieva T, Berglind A, Illei GG, Tummala R. Lupus low disease activity state (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the Phase IIb MUSE trial of anifrolumab. Ann Rheum Dis 2018;77:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Vollenhoven R, Voskuyl A, Bertsias G et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis 2017;76:554–61. [DOI] [PubMed] [Google Scholar]
- 74. Zen M, Iaccarino L, Gatto M et al. Prolonged remission in Caucasian patients with SLE: prevalence and outcomes. Ann Rheum Dis 2015;74:2117–22. [DOI] [PubMed] [Google Scholar]
- 75. Louthrenoo W, Kasitanon N, Morand E, Kandane-Rathnayake R. Comparison of performance of specific (SLEQOL) and generic (SF36) health-related quality of life questionnaires and their associations with disease status of systemic lupus erythematosus: a longitudinal study. Arthritis Res Ther 2020;22:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yeo AL, Koelmeyer R, Kandane-Rathnayake R et al. Lupus low disease activity state is associated with reduced direct healthcare costs in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2019; [DOI] [PubMed] [Google Scholar]
- 77. Sharma C, Raymond W, Eilertsen G, Nossent J. Association of achieving lupus low disease activity state fifty percent of the time with both reduced damage accrual and mortality in patients with systemic lupus erythematosus. Arthritis Care Res 2020;72:447–51. [DOI] [PubMed] [Google Scholar]
- 78. Babaoglu H, Li J, Goldman D, Magder LS, Petri M. Predictors of predominant Lupus Low Disease Activity State (LLDAS-50). Lupus 2019;28:1648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Babaoğlu H, Li J, Goldman D, Magder LS, Petri M. Time to lupus low disease activity state in the Hopkins Lupus Cohort: role of African American ethnicity. Arthritis Care Res (Hoboken) 2020;72:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Costedoat-Chalumeau N, Pouchot J, Guettrot-Imbert G et al. Adherence to treatment in systemic lupus erythematosus patients. Best Pract Res Clin Rheumatol 2013;27:329–40. [DOI] [PubMed] [Google Scholar]
- 81. Kelly A, Crimston-Smith L, Tong A et al. Scope of outcomes in trials and observational studies of interventions targeting medication adherence in rheumatic conditions: a systematic review. J Rheumatol 2019; doi: 10.3899/jrheum.190726. [DOI] [PubMed] [Google Scholar]
- 82. Salmasi S, Sayre EC, Avina-Zubieta JA, Esdaile JM, De Vera MA. Adherence to antimalarial therapy and risk of type 2 diabetes mellitus among patients with systemic lupus erythematosus: a population-based study. Arthritis Care Res (Hoboken) 2020; doi: 10.1002/acr.24147. [DOI] [PubMed] [Google Scholar]
- 83. Feldman CH, Costenbader KH, Solomon DH, Subramanian SV, Kawachi I. Area-level predictors of medication nonadherence among us Medicaid beneficiaries with lupus: a multilevel study. Arthritis Care Res (Hoboken) 2019;71:903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Scherlinger M, Mertz P, Sagez F et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun Rev 2020;19:102531. [DOI] [PubMed] [Google Scholar]
- 85. Barber MRW, Clarke AE. Socioeconomic consequences of systemic lupus erythematosus. Curr Opin Rheumatol 2017;29:480–5. [DOI] [PubMed] [Google Scholar]
- 86. Sebbag E, Felten R, Sagez F et al. The world-wide burden of musculoskeletal diseases: a systematic analysis of the World Health Organization Burden of Diseases Database. Ann Rheum Dis 2019;78:844–8. [DOI] [PubMed] [Google Scholar]
- 87. McCormick N, Marra CA, Sadatsafavi M, Avina-Zubieta JA. Socioeconomic status at diagnosis influences the incremental direct medical costs of systemic lupus erythematosus: a longitudinal population-based study. Semin Arthritis Rheum 2020;50:77–83. [DOI] [PubMed] [Google Scholar]
- 88. Kaplowitz ET, Ferguson S, Guerra M et al. Contribution of socioeconomic status to racial/ethnic disparities in adverse pregnancy outcomes among women with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2018;70:230–5. [DOI] [PubMed] [Google Scholar]
- 89. George A, Wong-Pack A, Peschken CA et al. Influence of education on disease activity and damage in systemic lupus erythematosus: data from the 1000 Canadian faces of lupus. Arthritis Care Res (Hoboken) 2017;69:124–32. [DOI] [PubMed] [Google Scholar]
- 90. Falasinnu T, Chaichian Y, Palaniappan L, Simard JF. Unraveling race, socioeconomic factors, and geographical context in the heterogeneity of lupus mortality in the United States. ACR Open Rheumatol 2019;1:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Parisis D, Bernier C, Chasset F, Arnaud L. Impact of tobacco smoking upon disease risk, activity and therapeutic response in systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun Rev 2019;18:102393. [DOI] [PubMed] [Google Scholar]
- 92. Kallas R, Li J, Petri M. Association of African-American ethnicity and smoking status with total and individual damage index in systemic lupus erythematosus. Clin Rheumatol 2020;39:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chasset F, Francès C, Barete S, Amoura Z, Arnaud L. Influence of smoking on the efficacy of antimalarials in cutaneous lupus: a meta-analysis of the literature. J Am Acad Dermatol 2015;72:634–9. [DOI] [PubMed] [Google Scholar]
- 94. Parodis I, Sjöwall C, Jönsen A et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev 2017;16:343–51. [DOI] [PubMed] [Google Scholar]
- 95. Wattiaux A, Bettendorf B, Block L et al. Patient perspectives on smoking cessation and interventions in rheumatology clinics. Arthritis Care Res (Hoboken) 2020;72:369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ruiz-Irastorza G, Garcia M, Espinosa G et al. First month prednisone dose predicts prednisone burden during the following 11 months: an observational study from the RELES cohort. Lupus Sci Med 2016;3:e000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ruiz-Irastorza G, Ruiz-Estevez B, Lazaro E et al. Prolonged remission in SLE is possible by using reduced doses of prednisone: an observational study from the Lupus–Cruces and Lupus–Bordeaux inception cohorts. Autoimmun Rev 2019;18:102359. [DOI] [PubMed] [Google Scholar]
- 98. Apostolopoulos D, Kandane-Rathnayake R, Raghunath S et al. Independent association of glucocorticoids with damage accrual in SLE. Lupus Sci Med 2016;3:e000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zen M, Iaccarino L, Gatto M et al. The effect of different durations of remission on damage accrual: results from a prospective monocentric cohort of Caucasian patients. Ann Rheum Dis 2017;76:562–5. [DOI] [PubMed] [Google Scholar]
- 100. Buttgereit F, da Silva JA, Boers M et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis 2002;61:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van Vollenhoven RF, Petri M, Wallace DJ et al. Cumulative corticosteroid dose over fifty-two weeks in patients with systemic lupus erythematosus: pooled analyses from the phase III belimumab trials. Arthritis Rheumatol 2016;68:2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Little J, Parker B, Lunt M et al. Glucocorticoid use and factors associated with variability in this use in the Systemic Lupus International Collaborating Clinics Inception Cohort. Rheumatology (Oxford) 2018;57:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Felten R, Arnaud L. Is it possible to stop glucocorticoids in systemic lupus? Joint Bone Spine 2020. [DOI] [PubMed] [Google Scholar]