Heart failure with preserved ejection fraction (HFpEF), a heterogeneous syndrome characterized by dyspnea and exercise intolerance, leads to impairment in quality of life and carries a 5-year mortality of >75% after heart failure (HF) hospitalization.1 Unfortunately, atrial fibrillation (AF), a frequent comorbidity of HFpEF, confers important prognostic implications. Observational studies demonstrate that over 60% of individuals with HFpEF will be burdened by comorbid AF at some point over their life course.2 In addition, in community-based cohorts, comorbid AF in HFpEF is associated with higher risks of adverse clinical outcomes, including HF hospitalization and death, compared with HFpEF without comorbid AF.3 While recent randomized clinical trials have demonstrated a promising signal of efficacy for catheter ablation in HF with reduced ejection fraction,4 parallel data in HFpEF are lacking. As such, the optimal management strategy of AF in HFpEF remains uncertain.
In this issue of the Journal of Cardiovascular Electrophysiology, Zhang et al.5 aim to shed light on this challenging clinical situation by evaluating associations of AF management strategies with long-term outcomes in a community-based HFpEF cohort. Of 447 elderly individuals with incident AF and prior or concurrent HFpEF, all-cause mortality at 10 years was substantial (83%). A small proportion (16%) of HFpEF patients with incident AF underwent rhythm control (pharmacologic or procedural) in the first year after AF diagnosis, of which a minority (n = 7) underwent catheter ablation. After covariate adjustment, rhythm control was not associated with improved survival compared with rate control.5 This study provides key insight into the low prevalence of rhythm control among elderly HFpEF patients, and the analysis is strengthened by the incorporation of a rate control comparator group. However, the lack of association between rhythm control and outcomes may be explained by both the low prevalence of rhythm control in this cohort (decreased power to detect a signal of benefit) and the fact that catheter ablation, a more effective method of sinus rhythm restoration, was sorely underrepresented. Indeed, the low frequency of catheter ablation may reflect the elderly nature of the cohort. Taken together, these findings provide a call for randomized controlled trial evidence in this area. Although there are two small, ongoing trials (NCT04160000 and NCT04282850), additional, large-scale clinical trials are required. However, there are considerations that must be addressed before embarking upon future clinical trials of AF management in HFpEF. The basis of these considerations lies in the urgent need to better understand the pathophysiology surrounding these two syndromes when they occur in combination, as opposed to their occurrence in isolation.
1 |. HISTORY OF HFpEF TRIALS: FINDING THE SILVER LINING
Despite numerous pharmacologic clinical trials of HFpEF, there are no universally accepted therapies to reduce morbidity and mortality or alter disease progression.6 The profound heterogeneity of the HFpEF syndrome may partially explain the current lack of disease-modifying therapies. The spectrum of risk across individuals with HFpEF is wide and mechanisms of HFpEF development and progression vary substantially. Thus, the evaluation of highly specific therapies in a general HFpEF cohort (one-size-fits-all approach) has not led to substantial progress. As a result, ongoing and future clinical trials of HFpEF have incorporated varying methods, including tailored inclusion criteria, alternative endpoints, and novel trial designs, to evaluate certain therapies within HFpEF subtypes that are most likely to benefit based upon specific disease pathogenesis and drug or device effects. Given that AF itself is a syndrome defined by varied pathophysiology and prognosis, investigators must be aware of the heterogeneity of HFpEF when evaluating therapies for AF in this complex subgroup. To effectively study therapeutic interventions in AF and HFpEF, it may be prudent to characterize the pathophysiologic variation among individuals who have both syndromes.
2 |. AF HETEROGENEITY MAY INFLUENCE TREATMENT RESPONSE IN HFpEF
The electrical and mechanical substrates for AF initiation and maintenance are heterogeneous, especially in the background of HFpEF. While current schema to define AF burden (paroxysmal, persistent, and permanent) indirectly provide insight into the underlying atrial substrate driving AF, further characterization of the arrhythmogenic substrate may ultimately identify individuals most likely to benefit from specific treatment strategies (i.e., rate vs. rhythm control) or procedural management (i.e., ablation protocol). For example, an international working group has described a histological/pathophysiological classification scheme for atrial cardiomyopathies, termed the EHRAS (EHRA/HRS/APHRS/SOLAECE) classification.7 According to the working group, a particular goal is to ultimately apply this mechanistic classification to assist in tailoring therapies for AF. We believe that such formal, pathophysiologic classification will be particularly important for effective, tailored treatment of AF in the setting of HFpEF. Such a classification schema may not be feasible to use as inclusion criteria for trials of AF therapies within HFpEF, as it relies upon atrial tissue architecture. However, the correlation of such categories of atrial cardiomyopathy with serum biomarkers, electrocardiographic parameters (e.g., P-wave or F-wave morphology), imaging parameters (e.g., the atrial strain on echocardiography and fibrosis on cardiac magnetic resonance imaging), and AF burden or duration on continuous monitoring may provide insight to identify specific populations of AF within HFpEF for targeted therapeutic interventions.
In addition, the findings from the current analysis in the Journal of Cardiovascular Electrophysiology lead one to ponder if the temporal relationship between AF and HFpEF may influence response to therapy. Chronic HFpEF may lead to AF through a sustained elevation in leftsided filling pressures and subsequent electromechanical remodeling. May this atrial substrate be distinct from that of a patient with longstanding AF who later develops HFpEF? Further investigation regarding the variation in atrial electromechanical remodeling that occurs based upon the timing of AF development in relation to HFpEF may provide insight into the likelihood of response to therapies.
3 |. POTENTIAL EFFECT MODIFICATION BY HFpEF PHENOTYPE ON AF TREATMENT EFFICACY
While further detailed understanding regarding the heterogeneity of AF in HFpEF may be worthwhile for future clinical trials, it is also paramount to acknowledge that the efficacy of AF treatment is likely dependent upon the HFpEF phenotype. Certain restrictive cardiomyopathies, including cardiac amyloidosis, may be considered under the overarching umbrella of HFpEF and carry an increased risk for atrial arrhythmias. In cardiac amyloidosis, the underlying mechanisms of AF are indeed unique from other forms of HFpEF (e.g., deposition of amyloid fibrils within the atria), and AF is poorly tolerated in this cohort. Such factors may influence response to therapies for AF, including the risk of certain ratecontrolling agents (e.g., adverse reactions to digoxin and calcium channel blockers) and efficacy of catheter ablation. Distinct investigations of AF management strategies within specific HFpEF populations may provide clinically important understandings.
4 |. TREATMENT OF LEFT ATRIAL MYOPATHIC SUBSTRATE
In addition to the hemodynamic sequelae resulting from loss of sinus rhythm, the association of AF with poor outcomes in HFpEF may be partially driven by left atrial (LA) mechanical dysfunction (termed LA myopathy). Reduced LA reservoir function is common in AF, indicates low LA compliance (due to increased LA myocardial stiffness and/or elevated LA pressure), and maybe further exacerbated by functional mitral regurgitation due to atrial dilation, which frequently coexists with AF.8 Independent of AF, LA myopathy is associated with poor outcomes in HFpEF.9 Further investigations are required to understand the relative contributions of LA electromechanical failure (AF) and pure mechanical failure (LA myopathy) in driving poor outcomes in patients with AF and HFpEF to identify individuals who may particularly benefit from the restoration of sinus rhythm. It is possible that despite the restoration of sinus rhythm, patients with profound LA mechanical failure may have a persistently elevated risk of clinical deterioration. Such patients may also benefit from therapies that specifically target LA myopathy. While such treatment options are limited, there are investigations underway. For example, LA unloading through an interatrial shunt device (IASD) to reduce LA pressure in HFpEF patients is currently being investigated in Phase 3, randomized, multicenter, sham-controlled trial (REDUCE LAP-HF-II, NCT03088033; n = 608).10 Patients with AF and HFpEF may particularly benefit from LA unloading, not only due to the severity of LA myopathy in this population but also because AF ablation may result in increased LA stiffness and paradoxically worsen symptoms in some patients,11 which may be alleviated by IASD placement. In addition, certain pharmacotherapies offer promise in the treatment of both AF and LA myopathy in HFpEF patients. Dapagliflozin, a sodium-glucose cotransporter 2 inhibitor that has been traditionally used for glucose-lowering, reduced recurrent AF events among patients with prevalent AF and type 2 diabetes compared with placebo in the DECLARE-TIMI 58 trial.12 In aggregate, an enhanced understanding of the interplay between electrical and mechanical failure of the LA in AF and HFpEF may carry important implications toward improving clinical outcomes.
The poor clinical trajectory among those with AF and HFpEF in concert with immense technological advances of catheter ablation have led us to a clinical quandary. There are several aspects regarding the pathophysiology of comorbid AF and HFpEF that remain unclear (Figure 1). Further elucidation of these mechanisms will assist in shaping the designs of future large-scale clinical trials of rhythm control of AF in HFpEF, including inclusion criteria and endpoint selection. As we embark upon randomized investigations of AF management in HFpEF, a granular understanding of the interplay between these two complex syndromes is essential to chart a steadfast course toward improving outcomes in this vulnerable cohort.
FIGURE 1.
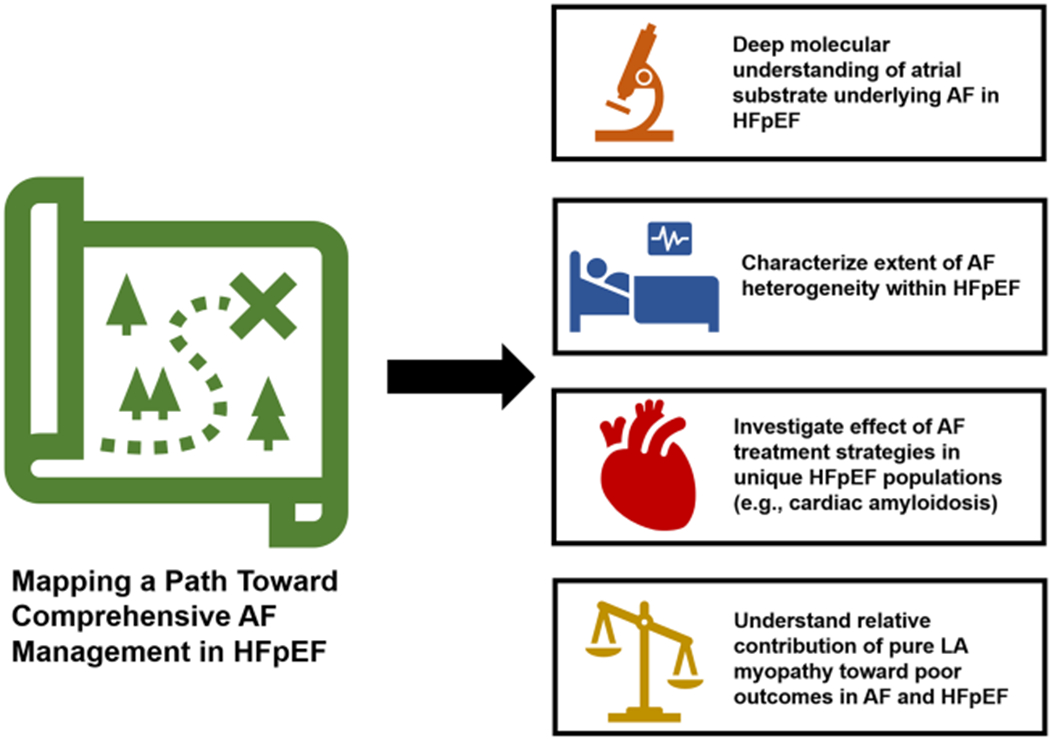
Mapping a path toward comprehensive AF management in HFpEF. AF, atrial fibrillation; HFpEF, heart failure with preserved ejection fraction; LA, left atrial
Acknowledgments
Disclosures: Dr. Ravi Patel is supported by the National Institutes of Health’s National Center for Advancing Translational Sciences (KL2TR001424). Dr. Rod Passman is on the advisory board for Abbott and Medtronic and receives royalties from UpToDate. Dr. Sanjiv Shah is the principal investigator of the Corvia REDUCE LAP-HF I and II trials, and has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Cardiora, Cyclerion, Cytokinetics, Eisai, Ionis, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Sanofi, Shifamed, Tenax, and United Therapeutics.
REFERENCES
- 1.Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 2.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378: 417–427. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Chamberlain AM, Hodge DO, et al. Outcomes of incident atrial fibrillation in heart failure with preserved or reduced ejection fraction: a community-based study. J Cardiovasc Electrophysiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel RB, Shah SJ. Drug targets for heart failure with preserved ejection fraction: a mechanistic approach and review of contemporary clinical trials. Annu Rev Pharmacol Toxicol. 2019;59:41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14: e3–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RB, Shah SJ. Therapeutic targeting of left atrial myopathy in atrial fibrillation and heart failure with preserved ejection fraction. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freed BH, Daruwalla V, Cheng JY, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9:e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry N, Mauri L, Feldman T, et al. Transcatheter InterAtrial shunt device for the treatment of heart failure: Rationale and Design of the Pivotal Randomized Trial to REDUCE elevated left atrial pressure in patients with heart failure II (REDUCE LAP-HF II). Am Heart J. In press. [DOI] [PubMed] [Google Scholar]
- 11.Park JW, Yu HT, Kim TH, et al. Atrial fibrillation catheter ablation increases the left atrial pressure. Circ Arrhythm Electrophysiol. 2019; 12:e007073. [DOI] [PubMed] [Google Scholar]
- 12.Zelniker TA, Bonaca MP, Furtado RHM, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141: 1227–1234. [DOI] [PubMed] [Google Scholar]


