Abstract
Neuropsychiatric (NP) events occur in the majority of patients with SLE and predominantly affect the CNS in addition to the peripheral and autonomic systems. Approximately 30% of all NP events are attributable to SLE (NPSLE) and present most frequently around the time of SLE onset. NPSLE is associated with increased morbidity and mortality and the proposed pathogenesis includes both ischaemic and neuroinflammatory mechanisms. Following diagnosis and causal attribution, the treatment of NPSLE is tailored to the type of NP event, the predominant putative pathogenic pathway and the activity and severity of the clinical event. There is a dearth of controlled clinical trials to guide management, but therapeutic options include symptomatic, antithrombotic and immunosuppressive agents that are supported by observational cohort studies. Our objective was to review what is currently known about NPSLE and to identify deficiencies in diagnostic biomarkers, novel therapies and clinical trials for this manifestation of SLE.
Keywords: SLE, neuropsychiatric, pathogenesis, biomarkers, neuroimaging, clinical trials
Rheumatology key messages
Neuropsychiatric SLE (NPSLE) represents one-third of all NP events in SLE patients.
The pathogenesis of NPSLE includes ischaemic and neuroinflammatory mechanisms.
Multidisciplinary research in NPSLE should focus on diagnostic biomarkers, novel therapies and clinical trials.
Clinical manifestations of neuropsychiatric SLE and attribution
Neuropsychiatric involvement (NP) is one of the most complex and challenging features of SLE, encompassing the CNS, peripheral nervous system (PNS) and autonomous nervous system (ANS). NPSLE can be mild or severe, focal or diffuse, acute or chronic, with a negative impact on the patient’s quality of life [1] and a tenfold to threefold increase in mortality rate compared with the general population [2] and with SLE patients without NPSLE [3], respectively.
The full disease burden of NPSLE is unclear, because robust epidemiology studies are lacking or biased by different methodology designs, such as lack of consistency in inclusion criteria and case definitions of NP events. In a meta-analysis including 5057 SLE patients, the prevalence of NPSLE varied from 17.6 to 44.5% in retrospective and prospective studies, respectively [3]. In a subanalysis of the 10 higher-quality prospective studies including 2049 patients, the overall prevalence of NP syndromes was 56.3%, predominantly affecting the CNS (93.1%) rather than the PNS (6.9%). Overall, up to half of SLE patients will develop NPSLE during their disease course, mostly within the first 3–5 years from SLE onset [4].
In 1999 the American College of Rheumatology published a standard nomenclature and a set of case definitions for 19 NP syndromes (12 CNS and 7 PNS) deemed to occur in SLE that is now widely used in clinical practice and for research [5]. Among the 19 NP syndromes, some are frequent (6.4–80%), others are common (7–20%) and the remaining are infrequent (0.6–11%) or rare (0.08–2%) (see Table 1 for details). Some NP syndromes that can occur in SLE, such as small fibre neuropathy, chronic inflammatory demyelinating polyneuropathy, posterior reversible encephalopathy syndrome and neuromyelitis optica spectrum disorder are not included in this classification.
Table 1.
Neuropsychiatric syndromes, according to the 1999 ACR classification stratified by frequency, in SLE patients
| NP clinical syndromes | Frequency range, % | |
|---|---|---|
| Cognitive dysfunction (mild)a,c | 6.6–80 | Frequent |
| Mood disordera | 7.4–65 | |
| Anxietya | 6.4–40 | |
| Headachea | 12.2–28.3 | |
| Seizuresa | 7.0–20 | Common |
| Cerebrovascular diseasea | 8.0–15 | |
| Psychosisa | 0.6–11 | Infrequent |
| Acute confusional statusa | 0.9–7 | |
| Mononeuropathyb | 0.9–6.9 | |
| Polyneuropathyb | 1.5–5.4 | |
| Myelopathya | 0.9–3.9 | |
| Demyelinating syndromea | 0.9–2.7 | |
| Aseptic meningitisa | 0.3–2.7 | Rare |
| Autonomic disorderb | 0.08–1.3 | |
| AIDP (GBS)b | 0.08–1.2 | |
| Cranial neuropathyb | 1.0 | |
| Movement disorders (chorea)a | 0.9 | |
| Myasthenia gravisb | 0.2 | |
| Plexopathyb | NR | |
CNS and
PNS clinical manifestations.
Severe cognitive dysfunction is less frequent (∼3–5%).
AIDP, acute inflammatory demyelinating polyradiculopathy; GBS, Guillain–Barré syndrome; NR, not reported.
Adapted with permission from Schwartz et al. [17].
None of the NP syndromes that occur in SLE have features that are specific for SLE. Determination of the correct attribution of NP events in SLE patients is a challenging but critical step in the treatment of individual patients and in performing research studies. Erroneous attribution can lead to suboptimal treatment of SLE patients presenting with NP events and to incorrect designation of patient groups in research studies. Thus significant effort has been made to define rules to achieve a confident attribution of NP events to SLE or non-SLE causes. The seminal work in this area is based on the SLICC inception cohort [6, 7]: two attribution rules of different stringency were developed to determine the attribution of NP events based on the following three factors: the interval between onset of the NP event(s) in relation to the diagnosis of SLE (i.e. the greater the interval, the lower the likelihood of causality); concurrent non-SLE factor(s) (i.e. identification of potential causes or contributing factors for each NP syndrome in the glossary accompanying the ACR case definitions) [5]; and the high frequency of some NP events in the general population (i.e. making it too challenging to confidently attribute these events to SLE) [8]. In the latter context, isolated headaches, anxiety, mild depression (mood disorders lacking criteria for ‘major depressive-like episodes’), mild cognitive impairment (defined as deficits in less than three of eight specified cognitive domains) and polyneuropathy without electrophysiological confirmation were not attributed to SLE [6].
Building upon this work, the Italian attribution algorithm was developed and validated against the ‘clinical judgement’ of attribution by a team of experts in two independent cohorts of SLE patients [9]. This Italian algorithm added a fourth item (i.e. ‘favouring factors’) to the three SLICC items and included imaging, laboratory test results and patient’s past history of NP events to determine the attribution of a new NP event to SLE. The individual components of the fourth item were derived from the EULAR recommendations on NPSLE and an expert panel. Each of the four items was weighted, generating a numerical score ranging from 0 to 10 points, where a higher score indicates a greater likelihood for attribution to SLE. Using the physician determination of attribution as the comparator, an optimal cut-off score of ≥7 was found to have a sensitivity of 87.9% and specificity of 82.6%. Adding this fourth component increased the ability to distinguish when an NP syndrome is attributable to SLE vs a competing comorbidity [10]. More recently, investigators in Leiden analysed the utility of repeated assessment in the attribution of NP events, emphasizing the value of multidisciplinary re-evaluation over time, to achieve the goal of a correct attribution [11]. Although all these models can be supportive and help clinician’s reasoning, none of them perform optimally in clinical practice and, at their best, only one-third of NP events can be attributed to SLE, leaving a ‘grey zone’ of uncertainty that still characterizes the diagnostic challenge of NPSLE. Lacking specific, reliable and validated imaging, laboratory and clinical biomarkers for NPSLE, the correct diagnosis relies upon the exclusion of other causes, the clinical expertise provided by a multidisciplinary team coupled with a careful follow-up of patients and outcomes of their NP events.
Although a significant effort has been made to define CNS disease in SLE patients, involvement of the PNS and ANS has received less attention. Recently two large multicentric studies (one retrospective and one prospective) have focussed on PNS involvement in SLE, yielding similar results. In a large retrospective study on 1224 SLE patients from Italy, the overall prevalence of PNS involvement was 6.9% (97 PNS events in 85 patients; two-thirds of them defined as SLE related). Polyneuropathy was the most frequent (39.2%), followed by cranial neuropathy (30.9%) and single (12.4%) or multiple (8.2%) mononeuritis. Patients with PNS disease were older at SLE onset, had higher SLEDAI-2K and SLICC/ACR Damage Index scores, as well as a higher frequency of hypertension and livedo reticularis [12]. In a larger multiethnic/racial, prospective SLE inception cohort (1827 SLE patients), 161 PNS events were found in 131 patients (7.6%) and the majority were deemed SLE related; peripheral polyneuropathy (41%), mononeuropathy (27.3%) and cranial neuropathies (24.2%) were the most frequent. Patients with PNS involvement were older at SLE diagnosis and had higher SLEDAI-2K scores. Although impairing health-related quality of life (HRQoL), the majority of neuropathies resolved or improved over time and cranial neuropathies had the steepest trajectory to resolution [13].
Overview of proposed pathogenesis
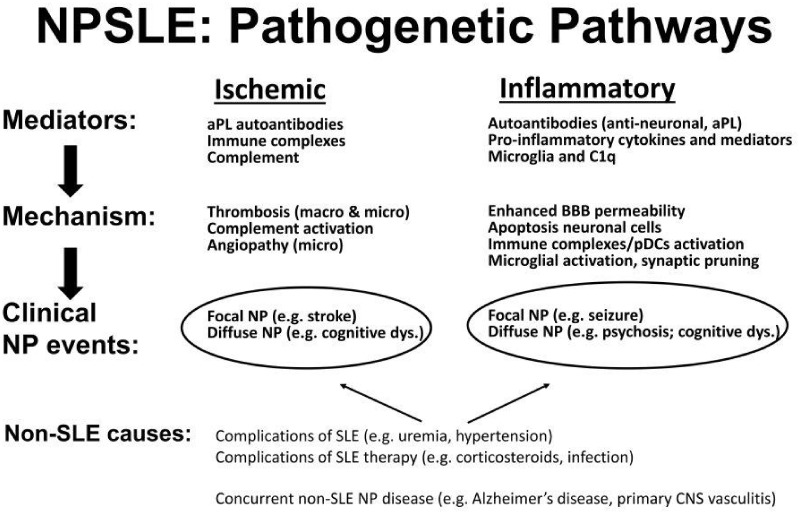
It is intuitive that no single, but rather multiple and interrelated pathophysiological mechanisms, including genetic susceptibility [14], account for the heterogeneous clinical phenotypic landscape of NPSLE. It has long been proposed that there are two main, and probably complementary, pathogenetic pathways underlying NPSLE (Fig. 1): the first is an ischaemic pathway involving large and small blood vessels, mediated by aPL antibodies, immune complexes and intravascular thrombosis. The corresponding clinical phenotypes are predominantly focal NP events such as stroke, seizures, movement disorders and some myelopathies. The second mechanism is an autoimmune-mediated neuroinflammatory pathway with complement activation, increased permeability of the blood–brain barrier (BBB), intrathecal migration of neuronal autoantibodies, local production of immune complexes and pro-inflammatory cytokines and other inflammatory mediators. The clinical phenotypes associated with this pathway are mostly diffuse NP manifestations such as psychosis, mood disorders, cognitive dysfunction and acute confusional states [4, 15–18].
Fig. 1.
Two autoimmune pathogenic pathways for NPSLE
Ischaemic injury involving both large- and small-calibre vessels mediated by aPL antibodies, immune complexes and complement activation. Injury due to inflammation in which enhanced permeability of the BBB in association with antineuronal antibodies and formation of immune complexes lead to production of pro-inflammatory mediators and microglial activation. Both pathways may result in either focal or diffuse NP manifestations for which other non-SLE causes must be considered. pDC: plasmacytoid dendritic cell; dys: dysfunction.
Support for the ischaemic pathway is provided by a recent brain autopsy study [19] reporting the presence of large and diffuse small vessel vasculopathy and vascular occlusion with microthrombi. In addition to pro-thrombotic effects of circulating aPL antibodies, there was deposition of complement activation products (C4d and C5b-9) on the endothelial cell surface and frank vasculitis in 31% of cases of NPSLE. Mice deficient in C3 and C5 components are resistant to thrombosis and endothelial cell activation induced by aPL antibodies [20, 21]. In addition, elevated anti-C1q antibodies and decreased CH50 were associated with diffuse NPSLE, while decreased C4 was associated with focal NPSLE in patients with aPL antibodies [22]. These data suggest that inflammatory mechanisms, including complement activation and deposition, may be a key factor in the interaction between circulating autoantibodies and thrombo-ischaemic lesions observed in NPSLE.
In regard to the proposed neuroinflammatory pathway for NPSLE, autoantibodies such as anti-neuronal, anti-NR2, anti-ribosomal P and anti-endothelial antibodies have been implicated as important mediators [23]. Pre-clinical studies have demonstrated that when anti-NR2 and anti-ribosomal P antibodies gain access to the CNS through a permeabilized BBB they induce neuronal death or apoptosis. Anti-NR2 antibodies in the cerebrospinal fluid (CSF) were associated with impairment of motor functioning and visuospatial processing in SLE patients [24]. Anti-NR2 antibodies can also activate endothelial cells through the nuclear factor κB pathway, leading to damage of the BBB [25, 26]. In a recent meta-analysis, CNS involvement, depression and psychosis were associated with anti-ribosomal P antibodies [27] and a study of the SLICC inception cohort found an association with clinically distinct NP events attributed to SLE [28]. Other novel autoantibodies [e.g. anti-BC RNA, anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH), anti-ubiquitin carboxyl terminal hydrolase-L1 (UCH-L1), anti-suprabasin antibodies] and their potential association with NPSLE are under study [29–32] (Table 2).
Table 2.
Novel autoantibodies that are potentially relevant in NPSLE
| Autoantibody | Antigen | Effect of autoantibodies | Clinical findings |
|---|---|---|---|
| Anti- suprabasin [26] | Suprabasin (SBSN) gene has been originally identified in mouse and human differentiating keratinocytes as an epidermal differentiation marker. SBSN is regarded as a stratified epithelium-specific secreted protein located subcellularly in vesicles and secreted to the extracellular region. | Anti-SBSN antibodies induce the expression of genes related to astrocyte damage and IL-6 production in astrocytes stimulated with LPS. Astrocytes exposed to anti-SBSN antibodies have significantly altered senescence and autophagy pathways. | Immune complex–associated SBSN was found only in the CSF of NPSLE patients. The titre of anti-SBSN antibodies was significantly higher in the CSF of NPSLE patients compared with SLE, multiple sclerosis and normal pressure hydrocephalus. CSF anti-SBSN antibodies could be a useful marker for distinguishing NPSLE patients from SLE patients without neuropsychiatric manifestations. To date no clinical correlations have been reported. |
| Stronger deposition of SBSN co-localize with glial fibrillary acidic protein (GFAP) staining in the astrocytes of NPSLE patients compared to the healthy individuals. | |||
| Anti- UCH-L1 [27] | UCH-L1 is a de-ubiquitinating enzyme. It is a neuronal cytoplasmic protein mainly expressed in large neurons such as Purkinje cells, brain stem and basal ganglia neurons, and has a 50-fold higher concentration in the brain than in other tissues. The most important function of ubiquitin protein is to regulate the ubiquitin proteasome system and synaptic remodelling. Elevated CSF and serum levels of UCH-L1 were detected in traumatic brain injury, neonatal hypoxic ischaemic encephalopathy, epilepsy and toxic encephalopathy. It could be the consequence of non-specific neuronal damage. Abnormal function of UCH-L1 is also involved in the pathogenesis of neurodegenerative diseases. | CSF anti-UCH-L1 have been detected in NPSLE but not in other conditions, indicating that specific autoimmune responses have been induced by UCH-L1 in NPSLE patients and it has been proposed as a potential biomarker of neuronal damage in NPSLE. | CSF UCH-L1 levels were found significantly increased in the severe NPSLE patients and associated with increased generalized disease activity (measured by SLEDAI-2K). |
| CSF anti-UCH-L1 levels were significantly elevated in patients with NPSLE in comparison to SLE without NP involvement and other connective tissue diseases and nervous system disorders. CSF anti-UCH-L1 levels were also associated with SLE organ involvement, e.g. cardiac involvement (P = 0.043), proteinuria (P = 0.048) and haematological manifestations (P = 0.016). | |||
| Serum anti-UCH-L1 levels were positively correlated with the matched CSF anti-UCH-L1 levels among patients with NPSLE. | |||
| anti-BC RNA [28] | Neuronal regulatory brain cytoplasmic (BC) RNAs are non-protein coding, small cytoplasmic RNAs (scRNAs), expressed in neurons and are localized to synaptodendritic domains. | Anti-BC antibodies target both primate BC200RNA and rodent BC1 RNA. | Once anti-BC RNA antibodies have gained access into CNS they induce a lack of BC1 RNA, which causes phenotypic abnormalities including: epileptogenic responses and cognitive dysfunction. |
| BC RNAs control local protein synthesis by interacting with eukaryotic initiation factors (EIFs) 4A and 4B, thus repressing translation in the basal default state. | SLE anti-BC antibodies effectively compete with RNA transport factor heterogeneous nuclear ribonucleoprotein A2 (hnRNP A2) for dendritic targeting elements (DTEs) access and significantly diminish BC RNA delivery to synaptodendritic sites of function. | ||
| Anti- GAPDH [29] | GAPDH is expressed on the neuronal cell surface and is involved in cell–cell interactions. GAPDH binds to laminin, which is a component of the extracellular matrix with a prominent role in neuroplasticity. Binding of GAPDH to laminin may promote neurite extension/elongation. | Anti-GADPH antibodies block binding to laminin and/or to other adhesion and synaptic molecules in the CNS, inducing neurite retraction and impairment of neuronal plasticity. | Serum anti-GAPDH autoantibodies are increased in both SLE patients with and without NP symptoms and associated with generalized disease activity (SLEDAI-2K, ESR, IgG and IgM levels), cognitive dysfunction, increased intracranial pressure and psychiatric manifestations such as anxiety, depression and psychosis. |
| In mice models (C57BL6/J mice), anti-GADPH administration resulted in behavioural changes associated with a detrimental cognitive and emotional profile. |
Several pro-inflammatory cytokines [B cell activating factor (BAFF), TNF-like weak inducer of apoptosis (TWEAK), IFN-α, IFN-γ, IL-2, IL-6, IL-8, IL-10) and mediators (i.e. MMP-9, S100β, plasminogen activator inhibitor-1 (PAI-1), osteopontin (OPN)] have also been implicated in the pathogenesis of NPSLE. Initial studies in SLE patients revealed associations of elevated CSF IL-6 levels with seizures and IFN-α with lupus psychosis. Further evidence suggested a role for other cytokines such as IL-2, IL-8 and IL-10 that are mainly produced by neuronal, glial and infiltrating immunocompetent cells [33], but also by BBB endothelium following surface binding of anti-NR2 and anti-ribosomal P antibodies [25]. Calcium-binding protein S100β and TWEAK are other potentially important pro-inflammatory mediators produced mainly by astrocytes and their overproduction by activated glial cells leads to a loss of neuronal cells and increased permeability of the BBB [34–36]. Another interesting molecule is OPN, a secreted glycoprotein (44–66 kDa) highly expressed in osteoblasts, macrophages, activated T cells and renal tubular cells promoting immune cell infiltration [37]. In a recent study, the concentration of full-length OPN in the CSF was significantly higher in NPSLE than in non-NPSLE and it decreased after treatment, thus representing a potential new biomarker [38].
Microglial cells are considered the main antigen presenting cells (APCs) within the CNS and are found in close proximity to the brain’s microvasculature. They play a fundamental role in regulating BBB function and in shaping brain circuits and development (‘synaptic pruning’). Interestingly SLE sera induce reactive phenotypes in microglia [39] and their potential role has been further explored in a murine study of long-term neuronal dysfunction mediated by transient exposure to anti-NR2 antibodies [40]. In these elegant experiments, anti-NR2 antibodies impaired dendritic arbourization, that was dependent on locally activated microglia and the presence of complement component C1q. In the same study, centrally acting inhibitors of angiotensin-converting enzyme (ACE; e.g. captopril) prevented microglial activation and preserved cognitive performance in the murine model. This raises the possibility that ACE inhibition could be a potential candidate for clinical trials to determine the effect on mitigating cognitive dysfunction .
Current treatments of NPSLE
In the absence of high-level evidence for the treatment of NPSLE, it is necessary to develop pragmatic therapeutic strategies supported by expert opinion, published observational cohort data on NPSLE and extrapolation from experience with other organ system disease in SLE. This is in line with the EULAR recommendations for the management of NPSLE published >10 years ago [41] and with the recent 2019 update of EULAR recommendations for the management of SLE [42]. Identification of the most likely cause and contributing factors to the NP event is determined by careful clinical assessment and the use of appropriate diagnostic tests that will vary depending on the clinical presentation. These may include a search for autoantibodies, examination of CSF primarily to exclude infection, neuroimaging, formal cognitive assessment and electrophysiological testing. Upon completion of this exercise, the patient’s NP event should be attributed exclusively to SLE, exclusively to non-SLE factors or to a combination of both. Regardless of attribution, it is important to distinguish between ongoing activity and organ damage, as only the former is reversible, although the consequences of some forms of damage may be modifiable (e.g. stroke rehabilitation). These determinations provide a framework for the selection of treatment modalities and outcomes for measuring efficacy in individual patients.
First, frequent non-SLE factors such as metabolic abnormalities, infection, hypertension and other cardiovascular risk factors should be treated appropriately. Second, the use of non-SLE-specific interventions should be considered. Psychotherapy had a beneficial effect on symptoms of anxiety, depression and quality of life in a controlled clinical trial of 20 weekly sessions in 80 SLE patients [43]. Pharmacotherapy with anxiolytics and antidepressants are also used, although no controlled trials have been performed specifically in SLE populations. The importance of detecting and treating mood disorders in SLE is emphasized by their association with poor overall medication adherence [44] and with suicidal behaviours [45]. Observational studies report a positive outcome with antidepressants [46] and the treatment of even mild anxiety and depression may improve cognitive complaints or function. However, the use of antidepressants is highly variable, in between 7% [47] and 70% [46] of SLE patients with mood disorders. The use of antiseizure drugs in SLE has also not been subjected to controlled clinical trials. However, observational cohort studies suggest that seizures in SLE patients frequently have a favourable outcome, as indicated by a lower recurrence rate, more frequent discontinuation of antiseizure medications and no detectable impact on patient self-reported HRQoL [48]. Antipsychotic medications are used in the majority of patients with lupus psychosis [49], which is a rare but dramatic presentation of NPSLE. Agents used to treat cognitive dysfunction in Alzheimer’s disease (e.g. cholinesterase inhibitors, memantine) and attention deficit disorder (e.g. methylphenidate) are worthy of consideration, but data in SLE are not supportive. For example, a single-centre controlled study of memantine, that is a non-competitive inhibitor of glutamate at the level of the N-methyl-D-aspartate receptor and used as a symptomatic treatment for moderate–severe Alzheimer’s disease, found no significant benefit compared with placebo on a computerized cognitive test battery in a group of 51 SLE patients treated over 12 weeks [50].
Behavioural rehabilitation of cognitive dysfunction is based on repeated practice and stimulation of impaired cognitive skills via drill-type exercises of increasing difficulty. While some studies have demonstrated improved test performance in other patient populations with this approach [51], these improvements are not typically associated with positive changes in daily functioning [52] and systematic studies in patients with SLE are lacking. Only one study of the feasibility and effectiveness of a multicontext rehabilitation strategy emphasizing the generalizability of training from the therapeutic environment to real-life situations has been conducted in patients with SLE [53]. This uncontrolled, non-randomized study included 17 patients with self-reported cognitive difficulties and associated limitations in adaptive functioning or emotional distress and demonstrated a 100% retention rate with reports of improved quality of life and memory self-efficacy. However, controlled studies are required to delineate the therapeutic components of this intervention.
When considering more lupus-specific treatment options for NP events attributed to SLE, it is helpful to first decide if the pathogenesis is primarily related to an ischaemic or inflammatory disease pathway, as this will guide the selection of more lupus-specific therapies.
Ischaemic-mediated injury
Primary prevention of NPSLE events attributed to cerebral ischaemia such as transient ischaemic attack and stroke is hypothetically linked to reducing the prothrombotic risk of aPL antibodies. A recent review of primary prevention in APS concluded that current evidence does not support either the use of low-dose aspirin or warfarin and that large, well-designed clinical trials are required to address this [54]. Secondary prevention of focal NP disease attributed to aPL antibodies requires lifelong anticoagulation [55] despite the lack of controlled clinical trials in NPSLE. However, controlled trials in patients with APS found no significant difference between low-intensity [target international normalized ratio (INR) 2.0–3.0] and high-intensity (target INR >3.0) warfarin in the prevention of recurrent thrombosis [56, 57]. A minority of patients in these studies had arterial thrombosis and the optimal target INR in such cases is controversial [58, 59]. Direct oral anticoagulants cannot be recommended at this time, as a study of high-risk patients with APS found rivaroxaban to be associated with an increased risk of thromboembolic events relative to warfarin [60]. Potential adjunctive therapies are antiplatelet agents, antimalarials and statins, particularly for those with arterial thrombosis and recurrent venous thrombosis while on warfarin [58].
Inflammation-mediated injury
Immunosuppressive therapy with high-dose corticosteroids, azathioprine, cyclophosphamide and mycophenolate mofetil is used to varying degrees [49, 61–64] in the treatment of NPSLE linked to an immune-inflammatory pathogenesis. In large part these treatment regimens were selected on the basis of their efficacy for LN. In NPSLE, only two of these agents (oral prednisone and intravenous cyclophosphamide) have been subjected to clinical trials in NPSLE [61, 62], and both had positive outcomes. Observational cohort studies [48, 65] have reported a lower risk of seizures in patients with SLE taking antimalarial drugs, even after adjusting for confounding variables. The precise mechanism responsible for this beneficial effect is unclear but it may result from a combination of the known anti-inflammatory [66, 67] and antithrombotic [68] properties of antimalarial drugs. Another cohort study of NPSLE and SLE patients, using MRI, found less brain atrophy and loss of white matter fibre tract integrity among those taking antimalarials [69]. In virtually all available studies, immunosuppressive therapy has been used in conjunction with corticosteroids and in addition to symptomatic therapies.
Information is even more limited on the efficacy of biologic therapies in NPSLE. Open studies of B-lymphocyte depletion with rituximab, used alone or in combination with conventional immunosuppressive agents including cyclophosphamide, have reported positive results [70–73] but requires further study. A post-hoc analysis of two phase III belimumab studies that included 45 patients with NPSLE, suggested a beneficial response to belimumab only in patients who had headache and not in those with other major NPSLE events [74]. Anifrolumab, a type I IFN receptor antagonist, had a positive result is a recent phase 3 clinical trial of SLE [75], but patients with severe NPSLE were excluded from the study and a subset analysis of patients in the trial with mild NPSLE is awaited.
Unmet needs in the diagnosis and treatment of NPSLE
There are a number of unmet needs related to NPSLE that need to be addressed to improve the clinical outcomes of this manifestation of SLE (Table 3).
Table 3.
Unmet needs in NPSLE
| Diagnostic biomarkers for determining the attribution of NP events to SLE |
| Potential candidates: CSF and serum proteins, neuroimaging |
| Novel therapies for NPSLE |
| Potential targets: BBB, pro-inflammatory cytokines, microglia |
| Advancing clinical trials for NPSLE. |
| Potential options: drugs for non-emergent NP events (e.g. mood disorders), validate outcome measures (e.g. self-report instruments, neuroimaging for brain structure and function) |
Lack of diagnostic biomarkers for NPSLE
Despite efforts to identify laboratory or neuroimaging biomarkers of NP involvement in SLE, so far none have been shown to be sufficiently accurate or reliable for use in clinical practice. A number of serum and/or CSF autoantibodies have been proposed as candidate biomarkers, but very few have passed the exploratory phase and are used in diagnostic and therapeutic decision making. Candidate autoantibody biomarkers include aPL, anti-ribosomal P and, to a lesser extent, anti-neuronal and anti-NR2 antibodies [23, 27, 76–79].
Different cytokines, chemokines, complement cascade products and other pro-inflammatory mediators are increased in the serum or CSF of patients with NPSLE [80]. Elevated CSF IL-6 levels have shown the strongest positive correlation with NP syndromes, especially with diffuse NPSLE such as acute confusional state [81–88]. Further advances may come from the identification of new and more specific neuronal surface antigens, the growing contribution of the omics technologies (genomics, transcriptomics and immunoproteomics) and a better understanding of BBB regulation.
Conventional MRI (cMRI) is the current neuroimaging gold standard for the assessment of patients with NPSLE, but clinicians still face the clinical–radiological paradox [80]. In fact, 40–50% of patients with a clinical diagnosis of NPSLE have no abnormalities on cMRI [84, 86, 87] and, conversely, many of the chronic abnormalities identified by cMRI are not associated with a clinically overt NP syndrome [89]. The reason for this apparent mismatch is due to the fact that nervous system tissue microarchitecture abnormalities and functional derangements are not necessarily aligned. Thus a multimodal approach combining morphological and functional neuroimaging techniques is required to overcome this impasse [4, 18, 90]. Neuroimaging is a rapidly evolving field and the availability of newer applications is presenting opportunities to better understand the pathogenesis of NPSLE [91]. In addition, there is the potential to improve the attribution of NP events to SLE and non-SLE causes and to develop objective neuroimaging outcomes to measure the response to new therapies [92–99].
Lack of novel therapies for NPSLE
The search for novel targets in the treatment of NPSLE has been hampered by a lack of information on precise pathogentic mechanisms and the unique characteristics of the brain that make it less accessible to investigation. Recent information from animal models and neuroimaging have provided some valuable insights.
Dysfunction of the BBB exposes the brain to blood components that are normally excluded. These include brain cross-reactive autoantibodies and non-immune proteins such as albumin, thrombin and activated protein C that cause inflammation, neuronal hyperexcitability and degeneration [100, 101]. Restoration of normal BBB function, through modification of non-immunological variables (e.g. smoking, hypertension and stress) or neutralizing the autoantbodies that have been shown to permeabilize the BBB in vitro are potential therapeutic strategies. Complement activation induced by autoantibodies and immune complexes is another potential target in NPSLE.
Pro-inflammatory cytokines, including type I IFN, IL-6 and others, produced by resident brain and infiltrating immunocompetent cells have been associated with NPSLE events [33] and provide another potential therapeutic target in patients with NPSLE. The use of commercially available biologics or Janus kinase inhibitors are two strategies worthy of consideration in this regard.
Microglial activation, associated synaptic pruning linked to C1q tagging and loss of synaptic density has been implicated in neurodegenerative disorders [102] and more recently in the pathogenesis of NPSLE [40]. Potential therapeutic strategies include neutralizing the autoantibodies and complement proteins that cause in vitro microglial activation or directly targeting microglia themselves. The latter include ACE inhibitors that cross the BBB.
Lack of clinical trials in NPSLE
There have been only three controlled clinical trials in NPSLE [50, 61, 62], a remarkable fact in view of the frequency and clinical significance of this manifestation of SLE. What are the reasons for this and what steps are required to facilitate this type of scientific discovery?
Most controlled clinical trials of new therapies in SLE have excluded patients with severe NP manifestations. Although it would be unethical to recruit patients with acute life-threatening NP events to clinical trials, other types of NP events that are common and not life-threatening could be studied. For example, SLE patients have a high frequency of mood disorders, selected cerebrovascular disease and cognitive impairment, all of which would be suitable for the study of efficacy and tolerability of symptomatic, anticoagulant and immunosuppressive therapies. Mood disorders occur in 12.7% of SLE patients [46], but there are no controlled clinical trials to determine the optimum pharmacotherapy. Clinical trials of NPSLE events that are less frequent (e.g. lupus psychosis, seizure disorders) will require a large, multicentric and multidisciplinary effort using standardized case definitions [5] and attribution rules [9, 103].
Validated outcome measures for NPSLE are essential to study the effectiveness of any therapeutic intervention. Clinical outcomes could include both generic and NP-specific instruments. For example, changes in the 36-item Short Form health Survey summary and subscale scores, in particular those related to mental health, are strongly associated with physician-determined clinical outcomes of NP events in SLE patients even after adjusting for potential confounder variables [104]. Validated self-report instruments for the assessment of mood have performed well in observational studies of SLE cohorts [105] and could be used in clinical trials. A multidisciplinary effort is required to identify the optimal generic and NP-specific instruments and to derive methods for distinguishing between ongoing disease activity that is potentially reversible in contrast to irreversible organ damage.
Neuroimaging of both brain structure and function may also provide objective outcome measures for future clinical trials. In a recent elegant review of cognitive dysfunction in SLE [106] a number of potential neuroimaging techniques were highlighted. These included cMRI for the assessment of hippocampal volume, diffusion tensor imaging and magnetic resonance spectroscopy for assessment of white matter tract integrity and PET for measurement of microglial activation.
In summary, although neuropsychiatric manifestations of SLE have been recognized for >100 years, the elucidation of pathogenic mechanisms, correct attribution of individual NP presentations to SLE and non-SLE causes and high-quality evidence to support optimal treatments has lagged behind knowledge on other manifestations of SLE. The recent insights on NPSLE summarized in this review provide a basis for further advances. In particular, we propose that future research should focus on the discovery of biomarkers for NPSLE and conducting clinical trials of novel and established drugs in SLE patients with NP manifestations. Although this will require a multidisciplinary effort, leadership for these initiatives should be provided by rheumatologists.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article. This paper was published as part of a supplement supported by an educational grant from GSK.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Hanly JG, Urowitz MB, Su L et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2010;69:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zirkzee EJ, Huizinga TW, Bollen EL et al. Mortality in neuropsychiatric systemic lupus erythematosus (NPSLE). Lupus 2014;23:31–8. [DOI] [PubMed] [Google Scholar]
- 3. Unterman A, Nolte JE, Boaz M et al. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum 2011;41:1–11. [DOI] [PubMed] [Google Scholar]
- 4. Govoni M, Bortoluzzi A, Padovan M et al. The diagnosis and clinical management of the neuropsychiatric manifestations of lupus. J Autoimmun 2016;74:41–72. [DOI] [PubMed] [Google Scholar]
- 5. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 6. Hanly JG, Urowitz MB, Sanchez-Guerrero J et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum 2007;56:265–73. [DOI] [PubMed] [Google Scholar]
- 7. Hanly JG, Urowitz MB, Su L, et al. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis Rheum 2008;59:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ainiala H, Hietaharju A, Loukkola J et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum 2001;45:419–23. [DOI] [PubMed] [Google Scholar]
- 9. Bortoluzzi A, Scire CA, Bombardieri S et al. Development and validation of a new algorithm for attribution of neuropsychiatric events in systemic lupus erythematosus. Rheumatology (Oxford) 2015;54:891–8. [DOI] [PubMed] [Google Scholar]
- 10. Bortoluzzi A, Fanouriakis A, Appenzeller S et al. Validity of the Italian algorithm for the attribution of neuropsychiatric events in systemic lupus erythematosus: a retrospective multicentre international diagnostic cohort study. BMJ Open 2017;7:e015546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magro-Checa C, Zirkzee EJ, Beaart-van de Voorde LJJ et al. Value of multidisciplinary reassessment in attribution of neuropsychiatric events to systemic lupus erythematosus: prospective data from the Leiden NPSLE cohort. Rheumatology (Oxford) 2017;56:1676–83. [DOI] [PubMed] [Google Scholar]
- 12. Bortoluzzi A, Piga M, Silvagni E et al. Peripheral nervous system involvement in systemic lupus erythematosus: a retrospective study on prevalence, associated factors and outcome. Lupus 2019;28:465–74. [DOI] [PubMed] [Google Scholar]
- 13. Hanly JG, Li Q, Su L et al. Peripheral nervous system disease in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Rheumatol 2020;72:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ho RC, Ong H, Thiaghu C et al. Genetic variants that are associated with neuropsychiatric systemic lupus erythematosus. J Rheumatol 2016;43:541–51. [DOI] [PubMed] [Google Scholar]
- 15. Hanly JG, Kozora E, Beyea SD, Birnbaum J. Review: nervous system disease in systemic lupus erythematosus: current status and future directions. Arthritis Rheumatol 2019;71:33–42. [DOI] [PubMed] [Google Scholar]
- 16. Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol 2014;10:579–96. [DOI] [PubMed] [Google Scholar]
- 17. Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol 2019;15:137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore E, Huang MW, Putterman C. Advances in the diagnosis, pathogenesis and treatment of neuropsychiatric systemic lupus erythematosus. Curr Opin Rheumatol 2020;32:152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen D, Rijnink EC, Nabuurs RJ et al. Brain histopathology in patients with systemic lupus erythematosus: identification of lesions associated with clinical neuropsychiatric lupus syndromes and the role of complement. Rheumatology (Oxford) 2017;56:77–86. [DOI] [PubMed] [Google Scholar]
- 20. Cohen D, Buurma A, Goemaere NN et al. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol 2011;225:502–11. [DOI] [PubMed] [Google Scholar]
- 21. Pierangeli SS, Girardi G, Vega-Ostertag M et al. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum 2005;52:2120–4. [DOI] [PubMed] [Google Scholar]
- 22. Magro-Checa C, Schaarenburg RA, Beaart HJ et al. Complement levels and anti-C1q autoantibodies in patients with neuropsychiatric systemic lupus erythematosus. Lupus 2016;25:878–88. [DOI] [PubMed] [Google Scholar]
- 23. Ho RC, Thiaghu C, Ong H et al. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmun Rev 2016;15:124–38. [DOI] [PubMed] [Google Scholar]
- 24. Lauvsnes MB, Omdal R. Systemic lupus erythematosus, the brain, and anti-NR2 antibodies. J Neurol 2012;259:622–9. [DOI] [PubMed] [Google Scholar]
- 25. Yoshio T, Okamoto H, Hirohata S, Minota S. IgG anti-NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells. Arthritis Rheum 2013;65:457–63. [DOI] [PubMed] [Google Scholar]
- 26. Wang JY, Zhao YH, Zhang JH, Lei HW. Anti-N-methyl-D-aspartic acid receptor 2 (anti-NR2) antibody in neuropsychiatric lupus serum damages the blood-brain barrier and enters the brain. Med Sci Monit 2019;25:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi MY, FitzPatrick RD, Buhler K, Mahler M, Fritzler MJ. A review and meta-analysis of anti-ribosomal P autoantibodies in systemic lupus erythematosus. Autoimmun Rev 2020;19:102463. [DOI] [PubMed] [Google Scholar]
- 28. Hanly JG, Urowitz MB, Siannis F et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum 2008;58:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ichinose K, Ohyama K, Furukawa K et al. Novel anti-suprabasin antibodies may contribute to the pathogenesis of neuropsychiatric systemic lupus erythematosus. Clin Immunol 2018;193:123–30. [DOI] [PubMed] [Google Scholar]
- 30. Li X, Sun J, Mu R et al. The clinical significance of ubiquitin carboxyl hydrolase L1 and its autoantibody in neuropsychiatric systemic lupus erythematosus. Clin Exp Rheumatol 2019;37:474–80. [PubMed] [Google Scholar]
- 31. Muslimov IA, Iacoangeli A, Eom T et al. Neuronal BC RNA transport impairments caused by systemic lupus erythematosus autoantibodies. J Neurosci 2019;39:7759–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun J, Li X, Zhou H et al. Anti-GAPDH autoantibody is associated with increased disease activity and intracranial pressure in systemic lupus erythematosus. J Immunol Res 2019;2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine 2016;77:227–37. [DOI] [PubMed] [Google Scholar]
- 34. Lapa AT, Postal M, Sinicato NA et al. S100β is associated with cognitive impairment in childhood-onset systemic lupus erythematosus patients. Lupus 2017;26:478–83. [DOI] [PubMed] [Google Scholar]
- 35. Wen J, Doerner J, Weidenheim K et al. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/LPR mice. J Autoimmun 2015;60:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wen J, Xia Y, Stock A et al. Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. J Autoimmun 2013;43:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. FASEB J 1993;7:1475–82. [PubMed] [Google Scholar]
- 38. Kitagori K, Yoshifuji H, Oku T et al. Utility of osteopontin in cerebrospinal fluid as a diagnostic marker for neuropsychiatric systemic lupus erythematosus. Lupus 2019;28:414–22. [DOI] [PubMed] [Google Scholar]
- 39. Wang J, Yang C, Zhao Q et al. Microglia activation induced by serum of SLE patients. J Neuroimmunol 2017;310:135–42. [DOI] [PubMed] [Google Scholar]
- 40. Nestor J, Arinuma Y, Huerta TS et al. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J Exp Med 2018;215:2554–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bertsias GK, Ioannidis JP, Aringer M et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 2010;69:2074–82. [DOI] [PubMed] [Google Scholar]
- 42. Fanouriakis A, Kostopoulou M, Alunno A et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 43. Conceicao CTM, Meinao IM, Bombana JA, Sato EI. Psychoanalytic psychotherapy improves quality of life, depression, anxiety and coping in patients with systemic lupus erythematosus: a controlled randomized clinical trial. Adv Rheumatol 2019;59:4. [DOI] [PubMed] [Google Scholar]
- 44. Julian LJ, Yelin E, Yazdany J et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum 2009;61:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karassa FB, Magliano M, Isenberg DA. Suicide attempts in patients with systemic lupus erythematosus. Ann Rheum Dis 2003;62:58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanly JG, Su L, Urowitz MB et al. Mood disorders in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Rheumatol 2015;67:1837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Exel E, Jacobs J, Korswagen LA et al. Depression in systemic lupus erythematosus, dependent on or independent of severity of disease. Lupus 2013;22:1462–9. [DOI] [PubMed] [Google Scholar]
- 48. Hanly JG, Urowitz MB, Su L et al. Seizure disorders in systemic lupus erythematosus results from an international, prospective, inception cohort study. Ann Rheum Dis 2012;71:1502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hanly JG, Li Q, Su L et al. Psychosis in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Rheumatol 2019;71:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petri M, Naqibuddin M, Sampedro M, Omdal R, Carson KA. Memantine in systemic lupus erythematosus: a randomized, double-blind placebo-controlled trial. Semin Arthritis Rheum 2011;41:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cicerone KD, Dahlberg C, Malec JF et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil 2005;86:1681–92. [DOI] [PubMed] [Google Scholar]
- 52. Wilson BA. Cognitive rehabilitation: how it is and how it might be. J Int Neuropsychol Soc 1997;3:487–96. [PubMed] [Google Scholar]
- 53. Harrison MJ, Morris KA, Horton R et al. Results of intervention for lupus patients with self-perceived cognitive difficulties. Neurology 2005;65:1325–7. [DOI] [PubMed] [Google Scholar]
- 54. Vadgama TS, Smith A, Bertolaccini ML. Treatment in thrombotic antiphospholipid syndrome: a review. Lupus 2019;28:1181–8. [DOI] [PubMed] [Google Scholar]
- 55. Hanly JG, Li Q, Su L et al. Cerebrovascular events in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Care Res (Hoboken) 2018;70:1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crowther MA, Ginsberg JS, Julian J et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003;349:1133–8. [DOI] [PubMed] [Google Scholar]
- 57. Finazzi G, Marchioli R, Brancaccio V et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005;3:848–53. [DOI] [PubMed] [Google Scholar]
- 58. de Amorim LC, Maia FM, Rodrigues CE. Stroke in systemic lupus erythematosus and antiphospholipid syndrome: risk factors, clinical manifestations, neuroimaging, and treatment. Lupus 2017;26:529–36. [DOI] [PubMed] [Google Scholar]
- 59. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum 2007;57:1487–95. [DOI] [PubMed] [Google Scholar]
- 60. Pengo V, Denas G, Zoppellaro G et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365–71. [DOI] [PubMed] [Google Scholar]
- 61. Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, Jara LJ et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis 2005;64:620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Denburg SD, Carbotte RM, Denburg JA. Corticosteroids and neuropsychological functioning in patients with systemic lupus erythematosus. Arthritis Rheum 1994;37:1311–20. [DOI] [PubMed] [Google Scholar]
- 63. Fanouriakis A, Pamfil C, Sidiropoulos P et al. Cyclophosphamide in combination with glucocorticoids for severe neuropsychiatric systemic lupus erythematosus: a retrospective, observational two-centre study. Lupus 2016;25:627–36. [DOI] [PubMed] [Google Scholar]
- 64. Mok CC, Lau CS, Wong RW. Treatment of lupus psychosis with oral cyclophosphamide followed by azathioprine maintenance: an open-label study. Am J Med 2003;115:59–62. [DOI] [PubMed] [Google Scholar]
- 65. Andrade RM, Alarcon GS, Gonzalez LA et al. Seizures in patients with systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA LIV). Ann Rheum Dis 2008;67:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Potvin F, Petitclerc E, Marceau F, Poubelle PE. Mechanisms of action of antimalarials in inflammation: induction of apoptosis in human endothelial cells. J Immunol 1997;158:1872–9. [PubMed] [Google Scholar]
- 67. A randomized trial of hydroxychloroquine in early rheumatoid arthritis: the HERA Study. Am J Med 1995;98:156–68. [DOI] [PubMed] [Google Scholar]
- 68. Jung H, Bobba R, Su J et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum 2010;62:863–8. [DOI] [PubMed] [Google Scholar]
- 69. Sarbu N, Toledano P, Calvo A et al. Advanced MRI techniques: biomarkers in neuropsychiatric lupus. Lupus 2017;26:510–6. [DOI] [PubMed] [Google Scholar]
- 70. Dale RC, Brilot F, Duffy LV et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology 2014;83:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Narvaez J, Rios-Rodriguez V, de la Fuente D et al. Rituximab therapy in refractory neuropsychiatric lupus: current clinical evidence. Semin Arthritis Rheum 2011;41:364–72. [DOI] [PubMed] [Google Scholar]
- 72. Saito K, Nawata M, Nakayamada S et al. Successful treatment with anti-CD20 monoclonal antibody (rituximab) of life-threatening refractory systemic lupus erythematosus with renal and central nervous system involvement. Lupus 2003;12:798–800. [DOI] [PubMed] [Google Scholar]
- 73. Tokunaga M, Saito K, Kawabata D et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis 2006;66:470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Manzi S, Sanchez-Guerrero J, Merrill JT et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis 2012;71:1833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Morand EF, Furie R, Tanaka Y et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- 76. Govoni M, Bombardieri S, Bortoluzzi A et al. Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology (Oxford) 2012;51:157–68. [DOI] [PubMed] [Google Scholar]
- 77. Hanly JG, Urowitz MB, Su L et al. Autoantibodies as biomarkers for the prediction of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis 2011;70:1726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Karassa FB, Afeltra A, Ambrozic A et al. Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis. Arthritis Rheum 2006;54:312–24. [DOI] [PubMed] [Google Scholar]
- 79. Tay SH, Fairhurst AM, Mak A. Clinical utility of circulating anti-N-methyl-d-aspartate receptor subunits NR2A/B antibody for the diagnosis of neuropsychiatric syndromes in systemic lupus erythematosus and Sjogren’s syndrome: an updated meta-analysis. Autoimmun Rev 2017;16:114–22. [DOI] [PubMed] [Google Scholar]
- 80. Magro-Checa C, Steup-Beekman GM, Huizinga TW, van Buchem MA, Ronen I. Laboratory and neuroimaging biomarkers in neuropsychiatric systemic lupus erythematosus: where do we stand, where to go? Front Med (Lausanne) 2018;5:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Asano T, Ito H, Kariya Y et al. Evaluation of blood-brain barrier function by quotient alpha2 macroglobulin and its relationship with interleukin-6 and complement component 3 levels in neuropsychiatric systemic lupus erythematosus. PLoS One 2017;12:e0186414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fragoso-Loyo H, Richaud-Patin Y, Orozco-Narvaez A et al. Interleukin-6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus. Arthritis Rheum 2007;56:1242–50. [DOI] [PubMed] [Google Scholar]
- 83. Trysberg E, Carlsten H, Tarkowski A. Intrathecal cytokines in systemic lupus erythematosus with central nervous system involvement. Lupus 2000;9:498–503. [DOI] [PubMed] [Google Scholar]
- 84. Wang JB, Li H, Wang LL, Liang HD et al. Role of IL-1β, IL-6, IL-8 and IFN-γ in pathogenesis of central nervous system neuropsychiatric systemic lupus erythematous. Int J Clin Exp Med 2015;8:16658–63. [PMC free article] [PubMed] [Google Scholar]
- 85. Yoshio T, Okamoto H, Kurasawa K et al. IL-6, IL-8, IP-10, MCP-1 and G-CSF are significantly increased in cerebrospinal fluid but not in sera of patients with central neuropsychiatric lupus erythematosus. Lupus 2016;25:997–1003. [DOI] [PubMed] [Google Scholar]
- 86. Lu XY, Zhu CQ, Qian J et al. Intrathecal cytokine and chemokine profiling in neuropsychiatric lupus or lupus complicated with central nervous system infection. Lupus 2010;19:689–95. [DOI] [PubMed] [Google Scholar]
- 87. Luyendijk J, Steens SC, Ouwendijk WJ et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum 2011;63:722–32. [DOI] [PubMed] [Google Scholar]
- 88. Hirohata S, Kanai Y, Mitsuo A et al. Accuracy of cerebrospinal fluid IL-6 testing for diagnosis of lupus psychosis. A multicenter retrospective study. Clin Rheumatol 2009;28:1319–23. [DOI] [PubMed] [Google Scholar]
- 89. Sibbitt WL Jr, Sibbitt RR, Brooks WM. Neuroimaging in neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 1999;42:2026–38. [DOI] [PubMed] [Google Scholar]
- 90. Nikolopoulos D, Fanouriakis A, Boumpas DT. Update on the pathogenesis of central nervous system lupus. Curr Opin Rheumatol 2019;31:669–77. [DOI] [PubMed] [Google Scholar]
- 91. Mackay M, Tang CC, Vo A. Advanced neuroimaging in neuropsychiatric systemic lupus erythematosus. Curr Opin Neurol 2020;33:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Costallat BL, Ferreira DM, Lapa AT et al. Brain diffusion tensor MRI in systematic lupus erythematosus: a systematic review. Autoimmun Rev 2018;17:36–43. [DOI] [PubMed] [Google Scholar]
- 93. Mikdashi JA. Altered functional neuronal activity in neuropsychiatric lupus: a systematic review of the fMRI investigations. Semin Arthritis Rheum 2016;45:455–62. [DOI] [PubMed] [Google Scholar]
- 94. Papadaki E, Fanouriakis A, Kavroulakis E et al. Neuropsychiatric lupus or not? Cerebral hypoperfusion by perfusion-weighted MRI in normal-appearing white matter in primary neuropsychiatric lupus erythematosus. Ann Rheum Dis 2018;77:441–8. [DOI] [PubMed] [Google Scholar]
- 95. Wang Y, Coughlin JM, Ma S et al. Neuroimaging of translocator protein in patients with systemic lupus erythematosus: a pilot study using [(11)C]DPA-713 positron emission tomography. Lupus 2017;26:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ercan E, Magro-Checa C, Valabregue R et al. Glial and axonal changes in systemic lupus erythematosus measured with diffusion of intracellular metabolites. Brain 2016;139:1447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lee SW, Park MC, Lee SK, Park YB. The efficacy of brain 18F-fluorodeoxyglucose positron emission tomography in neuropsychiatric lupus patients with normal brain magnetic resonance imaging findings. Lupus 2012;21:1531–7. [DOI] [PubMed] [Google Scholar]
- 98. Wang PI, Cagnoli PC, McCune WJ et al. Perfusion-weighted MR imaging in cerebral lupus erythematosus. Acad Radiol 2012;19:965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang Z, Wang Y, Shen Z et al. The neurochemical and microstructural changes in the brain of systemic lupus erythematosus patients: a multimodal MRI study. Sci Rep 2016;6:19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. De Luca C, Virtuoso A, Maggio N, Papa M. Neuro-coagulopathy: blood coagulation factors in central nervous system diseases. Int J Mol Sci 2017;18:2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Heinemann U, Kaufer D, Friedman A. Blood–brain barrier dysfunction, TGFβ signaling, and astrocyte dysfunction in epilepsy. Glia 2012;60:1251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gyorffy BA, Kun J, Torok G et al. Local apoptotic-like mechanisms underlie complement-mediated synaptic pruning. Proc Natl Acad Sci USA 2018;115:6303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hanly JG, Urowitz MB, Gordon C et al. Neuropsychiatric events in systemic lupus erythematosus: a longitudinal analysis of outcomes in an international inception cohort using a multistate model approach. Ann Rheum Dis 2020;79:356–62. [DOI] [PubMed] [Google Scholar]
- 104. Hanly JG, Urowitz MB, Jackson D et al. SF-36 summary and subscale scores are reliable outcomes of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis 2011;70:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kwan A, Marzouk S, Ghanean H et al. Assessment of the psychometric properties of patient-reported outcomes of depression and anxiety in systemic lupus erythematosus. Semin Arthritis Rheum 2019;49:260–6. [DOI] [PubMed] [Google Scholar]
- 106. Kello N, Anderson E, Diamond B. Cognitive dysfunction in systemic lupus erythematosus: a case for initiating trials. Arthritis Rheumatol 2019;71:1413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]