Abstract
BACKGROUND:
Social isolation has shown robust associations with clinical outcomes in the general population and in patients with cancer. In patients with ovarian cancer, social isolation has been found to be related to decreased survival and multiple biomarkers supporting tumor progression. However, to the authors’ knowledge, little is known regarding the relationship between social isolation and the molecular characteristics of ovarian tumors. Herein, the authors have used genome-wide transcriptional profiling to quantify associations between social isolation and epithelial-mesenchymal transition (EMT) polarization in ovarian tumors and transcriptome-driven, promoter-based bioinformatics analyses to identify gene regulatory pathways that may potentially underlie these changes.
METHODS:
Tumor was sampled during primary surgical resection and immediately frozen in liquid nitrogen. After RNA extraction, microarray analysis of the transcriptome was performed and samples were analyzed to assess associations between EMT-related gene transcripts and social isolation (as indicated by a Social Provisions Scale Attachment subscale score <15). Convergent validation was provided by a promoter-based bioinformatic analysis of transcription factor activity.
RESULTS:
Primary analyses of 99 women demonstrated a lower average expression of gene transcripts previously associated with epithelial differentiation in women with high social isolation (−0.143 ± 0.048 log2 mRNA abundance; P = .004), but no difference in mesenchymal differentiation as a function of social isolation (+0.007 ± 0.0064 log2 mRNA abundance; P = .900). Upregulated activity was shown for 3 of the 4 targeted EMT-related transcription factors, including GATA4 (P = .014); SMAD2, SMAD3, and/or SMAD4 (P < .001); and TWIST1 (P < .001). Analyses of SNAIL2/SLUG activity indicated a directional trend toward increased activity that did not reach statistical significance (P = .123).
CONCLUSIONS:
The findings of the current study demonstrated differential EMT polarization and EMT-related transcription factor activity according to social isolation, a known socioenvironmental risk factor.
Keywords: biobehavioral, epithelial-mesenchymal transition, ovarian cancer, social isolation, transcriptome, treatment resistance
LAY SUMMARY:
● Social isolation has shown robust associations with clinical outcomes in the general population and in patients with cancer. Herein, the authors examined the relationship between social isolation and the molecular characteristics of ovarian tumors.
● The authors investigated the epithelial-mesenchymal transition (EMT), a process whereby tumor cells lose epithelial characteristics and become more embryonic (mesenchymal), thereby enhancing invasiveness.
● Primary analyses demonstrated lower expression of genes previously associated with epithelial differentiation and increased activity of specific EMT-related transcription factors in individuals with high social isolation, indicating increased EMT polarization in these patients. These findings extend the understanding of how socioenvironmental factors may modulate tumor growth.
INTRODUCTION
Characteristics of the host macroenvironment are known to influence key molecular processes in the tumor microenvironment to enhance tumor growth and thereby modulate the progression of a variety of tumors, including ovarian carcinoma.1–4 Social isolation is a host biobehavioral factor that has demonstrated robust associations with clinical outcomes and mortality in both the general population5,6 and in individuals with cancer.7,8 For example, social isolation was associated with a greater risk of disease recurrence9 and poorer survival in several large-scale studies of patients with breast cancer9–11 and in a meta-analysis that examined results from 87 studies of patients with cancer.12 Among patients with ovarian cancer, we previously have reported that individuals experiencing greater social isolation had significantly shorter survival than those with higher levels of social support, adjusting for clinical covariates. 13 Chronic social isolation also has been shown to be a risk factor for an increased incidence of ovarian cancer.7 Paralleling these findings, patients with ovarian cancer who report greater social isolation have demonstrated poorer cellular immunity,14 higher levels of inflammation15 and of the stress hormone norepinephrine in tumor and ascites,16 and elevations in biomarkers of angiogenesis and invasion in the tumor microenvironment.17–19 Similarly, a preclinical model of social isolation in ovarian carcinoma demonstrated greater tumor volume and a greater number of tumor nodules in socially isolated animals.20 However, despite this research, to our knowledge little is known regarding the relationship between social isolation and the molecular characteristics of ovarian tumors.
A significant factor in cancer metastasis is the epithelial-mesenchymal transition (EMT), which enables tumor cells to transition to a more invasive (mesenchymal) phenotype. In addition to promoting metastasis, cells having undergone this transition demonstrate greater chemoresistance, radiotherapy resistance, and evasion of apoptosis.21–23 EMT also enhances immune suppression.24 In patients with early-stage breast cancer, increased mesenchymal polarization was observed in tumors of women reporting higher levels of social isolation. 25 A recent phase 2 trial in patients with early-stage breast cancer demonstrated that EMT can be modulated with a stress-blocking (β-adrenergic) and inflammation-blocking (COX-2) intervention,26 highlighting the role of stress and inflammatory stimuli in modulating the EMT. We previously have reported that patients with ovarian cancer who are experiencing higher levels of social isolation demonstrated increased expression of EMT-related gene transcripts in exosomes derived from peripheral blood.27 Although this finding is suggestive, to our knowledge it is not known to what extent this association reflects the biology of the tumor or what molecular signaling pathways might mediate the effects of host social support levels on tumor cell biology. To address this issue, we used genome-wide transcriptional profiling to quantify associations between social isolation and EMT polarization in tumor samples from patients with ovarian cancer, and subsequently used transcriptome-driven, promoter-based bioinformatics analyses to identify gene regulatory pathways that potentially may underlie these changes.
MATERIALS AND METHODS
Participants
Women aged >18 years with suspected ovarian cancer were recruited from 2 academic medical centers during a clinic visit prior to undergoing primary surgery as part of a larger study regarding biobehavioral factors and tumor progression. Inclusion was confirmed by a histological diagnosis of epithelial ovarian, peritoneal, or fallopian tube cancer. Exclusion criteria included nonepithelial ovarian tumors, metastases to the ovaries from other organs, the regular use of systemic corticosteroid medication within the last month, history of another cancer within the last 5 years, a comorbid condition with known immune system effects, current pregnancy, and inability to accurately answer questions (eg, dementia). Informed consent was obtained during the presurgical visit and patients completed psychosocial surveys at home prior to surgery. Tumor was sampled during primary surgical resection and immediately frozen in liquid nitrogen. Each tumor was classified by pathology according to grade, stage (International Federation of Gynecology and Obstetrics [FIGO] classification), and histologic subtype (serous, mucinous, endometrioid, and clear cell). All procedures were approved by the institutional review boards at the University of Iowa and the University of Miami. Tumor was obtained for 107 patients; 8 patients were missing social isolation data and thus the final sample included 99 women (95 from the University of Iowa and 4 from the University of Miami).
Psychosocial, Demographic, and Clinical Characteristics
The attachment subscale of the Social Provisions Scale (SPS)28 was used to assess current perceptions of social attachment and/or isolation. This 4-item subscale assesses perception of emotional connection versus isolation from others. Based on an a priori hypothesis derived from prior studies of biobehavioral risk factors in patients with ovarian cancer, 19,25–27 biobehavioral risk was defined by an SPS Attachment subscale score <15 (the median value). All analyses also controlled for patient age, body mass index (BMI), tumor grade (high vs low), and disease stage (categorical; FIGO stage I, II, III, or IV).
Tumor Tissue Gene Expression
Tumor fragments (0.1 g) were excised from tissue samples maintained in liquid nitrogen, homogenized in 600 uL of RNA-stabilizing lysis buffer (RNeasy RLT; Qiagen, Valencia, California), and supplemented with 400 uL of RNAse-free water (Qiagen) to produce a 1-mL nucleic acid lysate. One mL of QIAzol reagent (Qiagen) and 300 uL of chloroform were added, and lysates were centrifuged for 5 minutes at 1500 revolutions per minute in a 15-mL centrifuge tube maintained at 4 °C. Aqueous phase products were mixed with 1 volume of 70% ethanol and applied to an RNeasy Mini spin column (Qiagen). Total RNA was extracted and treated with RNAse-free DNAse (Qiagen) following the manufacturer’s protocol (supplemented by 1 additional wash in RW1 reagent and 1 additional wash in RPE reagent to remove excess salt). RNA purity and integrity were assessed using an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, California), and genome-wide transcriptional profiling was performed using Ambion TotalPrep cRNA synthesis with hybridization to Illumina HT-12 high-density oligonucleotide arrays (Illumina, San Diego, California) in the University of California at Los Angeles Neuroscience Genomics Core following the manufacturers’ standard protocols. Data were deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GSE103737). Raw gene expression values were quantile-normalized 29 and log2-transformed for analysis using standard linear statistical models relating transcript abundance to social support (high vs low) while controlling for age (continuous years), BMI (in mg/kg2), tumor grade (high vs low), and stage of disease (categorical; FIGO I, II, III, and IV). Parameter estimates from these analyses served as input into higher order bioinformatics analyses as described below.
EMT Polarization
The primary hypothesis regarding EMT-related gene expression was tested using a gene signature previously derived to discriminate between epithelial-polarized and mesenchymal-polarized breast cancer cells.30 Using this a priori defined gene set (276 of which were present in the HT-12 microarray data), we tested whether the average expression of the 169 mesenchymal-characteristic genes and the average expression of the 107 epithelial-characteristic genes differed significantly between patients with low versus high levels of social isolation while controlling for age, BMI, tumor grade, and disease stage. The average differential expression estimate across the a priori specified gene set was tested for statistical significance using a standard error of the mean derived by bootstrap resampling of linear model residual vectors across genes (ie, we accounted for any potential correlation among genes).31 In addition, ancillary sensitivity analyses controlled for serous versus nonserous histology or deleted the 25 nonserous tumors.
Transcription Factor Bioinformatics
Secondary hypotheses concerning the role of EMT-promoting transcription factor activity were tested using the Transcription Element Listening System (TELiS) promoter sequence-based bioinformatics analysis, as previously described.32 This analysis used as input a list of all genes showing a >25% difference in average expression between low and high social support groups (adjusting for age, BMI, tumor grade, and stage of disease), with upregulated and downregulated genes tested for the differential prevalence of transcription factor-binding motifs (TFBMs) for 4 groups of transcription factors involved in promoting EMT33,34: 1) Snail2/Slug (detected by the Jaspar SNAI2 position-specific weight matrix); 2) TWIST1 (detected by the Jaspar TWIST1); 3) SMAD2, SMAD3, and/or SMAD4 (detected by the Jaspar SMAD2/SMAD3/SMAD4); and 4) GATA4 (detected by the Jaspar GATA4). The log2-transformed ratios of TFBM prevalence in upregulated and downregulated promoters were computed for 9 combinations of 3 core promoter lengths (−300 base pair [bp], −600 bp, and −1000 to +200 bp with respect to the RefSeq gene transcription start site) and 3 TFBM detection stringencies (TRANSFAC MatSim values of 0.80, 0.90, and 0.95), with log-ratios averaged over all 9 parametric combinations and tested for statistical significance using a standard error of the mean derived via bootstrap resampling of linear model residual vectors across genes (ie, accounting for any potential correlation among genes).31
RESULTS
Participants
Patients predominantly were diagnosed with advanced stage (74.74%), high-grade (91.92%) serous (74.75%) disease and none had received neoadjuvant chemotherapy. The mean age of the participants was 59.2 years (±13.01 years) and the mean BMI was 28.87 kg/m2 (±6.77 kg/m2), with 72.73% being overweight, obese, or morbidly obese. The sample was largely white (96.96%) and non-Hispanic (96.96%); approximately 44.90% of patients had an educational level of ≤high school, 61.90% had an income of ≤$50,000, approximately 80.81% were married or living with partners, and 15.15% were current smokers (Table 1).
TABLE 1.
Patient Characteristics
| Characteristics | High Isolationa N = 42 | Low Isolation N = 57 | All Patients N = 99 |
|---|---|---|---|
| Mean age (SD), y | 62.57 (13.87) | 56.75 (11.88) | 59.22 (13.01) |
| Mean BMI (SD), kg/m2 | 29.83 (7.97) | 28.17 (5.70) | 28.88 (6.77) |
| Race, no. (%) | |||
| Nonwhite | 2 (4.76%) | 2 (3.51%) | 4 (4.04%) |
| White | 40 (95.24%) | 55 (96.49%) | 95 (95.96%) |
| Ethnicity, no. (%) | |||
| Non-Hispanic | 42 (100.0%) | 54 (94.74%) | 96 (96.96%) |
| Hispanic | 0 (0.00%) | 3 (5.26%) | 3 (3.04%) |
| Education, no. (%) (42 patients in the high isolation group and 56 patients in the low isolation group) | |||
| ≤High school | 21 (50.00%) | 23 (41.07%) | 44 (44.90%) |
| Some college/trade school | 13 (30.95%) | 22 (39.29%) | 35 (35.71%) |
| College degree | 6 (14.29%) | 7 (12.50%) | 13 (13.27%) |
| Advanced degree | 2 (4.76%) | 4 (7.14%) | 6 (6.12%) |
| Relationship status, no. (%) | |||
| Not married | 10 (23.81%) | 9 (15.79%) | 19 (19.19%) |
| Married/living with partner | 32 (76.19%) | 48 (84.21%) | 80 (80.81%) |
| FIGO stage of disease, no. (%) | |||
| I | 7 (16.67%) | 10 (17.54) | 17 (17.17) |
| II | 1 (2.38%) | 7 (12.29%) | 8 (8.08%) |
| III | 27 (64.28%) | 37 (64.91%) | 64 (64.65%) |
| IV | 7 (16.67%) | 3 (5.26%) | 10 (10.10%) |
| Grade, no. (%) | |||
| Low | 0 (0.00%) | 8 (14.03%) | 8 (8.08%) |
| High | 42 (100.0%) | 49 (85.97%) | 91 (91.92%) |
| Histology, no. (%) | |||
| Nonserous | 11 (26.19%) | 14 (24.56%) | 25 (25.25%) |
| Serous | 31 (63.26%) | 43 (75.44%) | 74 (74.75%) |
Abbreviations: BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; SD, standard deviation. Percentages are shown in parentheses unless the mean and standard deviation are specifically indicated.
High isolation indicates a Social Provisions Scale Attachment subscale score <15.
EMT Polarization
Primary analyses examined whether tumor samples from patients with high levels of social isolation demonstrated a greater abundance of EMT-related gene transcripts compared with patients experiencing low social isolation, controlling for age, BMI, tumor grade, and disease stage. In an analysis of 107 genes known to be associated with epithelial differentiation in cancer cells, the results demonstrated lower average expression of those transcripts in individuals with high levels of social isolation (−0.143 ± 0.048 log2 mRNA abundance; P = .004) (Fig. 1). Parallel analyses of 169 genes previously linked to mesenchymal differentiation in cancer cells demonstrated no difference with regard to average expression as a function of social isolation (+0.007 ± 0.064 log2 mRNA abundance; P = .900). Similar results were found in analyses that in addition controlled for serous versus nonserous histology (epithelial: −0.146 ± 0.070 [P = .042] and mesenchymal: +0.040 ± 0.112 [P = .721]) or omitted nonserous tumors (epithelial: −0.127 ± 0.062 [P = .044] and mesenchymal: −0.068 ± 0.124 [P = .584]).
Figure 1.
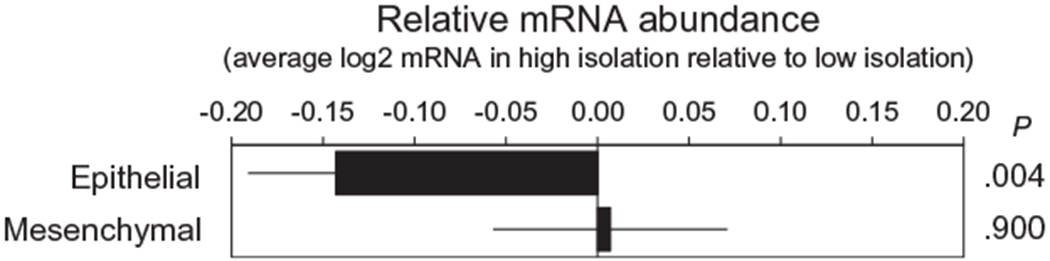
Differential expression of messenger RNA (mRNA) from epithelial and mesenchymal gene sets in tumor samples from patients experiencing high versus low levels of social isolation.
EMT Transcription Regulation
To provide convergent validation results from primary analyses using an alternative analytic strategy, we used a promoter-based bioinformatic analysis of transcription factor activity to assess the role of EMT-related transcription factors in structuring empirical differences in gene expression associated with social isolation. Instead of prespecifying selected epithelial or mesenchymal gene sets a priori, this analysis used a genome-wide, unbiased assessment of tumor transcriptome differences associated with social isolation and tested whether those differences might be structured in part by transcription factors known to promote EMT (ie, GATA4; SMAD2, SMAD 3, and/or SMAD 4; SNAIL2/SLUG; and TWIST1). In analyses comparing the prevalence of TFBMs in the promoters of 2307 gene transcripts showing a differential expression of >25% in patients with high versus those with low social isolation (598 were upregulated in patients with high isolation vs 1709 being downregulated), the results indicated upregulated activity for 3 of the 4 targeted transcription factors (Fig. 2), including GATA4 (mean log2 TFBM ratio in upregulated vs downregulated genes: +0.512 ± 0.207 standard error; P = .014); SMAD2, SMAD3, and/or SMAD4 (+0.888 ± 0.246; P < .001); and TWIST1(+0.709 ± 0.194; P < .001). Analyses of SNAIL2/SLUG activity indicated a directional trend toward increased activity, but that difference did not reach statistical significance (+0.147 ± 0.095; P = .123).
Figure 2.
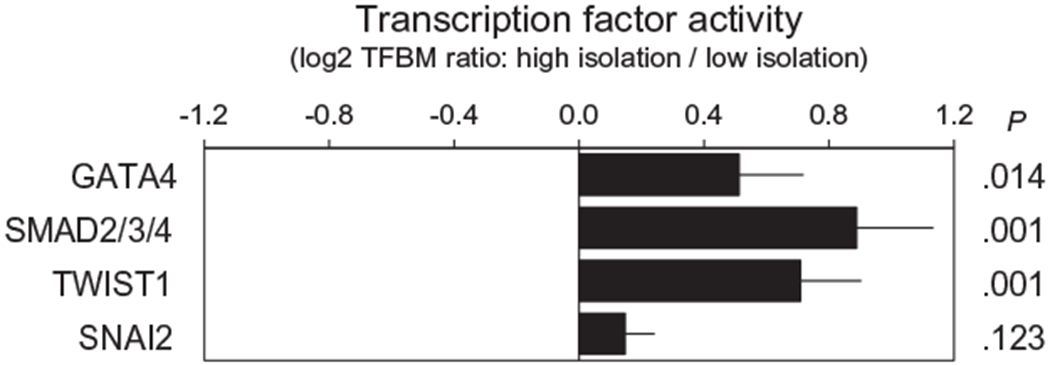
Transcription Element Listening System bioinformatics analysis of transcription factor-binding site prevalence in promoters of gene transcripts that differed in average expression by ≥25% in high versus low social isolation tumor samples. TFBM indicates transcription factor-binding motif.
DISCUSSION
The key finding of the current study was that ovarian tumors from patients reporting high levels of social isolation demonstrated increased transcriptional indications of EMT compared with tumors from patients with low social isolation. These findings were independent of potential confounders such as age, BMI, tumor grade, tumor stage, and histology, and emerged in parallel across 2 alternative modes of analysis. These included differential expression of a priori specified EMT indicator genes (particularly reduced expression of epithelial genes) and promotor-based bioinformatics analyses of empirical transcriptomic differences in terms of EMT-related transcription factor activity (ie, upregulated activity of TWIST1; GATA4; and SMAD2, SMAD3, and/or SMAD4, and a nonsignificant trend toward increased SNAIL2/SLUG activity).
These findings represent an important step toward gaining a better understanding of how a risk factor at the organismal level of functioning can impact the biology of the tumor. We previously demonstrated that social isolation is related to the increased expression of EMT-related gene transcripts in peripheral blood exosomes.27 The findings of the current study have extended this work by furthering our understanding of the pathways by which social risk factors may affect disease progression and by demonstrating that similar relationships are observable within tumor tissue. These results also represent what to the best of our knowledge is the first time that we have been able to map patient-level risk factors on the EMT status of the ovarian tumor itself. These data mirror research indicating increased EMT polarization of tumors from socially isolated patients with early-stage breast cancer. 25 The findings of the current study also are consistent with a model of socioenvironmental modulation of the tumor microenvironment via stress-response pathways that alter gene expression through activation of transcription factors that subsequently alter cellular processes.2 Because social isolation has been linked with higher levels of the sympathetic mediator norepinephrine in the ovarian tumor microenvironment,16 it is possible that these relationships are mediated in part by β-adrenergic signaling. This premise is consistent with a recent study demonstrating the ability of a pharmacologic intervention involving β-adrenergic and COX-2 antagonists to downregulate expression of EMT-related gene transcripts.26,35
It is interesting to note that the most prominent molecular correlate of social isolation in the current study was reduced epithelial polarization, rather than expression of mesenchymal gene transcripts. For example, when compared with nonisolated patients, tumors from socially isolated patients demonstrated markedly lower expression of multiple epithelial marker genes including EPCAM (48% reduction), CD24 (−39% reduction), CDH1 (−49% reduction), CDS1 (−21% reduction), SYK (−40% reduction), KLK7 (−62% reduction), and ELF3 (−49% reduction). This may reflect a preferential effect of social isolation-related neural influences on the activity of transcription factors that inhibit epithelial gene expression (eg, SNAI2 and TWIST1, which potently repress transcription of epithelial characteristic genes such as CDH1). Tumor cells now are known to lose epithelial features without a gain of mesenchymal features and to be able to reside in an intermediate or hybrid state in the EMT spectrum. Cells with this asymmetric dedifferentiation or hybrid EMT have been found to demonstrate aggressive features that are similar to or greater than those of cells demonstrating full EMT.36,37 It also is possible that social isolation does affect mesenchymal biology, but testing such effects would require assessment at a protein level. Future research replicating these findings and extending them to direct analyses of transcription factor activity may help to resolve this issue.
Limitations
The current study was based on a correlational analysis, and thus we were unable to establish whether the observed associations reflect a causal effect of social isolation on EMT gene expression or other aspects of ovarian cancer. These data were derived from a sample of limited geographic and demographic scope, and future research in other samples will be required to establish the generalizability of these findings. The health significance of the results from the current study also remains to be identified in future research. Finally, it should be noted that the indicator genes used to define epithelial and mesenchymal profiles in the current study were derived from breast cancer cell lines and are prognostically relevant for breast cancer30 but may not fully reflect the genomic signature of EMT in patients with epithelial ovarian cancer. However, the convergent validation of the upregulation of specific EMT-related transcription factor activity according to social isolation lends greater support to the role of social isolation in promoting EMT.
Conclusions and Clinical Significance
The results from the current study have demonstrated differential EMT polarization and EMT-related transcription factor activity according to social isolation, a known socioenvironmental risk factor for poor health. Because EMT polarization is reported to be related to treatment resistance,21–23 metastasis,23 and poorer survival of patients with ovarian cancer,38,39 addressing the potentially modifiable risk factor of social isolation in clinical practice using behavioral and support-based or pharmacological interventions may improve patient outcomes.
Acknowledgments
FUNDING SUPPORT
Supported in part by National Institutes of Health grants CA193249, CA140933, CA246540 (to Susan K. Lutgendorf), CA109298, CA209904, ACS Research Professor Award (to Anil K. Sood), AG017265 and AG043404 (to Steve W. Cole), and P30CA086862 (Principal Investigator: George Weiner). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Desire Christensen, MD, Sarah Strack, MS, Ellen M. Kinner, MA, David Bender, MD, David M. Lubaroff, PhD, and the women who participated in this study for their contributions to this research.
CONFLICT OF INTEREST DISCLOSURES
Susan K. Lutgendorf has received grants from the National Institutes of Health for work performed as part of the current study. Frank Penedo has received grants from the National Institutes of Health for work performed as part of the current study. Michael J. Goodheart has received grants from the National Institutes of Health for work performed as part of the current study. Laila Dahmoush has received grants from the National Institutes of Health for work performed as part of the current study. Jesusa M. G. Arevalo has received grants from the National Institutes of Health for work performed as part of the current study. Premal H. Thaker has acted as a paid consultant or speaker for Stryker, Iovance Biotherapeutics, AbbVie/Stemcentrx, Clovis Oncology, Unleash Immunolytics, and Celsion Corporation; has received research funding and personal fees from Merck and GlaxoSmithKline; has received personal fees from AstraZeneca; and is a Celsion shareholder. George M. Slavich has received grants from the National Institutes of Health for work performed as part of the current study. Anil K. Sood has received grants from the National Institutes of Health (CA109298 and CA209904) for work performed as part of the current study and has acted as a paid consultant for Merck and Kiyatec, is a shareholder in BioPath, and has received research funding from M-Trap for work performed outside of the current study. Steve W. Cole has received grants from the National Institutes of Health for work performed as part of the current study.
REFERENCES
- 1.Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019;9:702–710. [DOI] [PubMed] [Google Scholar]
- 2.Cole S, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le CP, Nowell CJ, Kim-Fuchs C, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obradovic MMS, Hamelin B, Manevski N, et al. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567:540–544. [DOI] [PubMed] [Google Scholar]
- 5.Alcaraz KI, Eddens KS, Blase JL, et al. Social isolation and mortality in US black and white men and women. Am J Epidemiol. 2019;188:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. Social relationships and physiological determinants of longevity across the human life span. Proc Natl Acad Sci U S A. 2016;113:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peres LC, Sinha S, Townsend MK, et al. Predictors of survival trajectories among women with epithelial ovarian cancer. Gynecol Oncol. 2020;156:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boen CE, Barrow DA, Bensen JT, et al. Social relationships, inflammation, and cancer survival. Cancer Epidemiol Biomarkers Prev. 2018;27:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroenke CH, Michael YL, Poole EM, et al. Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer. 2017;123:1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinzey A, Gaudier-Diaz MM, Lustberg MB, DeVries AC. Breast cancer and social environment: getting by with a little help from our friends. Breast Cancer Res. 2016;18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleisch Marcus A, Illescas AH, Hohl BC, Llanos AA. Relationships between social isolation, neighborhood poverty, and cancer mortality in a population-based study of US adults. PLoS One. 2017;12:e0173370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta-analysis. Crit Rev Oncol Hematol. 2010;75:122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutgendorf SK, DeGeest K, Dahmoush L, et al. Social Influences on Clinical Outcomes of Ovarian Cancer Patients. J Clin Oncol. 2012;30:2885–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutgendorf SK, Sood AK, Anderson B, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–7113. [DOI] [PubMed] [Google Scholar]
- 15.Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. [DOI] [PubMed] [Google Scholar]
- 16.Lutgendorf SK, DeGeest K, Dahmoush L, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2010;25:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutgendorf SK, DeGeest K, Sung CY, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. [DOI] [PubMed] [Google Scholar]
- 19.Lutgendorf SK, Lamkin DM, Jennings NB, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaker P, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and metastasis in ovarian carcinoma. Nat Med. 2006;12:939–944. [DOI] [PubMed] [Google Scholar]
- 21.Mittal V Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. [DOI] [PubMed] [Google Scholar]
- 22.Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loret N, Denys H, Tummers P, Berx G. The role of epithelial-to-mesenchymal plasticity in ovarian cancer progression and therapy resistance. Cancers (Basel). 2019;11:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soundararajan R, Fradette JJ, Konen JM, et al. Targeting the interplay between epithelial-to-mesenchymal-transition and the immune system for effective immunotherapy. Cancers (Basel). 2019;11:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bower JE, Shiao SL, Sullivan P, et al. Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr. 2018;2:pky029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaashua L, Shabat-Simon M, Haldar R, et al. Perioperative COX-2 and beta-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017;23:4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutgendorf SK, Thaker PH, Arevalo JM, et al. Biobehavioral modulation of the exosome transcriptome in ovarian carcinoma. Cancer. 2018;124:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutrona CE, Russell D. The provisions of social relationships and adaption to stress In: Jones H, Pearlman D, eds. Advances in personal relationships. A research annual 1. Greenwich, CT: Jai Press Inc; 1987: 37–67. [Google Scholar]
- 29.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. [DOI] [PubMed] [Google Scholar]
- 30.Choi YL, Bocanegra M, Kwon MJ, et al. LYN is a mediator of epithelial-mesenchymal transition and a target of dasatinib in breast cancer. Cancer Res. 2010;70:2296–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efron B, Tibshirani RJ. An Introduction to the Bootstrap Monographs on Statistics and Applied Probability. Boca Raton, FL: Chapman & Hall/CRC Press; 1994. [Google Scholar]
- 32.Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics. 2005;21:803–810. [DOI] [PubMed] [Google Scholar]
- 33.Bogachek MV, De Andrade JP, Weigel RJ. Regulation of epithelial-mesenchymal transition through SUMOylation of transcription factors. Cancer Res. 2015;75:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Lamouille S, Derynck R. TGF-beta–induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiller JG, Cole SW, Crone EM, et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res. 2020;26:1803–1811. [DOI] [PubMed] [Google Scholar]
- 36.Kroger C, Afeyan A, Mraz J, et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci U S A. 2019;116:7353–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastushenko I, Brisebarre A, Sifrim A, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–468. [DOI] [PubMed] [Google Scholar]
- 38.Yang D, Sun Y, Hu L, et al. Integrated analyses identify a master mi-croRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell. 2013;23:186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. [DOI] [PubMed] [Google Scholar]


