Abstract
Objective:
To investigate the effect of pregnancy and HIV infection on detection performances of Tuberculin skin test (TST) and QuantiFERON-TB Gold In-Tube (QFTGIT) for the diagnosis of LTBI among women living in high TB and HIV endemic setting.
Method:
A cross-sectional study was conducted among women with and without pregnancy and HIV infection. Three-hundred twenty women were enrolled in this study and were diagnosed by TST and QFTGIT for the detection of LTBI.
Results:
The overall prevalence of LTBI among the enrolled women were 55.6%, 46.3% and 51.1% as determined by TST, QFTGIT and concordant TST/QFTGIT results respectively. Our study revealed that pregnancy or HIV infection reduces the rate of detection of LTBI by TST and QFTGIT tests with utmost effect observed in HIV positive pregnant women. Besides, we observed that the concordance between TST and QFTGIT among women increased with the presence of pregnancy and/or HIV infection. Besides, history of contact with TB patients were significantly associated with the positivity of TST and QFTGIT.
Conclusion:
This study demonstrated that both pregnancy and HIV infection profoundly affect the detection performances of TST and QFTGIT, which may be associated with immunosuppression of anti-mycobacterial immunity in women with pregnancy and/or HIV infection.
Keywords: Pregnancy, HIV, M. tuberculosis, detection rate, latent TB infection, TST, QFTGIT
Introduction
Tuberculosis (TB) is a serious cause of morbidity and mortality globally, with the majority of deaths occurring in developing countries (1). World Health Organization (WHO) reported that 10 million new cases of TB and 1.5 million deaths occurred worldwide in 2018, and notably, 80–90% of the new TB cases and TB-associated deaths were in developing countries (1). Women accounted for 32% of the TB incidence rate, and TB is one of the top five killers among adult women aged 20–59 years. Active TB disease can be caused by direct exposure to Mycobacterium tuberculosis (M. tuberculosis) or reactivation of latent TB infection (LTBI). According to the recent WHO reports, about one-quarter of the world’s population is estimated to be latently infected with M. tuberculosis and only 5–10% of these individuals eventually develop active TB (1). However, the risk of developing active TB as a result of reactivation of the LTBI could substantially increase in the presence of several predisposing factors such as HIV infection and pregnancy (1, 2). HIV infection diminishes the Th1 cell-mediated immune response to M. tuberculosis while pregnancy deepens this suppression of anti-mycobacterial responses in HIV positive individuals (3–5). This shows that the co-existence of HIV and pregnancy markedly suppress M. tuberculosis-specific functional immune responses, thereby increase the risk of progression of LTBI to active TB.
On the other hand, suppression of anti-mycobacterial responses associated with HIV infection and pregnancy poses challenge on the performances of diagnostics of LTBI in women with these conditions as the commercially available tests for diagnosis of LTBI rely on detection of cell-mediated immune response to M. tuberculosis (6). Thus, accurate detection and subsequent prophylactic therapy of LTBI in individuals with HIV infection and pregnancy are important for TB elimination. WHO has recently issued a strong recommendation that either Tuberculin skin test (TST) or interferon-gamma (IFN-γ) release assays such as QuantiFERON-TB Gold-In Tube (QFTGIT) can be used for the diagnosis of LTBI (1, 7). However, the absence of an accurate gold standard test for diagnosis of LTBI is still a pressing challenge in the assessment of the true magnitude of LTBI and potential risk factors associated with its reactivation in various population groups especially in women with pregnancy and/or HIV infection. TST is the most common and widely used test for screening of LTBI mainly due to its low cost. TST is an in vivo test that assesses the host’s delayed-type hypersensitivity response to interdermally injected purified protein derivative (PPD) (6). This test causes false-positive results due to the cross-reactivity with non-tuberculous proteins that are present in mycobacteria species other than M. tuberculosis as well as in Bacille Calmette-Guérin (BCG) vaccine (8, 9). More recently, IFN-γ release assays such as QFTGIT have been used as an alternative test for the detection of M. tuberculosis infection with more specificity. QFTGIT is an enzyme-linked immunosorbent assay (ELISA)-based whole blood method that measures the level of IFN-γ released in response to early secreted antigenic target-6 (ESAT-6), culture filtrate protein-10 (CFP-10) and TB7.7, which are specifically present in M. tuberculosis but not in most nonpathogenic mycobacteria, including Mycobacterium bovis (M. bovis)-BCG (8, 9). TST and IFN-γ release assays require a competent immune response to identify people infected with M. tuberculosis and may not be a perfect test for measuring the progression to active disease (10, 11). The positivity rate of TST and IFN-γ release assays substantially varies between countries based on the TB burden. Ethiopia, which is one of the high TB burden countries, has been reported to have over 50% prevalence of LTBI as assessed by TST or IFN-γ release assays (12, 13). Besides, there are evidences that both pregnancy (14, 15) and HIV infection (16, 17) affects the performances of LTBI diagnostics during pregnancy and HIV infection. Differences in diagnostic properties between these tests have been previously demonstrated in various populations (18), including HIV-negative pregnant women (15). However, evidence on the detection performances of TST and QFTGIT for the diagnosis of LTBI in pregnant women, particularly in those with HIV infection, in a high TB and HIV burdened setting is scarce. We, therefore, investigated the effect of HIV and pregnancy on the detection performances of TST and QFTGIT for diagnosis of LTBI by determining and comparing detection rate and concordance of these tests in women with and without pregnancy and HIV infection.
Methods
Study participants
The recruitment and enrollment of study participants in this study is summarized in Fig. 1. Three-hundred thirty-one pregnant and non-pregnant women with and without HIV infection were recruited from five public hospitals (Yekatit 12, Ghandi Memorial, Zewditu, ALERT and St. Paul referral specialized hospitals) and two public health centers (Bole 17, and Arada health centers) in Addis Ababa, Ethiopia. Women with the age range of 18 to 49 years who consented for enrollment were consecutively selected by attending clinicians. Study participants with active TB, anemia, active hepatitis, abnormal pregnancy, isoniazid prophylaxis and immunosuppressive treatment, history of chronic infection or disorders and men were excluded from this study. Recruited women were tested by TST and QFTGIT assays for the diagnosis of LTBI. The positivity of TST and QFTGIT was interpreted and determined according to the existing guidelines recommended by the manufacturers and CDC. Of the 331 recruited participants, 320 women were tested by both TST and QFTGIT, and enrolled in this study whereas the remaining 11 women were excluded as they missed testing by either of the tests. The concordant and discordant results of TST and QFTGIT as well as indeterminate QFTGIT results were determined.
Fig. 1.
Flow chart of the recruitment and enrollment of participants in this study.
Tuberculin skin test (TST)
TST was performed by intradermal application of 0.1 ml of 2-TU PPD, RT23 (Statens Serum Institute (SSI), Denmark) per standard clinical practice. The diameter of induration was measured after 48–72 hours by well-experienced nurses. The TST response was considered positive when the induration was at least 5 mm for HIV positive participants and 10 mm for HIV negative participants (1, 3, 19, 20).
QuantiFERON-TB Gold In-Tube assay (QFTGIT)
A blood sample of study participants was further tested using QFTGIT assay kit (Cellestis Limited, Australia) and was performed according to the manufacturer’s protocol and as previously described (3, 14). 1 ml of the sample was collected in three QFTGIT evacuated tubes supplied by the manufacturer: one containing M. tuberculosis antigens (ESAT-6, CFP-10, and TB7.7), a positive control tube containing phytohemagglutinin (PHA), and a negative control tube, incubated overnight at 37°C, and plasma was collected and stored at −20°C. When required, frozen plasma was thawed and ELISA for IFN-γ measurement was performed according to the QFTGIT kit. Optical density was measured using a microplate reader (Molecular Devices Corporation, USA) fitted with a 450nm and 620nm filters. QuantiFERON-TB Gold Analysis Software (Cellestis Limited) was used to generate a standard curve and measure the actual concentration of IFN-γ in each of the three tubes. A positive IFN-γ response to M. tuberculosis antigens were predefined as > 0.35 IU/ml after subtraction of the negative control, and concentration < 0.35 IU/ml were set as a negative QFTGIT result according to the manufacturer, CDC (21) and previous studies (14, 20). A test result was set to be indeterminate if the negative control has IFN-γ level of > 8.0 IU/ml or a positive control has IFN-γ response of < 0.5 IU/ml after subtraction of the negative control (14, 20, 21).
CD4+ T cell count
Blood samples were collected from study participants in a heparinized tube. CD4+ T cell count was determined by a BD FACSCaliber flow cytometer (Becton Dickinson (BD) Biosciences, USA) in Zewditu hospital using the existing standard operating procedure.
Statistical analysis
Data was entered and analyzed using SPSS version 21.0 and Graphpad Prism 8.0.2. Descriptive statistics were used to describe the socio-demographic and clinical data. Continuous variables are expressed as median with interquartile range (IQR), and categorical variables as frequencies and percentages. Chi-square (χ2) was used to compare detection rates between two groups of study participants. The concordance between TST and QFTGIT was evaluated using a percentage of agreement and the kappa (k) coefficient analysis. Strength of agreement was considered ‘poor’ for κ ≤ 0.20, ‘fair’ for 0.20 < κ ≤0.40, ‘moderate’ for 0.40 < κ ≤ 0.60, ‘good’ for 0.60 < κ ≤ 0.80, and ‘excellent’ for 0.80 < κ ≤ 1.00 (22, 23). For the analysis of the agreement, participants with indeterminate test results were excluded. The risk factors associated with the positivity of TST and QFTGIT tests were assessed by univariate logistic regression analysis. A p-value < 0.05 were considered statistically significant.
Results
Socio-demographic and clinical data
A total of 320 participants were enrolled in this study and of these, 159 were pregnant (86 HIV positive and 73 HIV negative) and 161 were non-pregnant (107 HIV positive and 54 HIV negative) women. TST was first performed in all study participants, which was then followed by QFTGIT for detection of LTBI. The socio-demographic characteristics and clinical data of the study participants are shown in Table 1. The median age of the study participants was 29 years (IQR 25–33) with slightly higher years of age in HIV positive than HIV negative women. 67.5% (216/320) of the total participants were married and of these, the big majority were pregnant women with and without HIV while 57.4 % of the HIV negative non-pregnant women were single. Most of the study participants had an education level of primary and secondary level of formal education with virtually similar proportions among the four groups while 38.4% (123/320) and 29.8% (95/320) were housewives and private workers respectively (Table 1).
Table 1.
Socio-demographic and clinical data of the study participants categorized by pregnancy and HIV infection status.
| Variable | No. (%) of study participants | ||||
|---|---|---|---|---|---|
| HIV- non-pregnant n=54 | HIV- pregnant n=73 | HIV+ non-pregnant n=107 | HIV+ pregnant n=86 | Total n=320 | |
| Age (year) | |||||
| Median (IQR) | 26(22–30) | 26(22–30) | 33(29–37) | 29(25–31) | 29(25–33) |
| 18–33 | 46(85.2%) | 65(89.0%) | 59(55.1%) | 73(84.9%) | 243(75.9%) |
| 34–49 | 8(24.8%) | 8(11.0%) | 48(44.9%) | 13(15.1%) | 77(24.1%) |
| Marital status | |||||
| Single | 31(57.4%) | 3(4.1%) | 15(14.0%) | 5(5.8%) | 54(16.9%) |
| Married | 20(37.0%) | 70(95.9%) | 50(46.7%) | 76(88.4%) | 216(67.5%) |
| Divorced | 2(3.7%) | 0(0.0%) | 19(17.8%) | 5(5.8%) | 26(8.1%) |
| Widowed | 1(1.9%) | 0(0.0) | 23(21.5%) | 0(0.0%) | 24(7.5%) |
| Education level | |||||
| Illiterate | 2(3.7%) | 10(13.7%) | 16(14.9%) | 12(14.0%) | 40(12.5%) |
| Primary School | 13(24.1%) | 26(35.6%) | 37(34.6%) | 35(40.7%) | 111(34.7%) |
| Secondary School | 22(40.7%) | 23(31.5%) | 37(34.6%) | 31(36.0%) | 113(35.3%) |
| Other | 17(31.5%) | 14(19.2%) | 17(15.9%) | 8(9.3%) | 56(17.5%) |
| Occupation | |||||
| Private work | 4(7.4%) | 20(27.4%) | 40(37.4%) | 31(36.0%) | 95(29.8%) |
| Government work | 26(48.1%) | 9(12.3%) | 17(15.9%) | 7(8.1%) | 59(18.4%) |
| Housewife | 10(18.5%) | 38(52.1%) | 35(32.7%) | 40(46.5%) | 123(38.4%) |
| Other | 14(26.0%) | 6(8.2%) | 15(14.0%) | 8(9.3%) | 43(13.4%) |
| CD4+ T cell count (cells/μl) | |||||
| Median (IQR) | NA | NA | 390(276–561) | 384(269–605) | 385(275–572) |
| < 250 | NA | NA | 23 (21.5%) | 18 (20.9%) | 41(21.3%) |
| 250–500 | NA | NA | 49 (45.8%) | 41 (47.7%) | 90(46.6%) |
| > 500 | NA | NA | 35 (32.7%) | 27 (31.4%) | 62(32.1%) |
CD4+ T cell count was also measured from the blood sample of the 193 HIV positive participants; 86 pregnant and 107 non-pregnant women. The overall median count was 385/μl (IQR 275–572). Pregnant and non-pregnant women had similar CD4+ T-cell count with a median count of 384/μl (IQR 269–605) and 390/μl (IQR 276–561) respectively, p=0.9078. Furthermore, we stratified the CD4+ T cell count of the HIV positive pregnant and non-pregnant women into three groups as shown in Table 1. The majority of the HIV positive participants (90/193, 46.6%) in both pregnant (41/86, 47.7%) and non-pregnant (49/107, 45.8%) women had a CD4+ T cell count ranges 250–500/μl while 31.4 % (27/86) and 32.7% (35/107) of the pregnant and non-pregnant women had CD4+ T cell count of above 500/μl.
TST and QFTGIT results, and risk factors associated with the positive test results
Out of the enrolled study participants, 32.8% and 28.1% of them tested positive for M. tuberculosis infection according to TST and QFTGIT assays respectively (Table 2). Consistently, the frequency of TST positive participants were faintly higher than the QFTGIT positive women in all groups of the participants but no statistically significant. Besides, only 3/320 (0.9%) of the women (2 HIV positive pregnant and 1 HIV negative pregnant) had indeterminate QFTGIT results. 91.6% of the participants had concordant TST and QFTGIT results whereas 7.2% of them had discordant results. Of those participants with discordant results, most of them (82.6%) tested positive for TST but negative for QFTGIT with the remaining 17.4% had a negative TST and positive QFTGIT results (Table 2).
Table 2.
TST and QFT test results in pregnant and non-pregnant women with and without HIV infection
| Variable | No. (%) of study participants | ||||
|---|---|---|---|---|---|
| Total n=320 | HIV- non-pregnant n=54 | HIV- pregnant n=73 | HIV+ non-pregnant n=107 | HIV+ pregnant n=86 | |
| Positive TST | 105(32.8%) | 30(55.6%) | 26(35.6%) | 31(29.0%) | 18(20.9%) |
| Positive QFTGIT | 90(28.1%) | 25(46.3%) | 23(31.5%) | 25(23.4%) | 17(18.9%) |
| Indeterminate QFTGIT | 3(0.9%) | 0(0.0%) | 1(1.4%) | 0(0.0%) | 2(2.3%) |
| Concordant TST/QFTGIT | 293(91.6%) | 45(83.3%) | 65(89.0%) | 101(94.4%) | 82(95.3%) |
| Discordant TST/QFTGIT | 23(7.2%) | 9(16.7%) | 7(9.6%) | 6(5.6%) | 1(1.2%) |
We then assessed the potential risk factors that could be associated with the positivity of TST and QFTGIT tests in the study participant using the logistic regression analysis. Accordingly, age, alcohol intake, history of previous BCG vaccination and presence of recent parasitic infection were found to have no association with the positivity of TST nor QFTGIT assays (Table 3). However, history of contact with TB patients had statistically significant association with the positivity of both TST [OR: 2.51 (CI: 1.54–4.11), p<0.0001] and QFTGIT [OR: 2.13 (CI: 1.28–3.54), p=0.004] tests. Importantly, the presence of underlying condition of HIV infection or pregnancy had a significant inverse relationship with the positivity of TST and QFTGIT assays while the utmost effect was observed in women with both pregnancy and HIV infection (Table 3). Besides, we assessed whether TST and QFTGIT were affected by age of gestation and CD4+ T cell count in pregnant and HIV positive women respectively. Trimester of pregnancy showed no significant effect on both TST and QFTGIT test results. In addition, CD4+ T cell count appeared to have no statistically significant association with the positivity of TST nor QFTGIT although the proportion of HIV infected women with positive results of these tests were relatively higher in those with elevated CD4+ T cell count (Table 3).
Table 3.
Univariate logistic regression analysis of risk factors for positive TST and QFTGIT test results in women
| Variable | No. (%) of study participants | ||||
|---|---|---|---|---|---|
| Total | TST+ | OR (95% CI), p value | QFTGIT+ | OR (95% CI), p value | |
| Age (year) | |||||
| Median (IQR) | 29 (25–31) | 26 (22–30) | 26 (22–30) | ||
| 18–33 | 243(75.9%) | 77(31.7%) | 65(26.7%) | ||
| 34–49 | 77(24.1%) | 28(36.4%) | 1.23(0.72–2.11), p=0.447 | 25(32.5%) | 1.29(0.74–2.26), p=0.363 |
| Alcohol intake | |||||
| No | 227(70.1%) | 73(32.2%) | 61(26.9%) | ||
| Yes, Sometimes | 93(29.1%) | 32(34.4%) | 1.11(0.66–1.84), p=0.697 | 29(31.2%) | 1.24(0.73–2.10), p=0.430 |
| History of contact with TB patient | |||||
| No | 218(68.1%) | 57(26.1%) | 50(22.9%) | ||
| Yes | 102(31.9%) | 48(47.1%) | 2.51(1.54–4.11), p<0.0001 | 40(39.2%) | 2.13(1.28–3.54), p=0.004 |
| History of previous BCG vaccination |
|||||
| No | 178(55.6%) | 64(36.0%) | 56(31.5%) | ||
| Yes | 142(44.4%) | 41(28.9%) | 1.38(0.86–2.22), p=0.181 | 34(23.9%) | 0.69(0.42–1.14), p=0.150 |
| Parasitic infection* | |||||
| Absent | 291(90.9%) | 95(32.6%) | 82(28.2%) | ||
| Present | 29(9.1%) | 10(34.5%) | 0.92(0.41–2.06), p=0.841 | 8(27.6%) | 1.05(0.45–2.45), p=0.920 |
| Recent opportunistic infection* | |||||
| Absent | 315(98.4%) | 104(33.0%) | 89(28.3%) | ||
| Present | 5(1.6%) | 1(20.0%) | 0.55(0.06–4.60), p=0.546 | 1(20.0%) | 0.63(0.07–5.68), p=0.678 |
| HIV and Pregnancy | |||||
| HIV- NP | 54(16.9%) | 30(55.6%) | 25(46.3%) | ||
| HIV+ NP | 107(33.4%) | 31(29.0%) | 0.33(0.17–0.64), p=0.001 | 25(23.4%) | 0.35(0.18–0.71), p=0.003 |
| HIV- P | 73(22.8%) | 26(35.6%) | 0.44(0.22–0.91), p=0.026 | 23(31.5%) | 0.54(0.26–1.13), p=0.102 |
| HIV+ P | 86(26.9%) | 18(20.9%) | 0.21(0.10–0.45), p<0.0001 | 17(19.8%) | 0.29(0.14–0.63), p=0.001 |
| Trimester (only pregnant) | |||||
| 2nd trimester | 68(42.8%) | 20(29.4%) | 18(26.5%) | ||
| 3rd trimester | 91(57.2%) | 24(26.4%) | 0.86(0.43–1.73), p=0.672 | 22(24.2%) | 0.93(0.45–1.91), p=0.835 |
| CD4+ T cell count/μl (only HIV+) |
|||||
| <250 | 41(21.3%) | 7(17.1%) | 5(12.2%) | ||
| 250–500 | 90(46.6%) | 24(26.7%) | 0.503(0.19–1.34), p=0.170 | 20(22.2%) | 2.06(0.71–5.93), p=0.182 |
| >500 | 62(32.1%) | 18(29.0%) | 0.889(0.43–1.83), p=0.749 | 17(27.4%) | 2.85(0.96–8.48), p=0.060 |
Within 3 months of recruitment in this study
Detection rate of LTBI by TST and QFTGIT in women with and without pregnancy and HIV infection
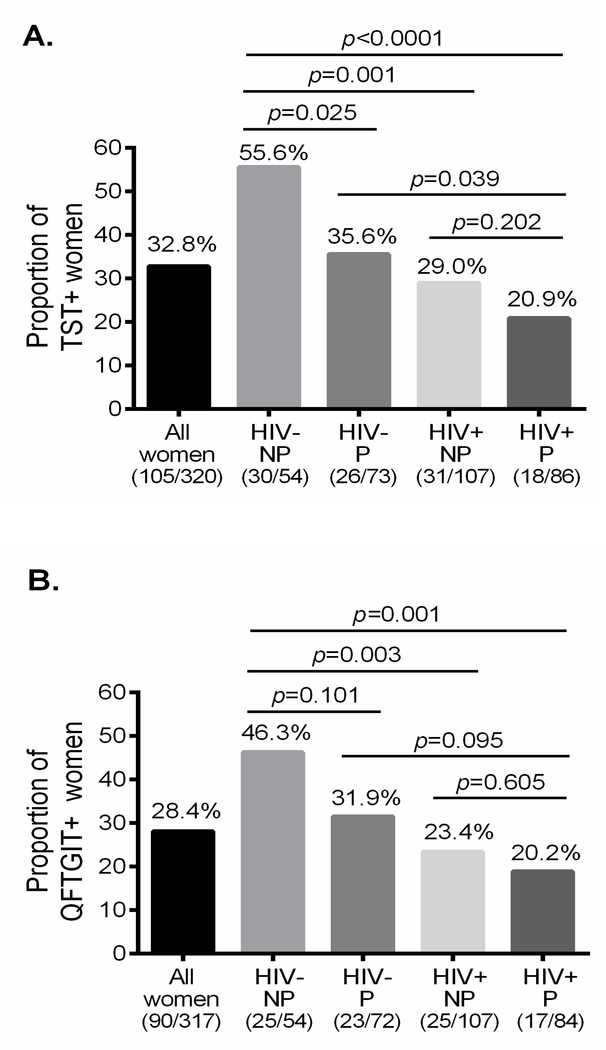
To further investigate the effect of pregnancy and HIV infection on detection performances of TST and QFTGIT for the diagnosis of LTBI, we determined and compared the rate of detection of LTBI in women with and without pregnancy and HIV infection. To precisely determine the proportion of women with LTBI, we selected study participants with valid results of TST and QFTGIT. The overall positivity rate of TST among all study participants was 32.8 %. Among the HIV negative non-pregnant women, 55.6% tested positive by TST and this frequency was significantly reduced to 35.6% and 29.0% in HIV negative pregnant (χ2=5.01, p=0.025) and HIV positive non-pregnant (χ2=10.77, p=0.001) women respectively (Fig 2A). The effect of HIV infection on the positivity of TST among pregnant women remained significant (χ2=4.26, p=0.039) while there was no substantial effect of pregnancy among HIV positive women (χ2=1.63, p=0.202) (Fig 2A). Notably, the positivity rate of TST was significantly lower in HIV positive pregnant women as compared to HIV negative non-pregnant women (χ2=17.65, p<0.0001). Besides, the detection rate of QFTGIT was determined and we found that the overall positivity rate was 28.1% (Fig. 2B). Similar to TST, QFTGIT results demonstrated that the detection rate was substantially reduced with the presence of HIV infection (χ2=8.81, p=0.003) while this reduction was not statistically significant among pregnant women (χ2=2.79, p=0.095). Importantly, the positivity rate of QFTGIT was significantly lower in HIV positive pregnant women as compared to HIV negative non-pregnant (χ2=10.54, p=0.001) (Fig. 2B).
Fig. 2.
Rate of detection of LTBI as assessed by TST, QFTGIT and TST/QFTGIT test results. The proportion of women who tested positive by TST (A), QFTGIT (B), and both TST and QFTGIT (C) among study participants in each group. Chi-square (χ2) was calculated to compare the detection rate of LTBI between the groups and p-values were determined. HIV+= HIV positive, HIV−= HIV negative, P= Pregnant and NP= Non-pregnant.
Furthermore, we determined the prevalence of LTBI as assessed by concordant positive TST/QFTGIT results among women with concordant TST/QFTGIT results after excluding participants with discordant and indeterminate results for this analysis, i.e., n=293. Accordingly, the overall prevalence of LTBI as detected by both TST and QFTGIT was 29.4%. HIV negative non-pregnant women had a LTBI detection rate of 51.1%, which was considerably reduced to 32.3% and 24.8 % in HIV negative pregnant (χ2=3.92, p=0.048) and HIV positive non-pregnant (χ2=9.80, p=0.002) women respectively (Fig. 2C). The magnitude of concordant positive TST/QFTGIT result in HIV infected pregnant women was 19.5%, which was significantly lower compared to that of HIV negative non-pregnant women (Fig. 2C).
Concordance of TST and QFTGIT test results
As the positivity rate of TST and QFTGIT was not significantly different and nearly similar, we analyzed the extent of agreement between TST and QFTGIT tests for the diagnosis of LTBI among the 317 study participants with valid test results, excluding the 3 participants with indeterminate QFTGIT. The overall agreement between TST and QFTGIT test results was 92.4% with a κ value of 0.82, indicating an excellent agreement (Table 4). Also, we evaluated whether pregnancy and HIV sero-status influence the level of concordance of the two screening tests for LTBI in women. We observed that HIV positive participants had an excellent concordance (97% agreement, κ=0.93 in pregnant and 94.4% agreement, κ=0.86 in non-pregnant women) whereas HIV negative participants had a fair-to-good agreements (83.3% agreement, κ=0.56 in non-pregnant and 90.3% agreement, κ=0.78 in pregnant women) (Table 4). This shows that the concordance of TST and QFTGIT results varies in women according to their status of pregnancy and HIV infection.
Table 4.
Concordance between TST and QFT.GIT test in women with valid results
| QFTGIT | Agreement κ value (95%CI) | ||
|---|---|---|---|
| QFTGIT+ | QFTGIT- | ||
| All participants (n=317) | |||
| TST+ | 86(27.1%) | 19(6.0%) | Agreement=92.7% |
| TST- | 4(1.3%) | 208(65.6%) | κ= 0.83 |
| HIV+ Pregnant (n=84) | |||
| TST+ | 17(20.2%) | 1(1.2%) | Agreement=97.8% |
| TST- | 0 (0.0%) | 66(78.6%) | κ= 0.96 |
| HIV- Pregnant (n=72) | |||
| TST+ | 21(29.2%) | 5(6.9%) | Agreement=90.3% |
| TST- | 2(2.8%) | 44(61.1%) | κ= 0.78 |
| HIV+ Non-pregnant (n=107) | |||
| TST+ | 25(23.4%) | 6(5.6%) | Agreement=94.4% |
| TST- | 0(0.0%) | 76(71.0%) | κ= 0.86 |
| HIV- Non-pregnant (n=54) | |||
| TST+ | 23(42.6%) | 7(13.0%) | Agreement=83.3% |
| TST- | 2(3.7%) | 22(40.7%) | κ= 0.67 |
Discussion
In this study, we demonstrated that detection performances of TST and QFTGIT for the diagnosis of LTBI in women substantially varies based on the status of pregnancy and HIV infection. The positivity rates of TST and QFTGIT in HIV positive women were significantly lower compared to the rates in HIV negative women, which is consistent with other studies (20, 24). TST and QFTGIT assays use different investigation approaches but both measure the T cell responses specific to M. tuberculosis antigens, indicating that these tests rely on the cell-mediated immune system. It has extensively been studied that HIV infection targets and substantially diminishes this particular arm of the immune system (4, 25–27). Hence, the immune responses measured in these tests may be reduced and thereby, cut-off size of induration for positivity of TST have been adapted between HIV negative (> 10mm) and HIV positive (> 5mm) (19, 20). Nevertheless, we still found that the positivity rate of TST in HIV positive women was significantly lower than HIV negative ones, suggesting that the interpretation of TST in HIV positive women still needs further refinement. On the other hand, QFTGIT measure the concentration of IFN-γ released in response to M. tuberculosis antigens. Notably, this assay has been used the same cut-off of IFN-γ concentration (> 0.35 IU/ml) to define positive test result of QFTGIT in all individuals according to the manufacturer, CDC (21) and previous studies (14, 20). HIV infection has been demonstrated to significantly decrease M. tuberculosis-specific IFN-γ responses in various population, including pregnant women (3, 25, 28). Thus, the reduced detection rate of LTBI by QFTGIT observed in this study could be related to the HIV-driven anti-mycobacterial immunosuppression. Altogether, these findings suggest that TST and QFTGIT testing for the diagnosis LTBI using the existing interpretation criteria may not accurately identify HIV positive women with M. tuberculosis infection.
Our study also determined the impact of pregnancy on the detection performances of TST and QFTGIT for the diagnosis of LTBI in the absence and presence of HIV infection. We and other groups have previously demonstrated that pregnancy impair M. tuberculosis-specific immune response, particularly IFN-γ responses (3, 29, 30) and the presence of underlying HIV infection further deepen the suppression (3). Consistently, we observed in the current study that pregnant women were significantly less likely to have a positive result of TST and QFTGIT as compared to non-pregnant women. Particularly, the positivity rates of TST and QFTGIT in HIV positive pregnant women were significantly lower than in HIV negative non-pregnant women. Important to mention, the cut-off value for positivity of TST and QFTGIT assays have not been adapted for pregnant women (14, 20, 21). The deepened anti-mycobacterial immunosuppression due to the combined impact of HIV and pregnancy may explain this low rate of detection of LTBI by TST and QFTGIT. Thus, pregnancy, concurrent with HIV infection, substantially reduces the detection performances of TST and QFTGIT in women.
The absence of a gold standard test for diagnosis of LTBI is a limitation of the study and we assume that the true positivity rate in each of the four groups was identical, implying that the differences in positivity rate among the groups are exclusively accounted to the performances of the tests. Thus, the altered detection performances of TST and QFTGIT for the diagnosis of LTBI in women with pregnancy and/or HIV infection may be associated with immunosuppression of anti-mycobacterial immunity triggered by pregnancy and HIV infection. This could probably means that these tests only identified women with a better immune competency, leaving those with weakened cell-mediated immune responses to M. tuberculosis due to pregnancy and/or HIV infection undetected by these tests. Besides, this study demonstrated that the concordance between TST and QFTGIT was increased in the presence of pregnancy or HIV infection with the highest agreement appearing in women with both conditions. In agreement with this finding, previous studies also showed an increased concordance of TST and QFTGIT in HIV positive individuals as compared to HIV negative controls (20) as well as in pregnant women compared to non-pregnant women (31). The increased agreement due to the underlying HIV infection and pregnancy could be related with the selection of participants with better immune competency and deteriorated sensitivity of the tests resulting in a higher concordance between TST and QFTGIT results.
In the current study, the positivity of TST and QFTGIT assays were significantly associated with history of contact with TB patients, consistence with previous studies (22, 32). However, age, alcohol intake, parasitic infection, trimester of pregnancy were not associated with the positive results of TST nor QFTGIT. Moreover, history of BCG vaccination had no association with the positivity of TST nor QFTGIT, which is similar with several other studies (32). This could be due to the overtime waning effect of childhood BCG vaccination in women of reproductive age. Yet, prior BCG vaccination were associated with the positivity of TST but not QFTGIT (31, 33) as the PPD used in TST is also present in the BCG vaccine while antigens used in QFTGIT are M. tuberculosis-specific (8, 9). Furthermore, although this study and others (14, 34) showed that HIV positive women with elevated CD4+ T cell count had a relatively higher detection rate of LTBI by these tests, there was no significant association between the positive TST and QFTGIT results, and CD4+ T cell count.
In conclusion, our study demonstrated the profound effect of pregnancy and HIV infection on the diagnostic performances of TST and QFTGIT for the detection of LTBI. Notably, the existence of pregnancy and/or HIV infection in women substantially reduces the detection rate of LTBI by TST and QFTGIT, but increased the concordance between these tests. In agreement with the new WHO recommendation, we suggest that either TST or QFTGIT can be used for the diagnosis of LTBI in women with pregnant and/or HIV infection as they have an excellent concordance between the tests. Yet, diagnosis of LTBI by TST and QFTGIT in HIV positive and pregnant women requires specially attention and defined result interpretation criteria as women with pregnancy and HIV infection are at higher risk of reactivation of LTBI to active TB disease. Thus, these data may suggest that a different thresholds or cut-off levels are required to define a positive results by TST and QFTGIT tests in women with pregnancy or HIV infection, particularly in HIV positive pregnant women. Future prospective studies with larger sample size are needed to determine the different threshold or cut-off level of these test results that would detect LTBI with a better sensitivity during pregnancy and HIV infection.
Highlights.
Pregnancy and HIV infection reduced the rate of detection of LTBI by TST and QFTGIT in women of reproductive age.
The concordance between TST and QFTGIT in adult women increased with the presence of pregnancy and HIV infection.
History of contact with TB patients were significantly associated with the positivity of TST and QFTGIT in women.
Acknowledgments
We acknowledge staff clinicians and laboratory technologists of the hospitals and health centers involved in this study for their support during patient recruitment, sample collection and performing CD4+ T cell counts; Armauer Hansen Research Institute (AHRI) for the financial and material support; and clinical nurses of AHRI: Genet Amare and Haregewine Yetesha for performing the TST. The authors would like to thank all the study participants who volunteered to participate in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was financially supported by the AHRI core budget. AHRI funding sources had no role in the study design, conduct and outcome of the manuscript.
Abbreviations:
- TB
Tuberculosis
- LTBI
Latent tuberculosis infection
- HIV
Human immunodeficiency virus
- M. tuberculosis
Mycobacterium tuberculosis
- CD
Cluster of differentiation
- Th
T helper
- IFN
Interferon
- BCG
Bacille Calmette-Guérin
- TST
Tuberculin skin test
- ELISA
Enzyme-linked immunosorbent assay
- QFTGIT
QuantiFERON-TB Gold In-Tube
- PPD
Purified protein derivative
- ESAT-6
Early secreted antigen target-6
- CFP-10
Culture filtrate protein-10
- PHA
phytohemagglutinin
- IQR
Interquartile range
- SSI
Staten’s Serum Institute
- CDC
Center for Disease Control and Prevention
- WHO
World Health Organization
Footnotes
Declarations of interest
None.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics statement
Prior to conducting this study, the study was reviewed and approved by Research Ethical Review Committee at Department of Medical Laboratory Science, Addis Ababa University, AHRI/ALERT Ethics Review committee, St. Paul Millennium Medical College ethical committee and the Ethiopian National Research Ethical Review Committee. Study participants were briefed regarding the purpose of the study, type and amount of specimen required before recruitment and written informed consent was obtained from all study participants enrolled in this study.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization (2019). Global Tuberculosis Report 2019. Geneva, Switzerland: Available at: https://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Jonsson J, Kühlmann-Berenzon S, Berggren I, Bruchfeld J. Increased risk of active tuberculosis during pregnancy and postpartum: a register-based cohort study in Sweden. European Respiratory Journal. 2020;55(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birku M, Desalegn G, Kassa G, Tegbaru B, Howe R, Tsegaye A, et al. Pregnancy suppresses Mycobacterium tuberculosis-specific Th1, but not Th2, cell-mediated functional immune responses during HIV/latent TB co-infection. Clinical Immunology. 2020:108523. [DOI] [PubMed] [Google Scholar]
- 4.Amelio P, Portevin D, Hella J, Reither K, Kamwela L, Lweno O, et al. HIV infection functionally impairs Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses. Journal of virology. 2019;93(5):e01728–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mofenson LM, Laughon BE. Human immunodeficiency virus, Mycobacterium tuberculosis, and pregnancy: a deadly combination. The University of Chicago Press; 2007. [DOI] [PubMed] [Google Scholar]
- 6.Moon H-W, Hur M. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection: an updated review. Annals of Clinical & Laboratory Science. 2013;43(2):221–9. [PubMed] [Google Scholar]
- 7.Organization WH. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. World Health Organization, 2018. 9241550236. [PubMed] [Google Scholar]
- 8.Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clinical infectious diseases. 1993:968–75. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Turner M, Elwood R, Schulzer M, FitzGerald J. A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax. 2002;57(9):804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma SK, Vashishtha R, Chauhan L, Sreenivas V, Seth D. Comparison of TST and IGRA in diagnosis of latent tuberculosis infection in a high TB-burden setting. PLoS One. 2017;12(1):e0169539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekele A, Ashenafi S, Aderay G, Assefa G, Aseffa A, Anderssen J, et al. LATENT TUBERCULOSIS AMONG ADULT ETHIOPIAN PATIENTS AT CHEST CLINIC, TIKURANBESSA SPECIALIZED HOSPITAL, ADDIS ABABA, ETHIOPIA. Ethiopian Medical Journal. 2016;54(4). [PubMed] [Google Scholar]
- 13.Teklu T, Legesse M, Medhin G, Zewude A, Chanyalew M, Zewdie M, et al. Latent tuberculosis infection and associated risk indicators in pastoral communities in southern Ethiopia: a community based cross-sectional study. BMC public health. 2018;18(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaCourse SM, Cranmer LM, Matemo D, Kinuthia J, Richardson BA, Horne DJ, et al. Effect of pregnancy on interferon gamma release-assay and tuberculin skin test detection of latent TB infection among HIV-infected women in a high burden setting. Journal of acquired immune deficiency syndromes (1999). 2017;75(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathad JS, Bhosale R, Sangar V, Mave V, Gupte N, Kanade S, et al. Pregnancy differentially impacts performance of latent tuberculosis diagnostics in a high-burden setting. PloS one. 2014;9(3):e92308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.König Walles J, Tesfaye F, Jansson M, Tolera Balcha T, Winqvist N, Kefeni M, et al. Performance of QuantiFERON-TB Gold Plus for detection of latent tuberculosis infection in pregnant women living in a tuberculosis-and HIV-endemic setting. PLoS One. 2018;13(4):e0193589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karam F, Mbow F, Fletcher H, Senghor CS, Coulibaly KD, LeFevre AM, et al. Sensitivity of IFN-γ release assay to detect latent tuberculosis infection is retained in HIV-infected patients but dependent on HIV/AIDS progression. PLoS One. 2008;3(1):e1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon-γ release assays and tuberculin skin testing for progression from latent TB infection to disease state. Chest. 2012;142(1):63–75. [DOI] [PubMed] [Google Scholar]
- 19.Cohn DL, O’Brien RJ, Geiter LJ, Gordin F, Hershfield E, Horsburgh C. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2000;49(6):1–54.10993565 [Google Scholar]
- 20.Lin W-C, Lin H-H, Lee SS-J, Sy C-L, Wu K-S, Chen J-K, et al. Prevalence of latent tuberculosis infection in persons with and without human immunodeficiency virus infection using two interferon-gamma release assays and tuberculin skin test in a low human immunodeficiency virus prevalence, intermediate tuberculosis-burden country. Journal of Microbiology, Immunology and Infection. 2016;49(5):729–36. [DOI] [PubMed] [Google Scholar]
- 21.Castro KG, Goldberg S, Jereb JA, LoBue P, Mazurek GH, Vernon A. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection--United States, 2010. 2010. [PubMed]
- 22.Ramos JM, Robledano C, Masiá M, Belda S, Padilla S, Rodríguez JC, et al. Contribution of interferon gamma release assays testing to the diagnosis of latent tuberculosis infection in HIV-infected patients: a comparison of QuantiFERON-TB Gold In Tube, T-SPOT. TB and tuberculin skin test. BMC infectious diseases. 2012;12(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khazraiyan H, Liaei ZA, Koochak HE, Ardalan FA, Ahmadinejad Z, Soltani A. Utility of QuantiFERON-TB Gold In-Tube Test in the Diagnosis of Latent TB in HIV-Positive Patients in a Medium-TB Burden Country. Journal of the International Association of Providers of AIDS Care (JIAPAC). 2016;15(2):101–6. [DOI] [PubMed] [Google Scholar]
- 24.Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals–a systematic review and meta-analysis. Journal of acquired immune deficiency syndromes (1999). 2011;56(3):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infection and immunity. 2011;79(4):1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray LW, Satti I, Meyerowitz J, Jones M, Willberg CB, Ussher JE, et al. Human Immunodeficiency Virus Infection Impairs Th1 and Th17 Mycobacterium tuberculosis–Specific T-Cell Responses. The Journal of infectious diseases. 2018;217(11):1782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esmail H, Riou C, du Bruyn E, Lai RP-J, Harley YX, Meintjes G, et al. The immune response to Mycobacterium tuberculosis in HIV-1-coinfected persons. Annual review of immunology. 2018;36:603–38. [DOI] [PubMed] [Google Scholar]
- 28.Petruccioli E, Chiacchio T, Navarra A, Vanini V, Cuzzi G, Cimaglia C, et al. Effect of HIV-infection on QuantiFERON-plus accuracy in patients with active tuberculosis and latent infection. Journal of Infection. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhard G, Noll A, Schlebusch H, Mallmann P, Ruecker AV. Shifts in the TH1/TH2 balance during human pregnancy correlate with apoptotic changes. Biochemical and biophysical research communications. 1998;245(3):933–8. [DOI] [PubMed] [Google Scholar]
- 30.Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR. The Th1: th2 dichotomy of pregnancy and preterm labour. Mediators of inflammation. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chehab BM, Kallail KJ, El Fakih RO, Zackula RE, Minns GO. Use of the QuantiFERON®-TB Gold Assay in pregnant patients. Kansas Journal of Medicine. 2010;3(2):24–30. [Google Scholar]
- 32.Yassin K, Ahmed EG, Musa AO, Hamdan HZ, Abuzied N, Fagear AA, et al. Prevalence of Latent Tuberculosis (LTB) Among Pregnant Women in a High Burden Setting in Sudan using Interferon Gamma (IFN-γ) Releasing Assay (IGRA). Current Women’s Health Reviews. 2019;15(3):214–7. [Google Scholar]
- 33.Worjoloh A, Kato–Maeda M, Osmond D, Freyre R, Aziz N, Cohan D. Interferon gamma release assay compared with tuberculin skin test for latent tuberculosis detection in pregnancy. Obstetrics and gynecology. 2011;118(6):1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latorre I, Martínez-Lacasa X, Font R, Lacoma A, Puig J, Tural C, et al. IFN-γ response on T-cell based assays in HIV-infected patients for detection of tuberculosis infection. BMC Infectious diseases. 2010;10(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]