Abstract
While there is an emerging consensus that engagement of the Mu opioid receptor by opioids may modulate various stages the HIV life cycle (e.g.: increasing cell susceptibility to infection, promoting viral transcription, and depressing immune responses to virally-infected cells), the overall effect on latency and viral reservoirs remains unclear. Importantly, the hypothesis that the increase in immune activation observed in chronic opioid users by direct or indirect mechanisms (i.e., microbial translocation) would lead to a larger HIV reservoir after ART-suppression has not been supported to date. The potential for a subsequent decrease in reservoirs after ART-suppression has been postulated and is supported by early reports of opioid users having lower latent HIV burden. Here, we review experimental data supporting the link between opioid use and HIV modulation, as well as the scientific premise for expecting differential changes in immune activation and HIV reservoir between different medications for opioid use disorder. A better understanding of potential changes in HIV reservoirs relative to the engagement of the Mu opioid receptor and ART-mediated immune reconstitution will help guide future cure-directed studies in persons living with HIV and opioid use disorder.
Keywords: opioid use disorder, medications of opioid use disorder, HIV latent reservoir, ART suppression, immune reconstitution, microbial translocation
Introduction
Opioid administration is known to negatively affect the host immune defenses, leading to increased susceptibility to infections such as pneumonia or, in the case of persons who inject drugs (PWIDs), endocarditis, osteomyelitis, septic arthritis and epidural abscesses (Ronan and Herzig, 2016; Edelman et al., 2019; Schranz et al., 2019; Visconti et al., 2019). This has led to an increased overall prevalence of hospitalizations related to infectious diseases in PWIDs [(Ronan and Herzig, 2016); reviewed in (Reardon, 2019)]. Indeed, opioids can directly affect immune function by interacting with cell surface receptors (e.g. μ opioid receptor, MOR) on immune effectors, inducing the secretion of pro-inflammatory cytokines, reducing Natural Killer (NK) cell activity, impairing T helper type 1 (Th-1) responses, and reducing antibody production. In addition, chronic opioid use leads to chronic constipation, dysbiosis and alterations of the mucosal barrier, and microbial translocation, all of which results in increased immune activation and chronic inflammation, as discussed below.
The use of intravenous drugs is associated with increased risk of HIV infection: according to reports by the US Center for Disease Control (Prevention, 2020), in 2018, PWIDs accounted for 7% of new US HIV diagnoses; in the period 2014–2018, the overall prevalence of HIV among US PWIDs was 9%. In addition, people living with HIV are more likely to be prescribed opioids for long-term management chronic pain. (Canan et al., 2019). The important role that immune activation and adaptive responses have on determining the course of Human Immunodeficiency Virus (HIV) infection, immune reconstitution after anti-retroviral therapy (ART), and levels of HIV reservoirs highlight the potential impact of MOR engagement on host and viral outcomes.
The goal of this manuscript is to review the existing literature covering the effects of MOR engagement on immune activation, and their impact on HIV persistence. We review the direct effects of opioids on chronic inflammation and immune activation, followed by a addressing their indirect effects by altered intestinal motility and function. Effects of opioid exposure on HIV infection are also reviewed, with reference to the viral cycle and the response to ART. We conclude by reviewing known and potential effects of opioid agonists and antagonists approved for the treatment of opioid use disorder, discussing their potential impact on the outcomes of HIV infection and treatment.
Opioids and leukocyte subsets: MOR and immunosuppression
Opioids can induce activation of T, B, NK cells and myeloid cells (neutrophils, monocytes and macrophages) (Zaki et al., 2006; Garcia et al., 2012; Zhu et al., 2016) which if sustained could contribute to immunosupression.
Engagement of the MOR at the surface of immune cells can directly affect immune function, resulting in secretion of pro-inflammatory cytokines [e.g., Monocyte chemoattractant protein (MCP)-1, Interferon (IFN)-γ inducible protein (IP)-10] in a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)/Protein kinase C (PKC) ζ -dependent manner (Happel et al., 2011; Al-Hashimi et al., 2013), reduction of NK cell activity through central dopaminergic signaling (Saurer et al., 2004), impaired T helper type 1 (Th-1) responses, and reduced antibody production (Roy et al., 2011; Ninkovic and Roy, 2013). MOR engagement can directly affect sustained immune responses, resulting in impaired Th-1 responses and reduced antibody production (Roy et al., 2011; Ninkovic and Roy, 2013);
While both lymphocytes and monocyte/macrophages express functional MOR, the effects of MOR activation can vary. In B cells, some effects have been noted: acute morphine administration induces loss of immature B-cell (among other subsets) in mice. This may be due to an effect on B-cell development, as the precursors are not depleted. Morphine reduces the B-cell mitogenic response to bacterial lipopolysaccharide (LPS) (Bryant et al., 1988), but MOR stimulation may enhance IgG and IgM production (Cheido et al., 2014). B cells are recovered upon cessation of morphine, in association with proliferation of the B-cell precursors. (Zhang et al., 2011). Whatever the effect on humoral responses, however, heroin and methadone users appear to have the same ability to mount an immune response to trivalent Influenza vaccine (Moroz et al., 2016), suggesting that compensatory mechanisms after prolonged opioid use may exist in vivo.
MOR activation is associated with NF-kB activation in myeloid cells (Gessi et al., 2016), while the effects on T-cells is affected by the T cells’ metabolic state. In activated T cells, treatment with MOR agonists results in decreased expression of transcription factors Activator protein (AP)-1, NF-kB and Nuclear factor of activated T cells (NFAT), indicating a possible impairment of multiple signaling pathways; however, the same factors are induced by MOR stimulation in resting cells (Wang et al., 2003; Martin-Kleiner et al., 2006). In T cells, morphine has also been shown to inhibit Tumor necrosis factor (TNF)-α-induced NF-kB activation (possibly by reversing I-kB ubiquitination) (Borner and Kraus, 2013). In nonhuman primates, long-term morphine administration increased circulating regulatory T cells (Treg) and the functional activity of Th17, indicating an overall dysregulation of these homeostatic mechanisms, with a potential suppression of Th-1 responses, which are necessary for viral control. Increasing overall frequencies of gut-homing (cluster differentiation (CD)161 and C-C chemokine receptor (CCR)6 and CCR5 expression in T-cells (Cornwell et al., 2013) were also observed, suggesting alterations in the ability of immune cells to target sites of infection. Opioid-induced changes on adaptive immune responses decreasing functionality would be detrimental to overall health. Importantly, not all opioids have the same immunosuppressive properties: in recent large retrospective study, patients using opioid analgesics without reported immunosuppressive activity (oxycodone, oxymorphone, tramadol) were at lower risk of developing infections requiring hospitalization [most frequent: pneumonia (56.1%), cellulitis (17.9%), bacteremia without pneumonia (15.3%), pyelonephritis (6.1%), and septic arthritis/osteomyelitis (3.8%)] than patients using immunosuppressive opioids (morphine, fentanyl, methadone) (Wiese et al., 2019). The potential for sustained immunosuppression of adaptive responses by opioids suggests that long-term opioid use (i.e. in individuals with OUD or undergoing chronic pain management) may reduce the overall immune system’s ability to control viral infections, and in the case of HIV, may result in impaired immune reconstitution after initiating ART, as further discussed below. As with humoral responses above, further analysis is needed to distinguish between the acute and sustained effects of MOR interactions in affecting cell-mediated responses in vivo as effects on immunosuppression or compensatory mechanisms if present may be affected by modality, frequency and duration of exposure.
Opioids and intestinal motility: Fueling Innate Immune Activation
Although acute stimulation of the MOR in peripheral blood mononuclear cells (PBMC) has been shown to result in secretion of the pro-inflammatory cytokines [e.g.: MCP-1, “Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted” (RANTES) and IP-10) in a NF-κB/PKCζ -dependent manner] (Happel et al., 2011; Al-Hashimi et al., 2013) and cellular activation (e.g., T cells (Brown et al., 2012), direct MOR engagement on lymphocytes is not thought to be the main driver of activation after chronic opioid use in vivo. At a systemic level, opioid-dependent systemic activation is thought to be driven indirectly by an increase trafficking of bacteria and microbial products (eg., bacterial or fungal products) from the small and gross intestine lumen resulting from a generalized disruption of the intestinal homeostasis, via an altered mucosal barrier (Meng et al., 2013), mucus secretion (Rogers and Barnes, 1989), and/or bile acid metabolism [in murine models, (Banerjee et al., 2016) reviewed in Wang and Roy (Wang and Roy, 2017)]. Specifically, a) opioid-induced overexpression of TOLL-like receptors (TLR) 2 and 4 in the intestinal mucosa leads to alterations in the epithelial tight junctions; in rodents this was shown to depend on the activation of myosin light chain kinase (MLCK), and to result in bacterial translocation to the liver; b) binding of opioids to cells of the enteric nervous system (ENS) plexa leads to decreased motility, resulting in chronic constipation and in increased intestinal transit times (Yuan et al., 1998; Holzer, 2004, 2008; Camilleri et al., 2017; Muller-Lissner et al., 2017) with an accumulation of bacterial products and c) chronic opioid exposure results in changes in the intestinal microbiota (increase in Gram+ bacteria of the phylum Firmicutes, previously associated with systemic inflammation), which is TLR-2-dependent and may be related to acidification of the bile secretion (Peng et al., 2012; Khansari et al., 2013; Meng et al., 2013; Banerjee et al., 2016). A potential added contributing factor is the alteration of the intestinal motility and homeostasis due to direct effects of opiates on the intestinal enteric glia (Galligan, 2015). Opioid use has also been reported to directly alter microbiota in humans and animal models (reviewed in (Banerjee et al., 2016; Wang and Roy, 2017; Le Bastard et al., 2018)); cirrhotic patients who use opioids show a decrease in the relative abundance of autochthonous taxa and Bacteroidaceae in intestinal microbiota (Acharya et al., 2017). Therefore, chronic opioid use is expected to indirectly impact immune activation by its effects on intestinal motility and permeability (Meng et al., 2019). It should be noted that polydrug use can also contribute to mucosal permeability dysregulation, as reported for use of stimulants (Volpe et al., 2014; Carrico et al., 2018) or alcohol (Engen et al., 2015; Monnig et al., 2016; Donnadieu-Rigole et al., 2018). Conversely, a reduction in immune activation has been reported by cannabinoids (Chandra et al., 2015; Rizzo et al., 2020), stressing the need to assess the added factors that may affect the integrity of the intestinal mucosal barrier.
Taken together, the observation of dysbiosis (verified in murine models) (rev. in (Akbarali and Dewey, 2019)) and increased translocation of bacterial residues supports a role for TLR-mediated inflammatory responses and immune activation as an outcome of opioid abuse (Banerjee et al., 2016). Importantly, dysbiosis, compromised intestinal wall, bacterial/microbial translocation, and myeloid activation caused by opioid share pathogenic features with HIV infection as both have been reported to result in dysbiosis and increased microbial translocation (Meng et al., 2015; Novati et al., 2015; Zevin et al., 2016; Allam et al., 2018; Bandera et al., 2018; Lujan et al., 2019; Weiner et al., 2019). As shown in Figure 1, an increase in dysbiosis, compromised gut integrity, and the resulting immune activation would provide a link between opioid use and the incidence of diseases of sustained systemic inflammation. Most recently, work by Hileman et al (Hileman, 2020) indicated that elevation of chronic inflammation-related biomarkers such as TNF-α receptors I and II, C-reactive protein (CRP), D-dimer, soluble (s) CD14 and sCD163, LPS-binding protein (LBP) and beta-D-glucan are associated with opioid use, independent of HIV infection (notably, zonulin levels are associated with HIV infection, independent of opioid use, indicating a viral-mediated intestinal mucosa injury). Although gut mucosa has been the predominant focus for the effects of MOR on microbial translocation studies, further investigation is still needed to address less studied mucosal barriers (eg., oral, lung, reproductive tract).
Fig 1.
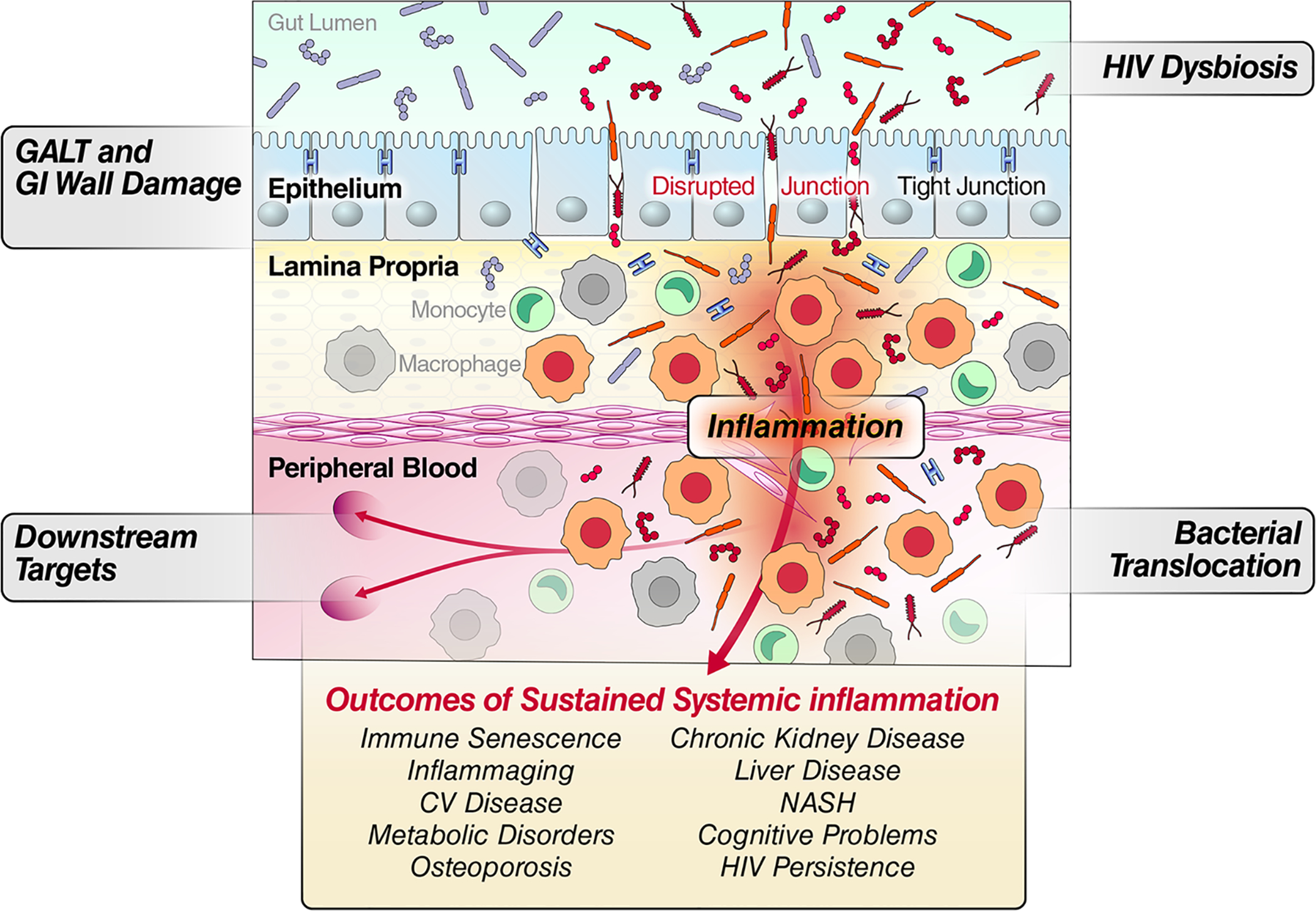
Conceptual model linking gut integrity damage, microbial translocation, persistent myeloid cell activation, and systemic disease outcomes from sustained inflammation.
The impact of OUD on systemic and intestinal mucosa immune activation has also been documented by the analysis of T-cell expression of the activation markers CD38 and HLA-DR, T cell proliferation [based on the expression of Kiel-67 (Ki67) antigen], and myeloid activation in association with markers of microbial translocation (as measured by LPS and sCD14) (Mehandru et al., 2015). Specifically, the analysis of 19 individuals with active OUD and 13 non-OUD individuals showed that the OUD group had significantly higher expression of CD38 in the peripheral blood and gastrointestinal (GI) tract as compared to the non-OUD group. Additionally, the expression of Ki67 on T cells was also significantly higher in the peripheral blood and GI tract of OUD individuals compared to the non-OUD group. Finally, the levels of sCD14 (indicative of myeloid activation) was significantly higher in the OUD individuals compared to non-OUD individuals. Combined, these data further evidence that chronic opioid is linked to increased activation in the gut-associated lymphoid tissue (GALT), increased expression of markers of myeloid cell activation, and increased presence of microbial translocation. Whether this effect is substantially different between type of opioid drug, medications for opioid use disorder or non-immunosuppressive opioid analgesics remains to be established.
Effect of opioids on HIV infection: Synergistics immune modulation
Opioid use results in host effects and biological outcomes that have the potential to affect HIV infectivity and promote viral replication, in part by modulating host immune function and sustaining chronic inflammation as noted above per intestinal stasis and microbial translocation. A recent study based on single-cell transcriptomics (Karagiannis et al., 2020) indicates that peripheral blood monocytes from chronic opioid users have reduced native expression of antiviral gene programs; in the same individuals, both T and NK lymphocytes have impaired responses as a result of LPS responses within PBMC. Importantly, the same study reports that acute in vitro treatment of PBMC with morphine impairs the LPS response as measured in in T, B and NK cells from non-OUD donors, suggesting an impaired direct causative pathway.
Apart from the effects in gastro-intestinal (GI) tract reviewed above, chronic opioid use in the presence of HIV infection affects other organs such as the central nervous system (CNS) as indicated by the fact that HIV-infected individuals with OUD have more cognitive impairment than non-OUD even after ART suppression (Applebaum et al., 2009; Hauser et al., 2012; Meyer et al., 2013). To date, the role of opioid-dependent dysregulation of immune cell functions in the CNS and in maintaining persistent chronic neuro inflammation after HIV infection has received more attention than opioid effects in other organ systems (Bell et al., 1998; Hauser et al., 2005; Brenchley et al., 2006; Burdo et al., 2006; Byrd et al., 2012; Gill and Kolson, 2014). For example, morphine can increase macrophage and CD8+ T cell infiltration, and viral load in the hippocampus and striatum (Bell et al., 1998; Reddy et al., 2012). In murine models, morphine promoted viral replication in the hippocampus, but decreased CCL5 in the frontal lobe (McLane et al., 2014). Indeed, MOR interaction by morphine was shown to synergize with HIV-1 Tat protein to induce astrocyte production of TNF-α, interferon(IFN)-γ, and CCL5/RANTES in both in vitro and in vivo mouse models (El-Hage et al., 2005; El-Hage et al., 2008a; El-Hage et al., 2008b) In myeloid cells, morphine enhances HIV infection and replication in macrophages and CNS myeloid cells (Banerjee et al., 2011; Reynolds et al., 2012). Opioid-associated mechanisms increasing myeloid production of HIV include suppression of type-1 IFN-related antiviral responses (Wang et al., 2012), alteration of the blood/brain barrier (Strazza et al., 2016) leading to transmigration of myeloid cells across the blood/brain barrier [(Leibrand et al., 2019), reviewed in (Strazza et al., 2011)], increased expression of HIV co-receptor molecules (Guo et al., 2002; Steele et al., 2003), and suppression of anti-HIV micro-RNAs (miRNAs) [(Dave and Khalili, 2010; Wang et al., 2011; Wang et al., 2015; Wang et al., 2020); reviewed in (Purohit et al., 2012)]. In addition, MOR stimulation may directly transactivate the HIV long-terminal repeat (LTR) through activation of cyclic adenosine monophosphate (cAMP)/ cAMP response element-binding protein (CREB)-dependent pathways (Banerjee et al., 2011). Of these, the effects of opioid on viral binding and negative modulation of the Type I interferon response are considered the most consequential. Specifically, morphine exposure is expected to increase viral replication by its induction of CCR5 and C-X-C chemokine receptor (CXCR)4, promoting viral infection, particularly of myeloid cells, and cellular trafficking (Li et al., 2003; Steele et al., 2003; Zheng et al., 2012). In addition, morphine is expected to disrupt type 1 interferon signaling, leading to a dysregulated cellular antiviral response and facilitating increased viral replication (Cheung et al., 1991; Wang et al., 2011). Heroin has also been reported to reduce the expression of Type I Interferon-induced restriction factors [Tripartite motif-containing protein (TRIM)5a, TRIM22, apolipoprotein B mRNA editing enzyme (APOBEC)3G], therefore increasing the permissiveness for HIV replication (Zhu et al., 2017). In a recent study looking at single-cell transcriptomics (Karagiannis et al., 2020), opioid users were also shown to have a blunted response (antiviral, peak inflammation and sustained inflammation genesets) to LPS further supporting that acute exposure to opioids may impair the ability of immune cells to restrict viral infection.
Consistent with an increase in viral replication following exposure to morphine, CD4 loss and viral setpoint after Simian immunodeficiency virus (SIV) infection in macaques were shown to be greater in animals undergoing long-term morphine administration (Kumar et al., 2004). Additional mechanisms potentially linking HIV infection and drugs of abuse, beyond the scope of this review, have been previously reported (reviewed in (Meng et al., 2015)).
As noted above, indirect mechanisms of HIV modulation by opioids include the increased representation of pro-inflammatory species/taxa in the intestinal microbiome. Individual microbes are well known to cause gut inflammation and disease in a susceptible host, but recent data implicate a role for whole intestinal microbial communities in the pathogenesis of numerous inflammatory disorders (58–63). In murine and human vaccine studies, the action of the microbiome has been implicated in determining inflammatory set points (65–69) that can have functional consequences such as altered vaccination outcomes. Effects of MOR on intestinal stasis and activation would be expected to intersect with effects of HIV infection, microbiota, and sustained activation. HIV-infected individuals with low abundance of colonic butyrate-producing bacteria have high levels of microbial translocation and immune activation (Dillon et al., 2017). Butyrate is a short chain fatty acid produced in the colon by bacteria as a breakdown product of dietary fiber which has been shown to regulate inflammatory immune responses and support intestinal barrier integrity (reviewed in (Koay et al., 2018)).
Figure 2 and Table 1 summarize the host changes associated with MOR modulation that may increase HIV infectivity upon exposure, contribute to increasing viral load by direct and indirect mechanisms, and impact HIV persistence after ART suppression. The available literature supports a higher frequency of HIV founder virus following intravenous exposure in PWIDs, supporting a link with higher inoculum dose by IV route or infectivity, or both. Available data also indicate a faster HIV disease progression in opioid users (McNeil, 1997; El-Hage et al., 2005; Meijerink et al., 2014), whereas data on the impact of opioid use in HIV persistence after ART-mediated viral suppression are still emerging as discussed below.
Fig 2.
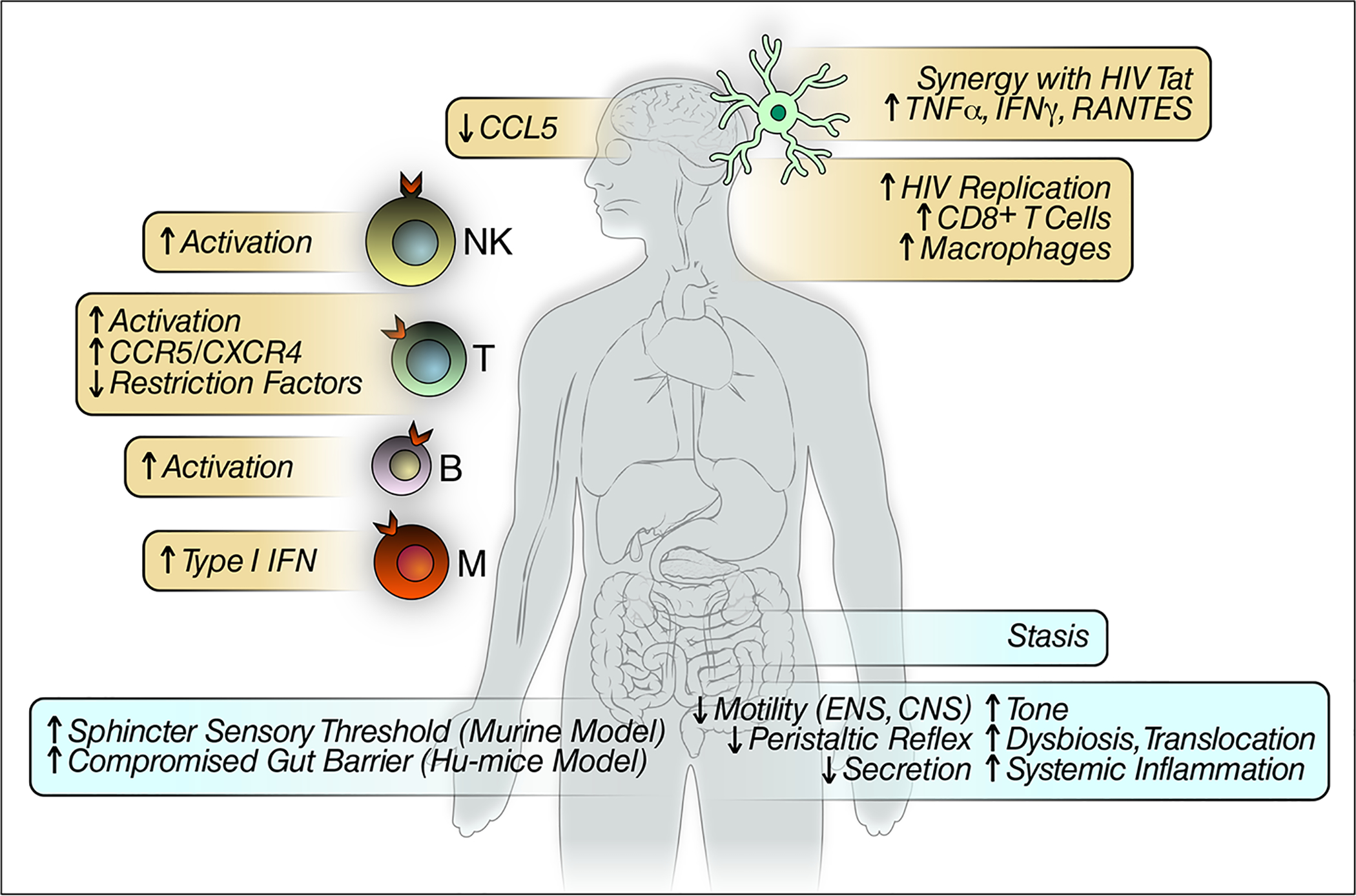
Summary of major cellular and systemic opioid effects expected to impact HIV infection and replication.
Table 1.
Potential opioid effects on molecular targets affecting HIV replication and pathogenesis in HIV-infected individuals on suppressive ART
| Target pathway | Effects | References |
|---|---|---|
| miRNAs | Opioids suppress anti-HIV miRNAs | (Dave and Khalili, 2010; Wang et al., 2011; Wang et al., 2015; Wang et al., 2020) |
| Antiviral restriction factors | Heroin reduces the expression of restriction factors TRIM5a, TRIM22, APOBEC3G, and IFN-a, -b | (Zhu et al., 2017) |
| Glucose transport and glycolysis | Glut1 expression is enhanced in rats treated with morphine | (Yousif et al., 2008) |
| Gut barrier, Microbial translocation | Opioid-induced Chronic activation of HIV-infected targets through TLRs | (Banerjee et al., 2016) |
| Direct activation of immune effectors | Opioid-induced activation of T, B, NK cells and myeloid cells | (Zaki et al., 2006; Garcia et al., 2012; Zhu et al., 2016) |
| Direct glial/astrocyte activation (CNS) | Morphine synergizes with HIV-1 Tat protein to activate glial/astrocytes | (El-Hage et al., 2005; El-Hage et al., 2008a; El-Hage et al., 2008b) |
| Enhanced transcription | Opioid-induced increased TLR4-mediated NFkB in microglia; decreased NFkB in T cells; induced AP-1 and CREB in neuronal cells; increased AP1 NFkB and NFAT in resting immune effectors. | (Bilecki et al., 2004; Martin-Kleiner et al., 2006; Borner and Kraus, 2013; Gessi et al., 2016) |
| Glycosylation patterns and immune activation | Opioid consumption (including methadone) associated with glycemic dysregulation and metabolic syndrome (Chronic inflammation and immune suppression) | (Giugliano et al., 1985; Mysels and Sullivan, 2010; Vallecillo et al., 2018) |
While we focus on immune activation, due to its significance in HIV disease, it is important to note that additional immune pathways may be affected by chronic exposure to opioids: for example, a) exposure to morphine has been long known to inhibit induced oxidative burst in PBMC (Peterson et al., 1987), and b) chronic exposure to morphine reduces chemotaxis, resulting in delayed recruitment of monocytes and granulocytes to wound site in murine models (Martin et al., 2010). Future studies leveraging novel discovery platforms (“omics” platforms such as transcriptomics, genomics, microbiomics, etc.) might prove helpful in addressing the intersection of these diverse, interconnected pathways in PWIDs living with HIV infection.
Opioids and ART-suppression of HIV: Synergy in activation continues
Currently no study has assessed the effect of opioids on HIV latent reservoir formation after ART-mediated suppression or what the effects of MOR-activation has on HIV persistence in target organs (e.g: CNS, GI tract). Chronic opioid use would predict a higher viral load at start of ART thus a potentially higher viral reservoir. The persistence of immune activation by opioids would be expected to compete against ART suppression, add to the retained levels of activation, and/or affect the rate and amount of CD4 recovery and immune reconstitution. In the absence of opioid use, long-term ART results in viral suppression (plasma VL < 50 copies/ml) but does not fully reverse the damage inflicted by chronic HIV replication. The extent of immune impairment associated with chronic residual immune activation on ART is not completely understood, but the increase in “exhausted” activate T memory cells is thought to contribute to the overall immune suppression that persist in chronic HIV, and approaches aimed at neutralizing checkpoint inhibitors such as PD-1 are being attempted in the pursuit of an HIV cure on ART [reviewed in (Henderson et al., 2020)].
A body of work on ART suppressed PLWH supports the persistence of T cell activation and inflammation (Valdez et al., 2002; French et al., 2009; Lederman et al., 2011) (further reviewed in (Hunt, 2012)), the lasting depletion and/or dysfunction of innate immune subsets (e.g., plasmacytoid dendritic cells (Chehimi et al., 2002; Sachdeva et al., 2008) and monocytes (Hearps et al., 2012)), and the retained expression of low level cell-associated HIV RNA. Persistent immune activation (as evidenced by the expression of CD38 and HLA-DR on CD8+ T cells) and exhaustion [e.g.: expression of the marker Programmed cell death protein (PD)1 on immune cells] are associated with impaired immune reconstitution (Hunt et al., 2003; Fernandez et al., 2006; Goicoechea et al., 2006; Nakanjako et al., 2011), and, as demonstrated in our studies, detection of cell-associated HIV RNA (Abdel-Mohsen et al., 2018). The observation that levels of soluble CD163 and CD14 (associated with microbial translocation) remain elevated despite ART (Burdo et al., 2011b; Burdo et al., 2011a; Patro et al., 2016) supports the retention of myeloid activation in addition to the T cell compartment, as recently reported (Hearps et al., 2012). Indeed, interleukin (IL)-6 levels have been reported as a correlate of low levels of viral replication on ART (Bastard et al., 2012). Furthermore, chronic inflammation markers (CRP, IL-6) and other acute phase reactants (e.g., D-dimer and cystatin C) remain elevated in subjects undergoing successful ART (VL < 400 copies/ml) (Neuhaus et al., 2010), indicating that, like cellular activation, the inflammatory cascade can remains active after viral suppression. Indeed, while immune reconstitution observed in patients with ART does improve neurological complications of HIV as demonstrated in different national Neuro-acquired immune deficiency syndrome (AIDS) cohorts (McArthur et al., 2010), there is retained levels of macrophage activation (i.e.: elevated neopterin) in the cerebrospinal fluid (CSF) (Abdulle et al., 2002; Eden et al., 2007), supporting the lack of complete reversal in myeloid activation in the CNS after ART suppression. Within the widely reported persistence of myeloid immune activation after ART suppression (Abdulle et al., 2002; Eden et al., 2007; McArthur et al., 2010; Hearps et al., 2012), the exposure of opioids would be expected to sustain activation levels irrespective of ART suppression.
However, as noted above, data indicate a potential difference in type of opioid used and effects on activation. While CD4 recovery following ART initiation might not be affected by chronic exposure to prescription opioids, as indicated in a large Veteran Administration cohort study (Edelman et al., 2016), recent publications indicate a higher retention of immune activation in OUD individuals with ART-suppressed HIV by maintaining higher levels of circulating intermediate and non-classical CD14+/CD16+ monocytes bearing CD163 (as well as increased soluble CD163) (Underwood et al., 2020) than ART-suppressed controls; this monocyte subset is more prone to HIV infection and are thought to be involved in supporting chronic inflammation in tissues (Fischer-Smith et al., 2001). Finally, expression of the glucose transporter 1 (Glut1) (and subsequently increased glucose intake into the cell) is induced by binding of the delta opioid receptor (DOR), receptor for enkephalins and cannabidiol which may mediate the reward mechanisms of MOR-specific opioids and is antagonized by buprenorphine and naloxone reviewed in Pradhan A.A (Pradhan et al., 2011); this has also been observed in rats treated with morphine (Yousif et al., 2008); to date, a similar association has not been established in humans. Increased expression of Glut1 has also been described CD4+ T cells from HIV infected individuals (Palmer et al., 2014), where it leads to increased anaerobic glycolysis and correlates with cellular activation markers and may be associated with disease progression. The existence of a possible synergy between MOR stimulation and HIV infection on the expression of Glut1 has not yet been explored; if confirmed, this pathway could contribute to linking MOR-induced anaerobic metabolism in HIV-infected CD4+ T cells, sustaining their chronic activation and viral transcription, with a mechanism for sustaining the persistence of HIV on ART.
Medications of Opioid Use Disorder and Immune Activation: MOR agonists and antagonists
OUD is currently treated with medications (MOUDs) such as opioid agonists (methadone or buprenorphine), or antagonist (extended-release naltrexone), together with other harm-reduction services. These interventions have greatly reduced HIV incidence by reducing opioid use and increasing access and adherence to ART (Volkow and Montaner, 2011; 2014), and contribute to significant public health improvement (Stimson et al., 2010; 2014).
The effect of MOUD on ART-mediated immune recovery is expected to differ, depending on the degree in which MOUD components (and indeed any other MOR agonist, such as prescription and non-prescription opioids) engage and stimulate MOR-dependent signaling. Specifically, it is anticipated that agonists and, to a lesser degree, partial agonists will have immunomodulatory effect similar to those observed in opioids (leading to chronic inflammation and immune activation, negatively impacting immune recovery), whereas individuals receiving naltrexone will experience a reduction in dysbiosis and bacterial translocation, leading to a greater degree of immune reconstitution (See Figure 3). Regarding partial agonists, the expectation may be less clear as studies with buprenorphine associate with better neurocognitive outcomes (Soyka et al., 2005; Pirastu et al., 2006; Rapeli et al., 2007; Rapeli et al., 2011; Soyka et al., 2011), suggesting that they might result in lower levels of neuroinflammation. Importantly, buprenorphine may to reduce the migration of CD14+/CD16+ monocytes into the CNS in response to CCL2 (Jaureguiberry-Bravo et al., 2018). The potential biologic effects of MOUDs on immune responses and chronic inflammation within target systems (Gastrointestinal, CNS, lymph), particularly relevant in persons living with HIV with OUD (e.g.: the impact of MOUDs on disease progression, immune activation or HIV reservoirs on ART), remain poorly understood, despite substantial evidence for opioid-induced immunosuppression (Sacerdote et al., 2012).
Fig 3.
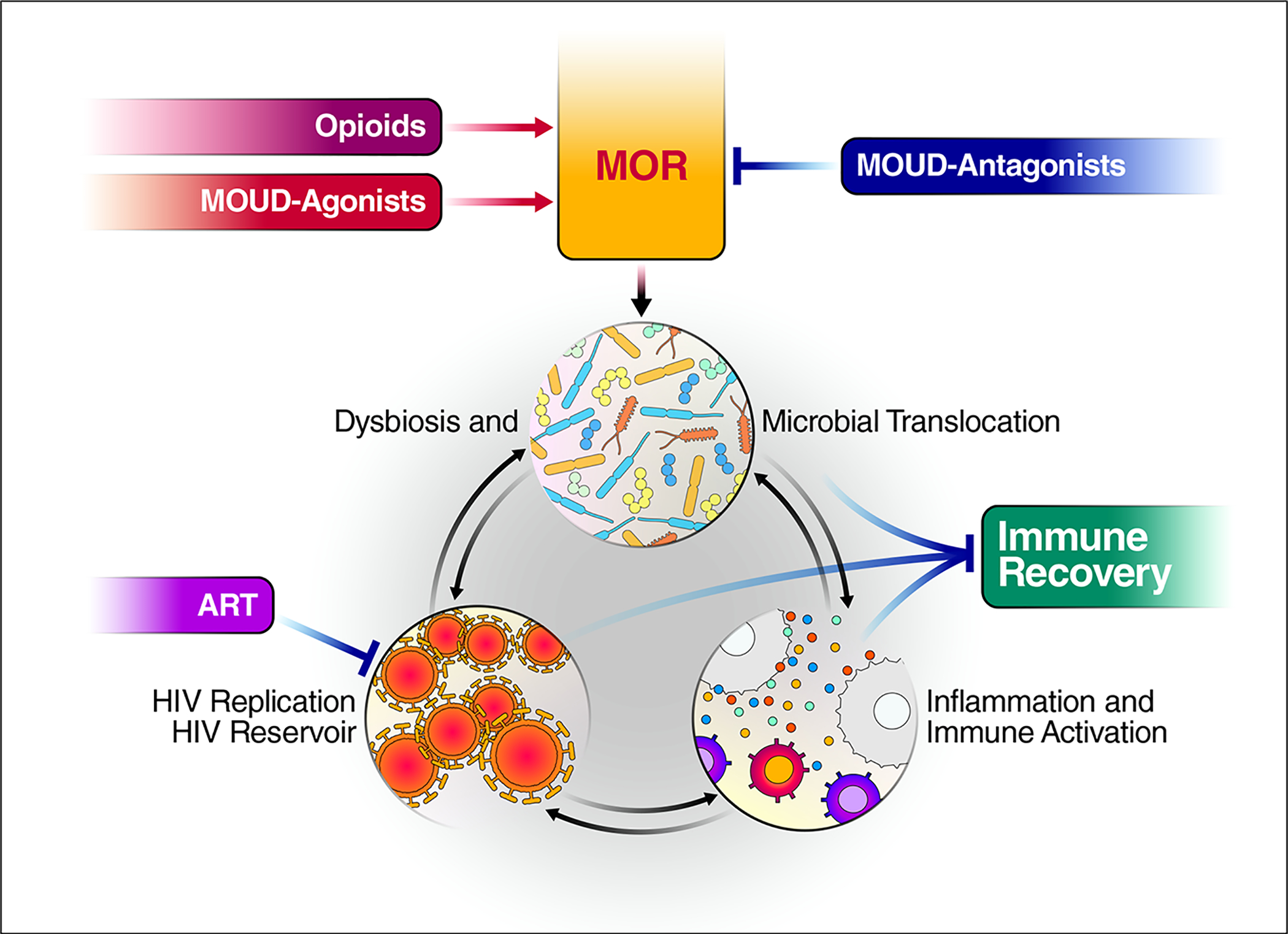
Expected interplay between medications of opioid use disorder (MOUD), μ-Opioid Receptor (MOR) and sustained dysbiosis/microbial translocation. The use of Opioids, MOUD-Agonists (methadone, buprenorphine) and MOUD-Antagonists (naltrexone) are the leading mechanisms shown. Blue lines illustrate how MOUD-Antagonists may block MOR activation resulting in a reduction in dysbiosis, a decrease of microbial translocation-inducted activation, and increased immune recovery on antiretroviral therapy (ART).
Opioids and Persistent HIV Reservoirs Dynamics on ART
Chronic opioid use in ART-suppressed persons living with HIV promotes a positive feedback of activation and dysbiosis as summarized in Figure 4 which alone or with added influences from polydrug, comorbidities and diet are expected to impact HIV persistence. It remains to be determined how opioid based immunosuppression may impact the “latent reservoir” in which cells carry an integrated copy of the viral genome that does not express viral transcripts or proteins. These latent proviruses are not affected by ART or cleared by the immune system with a fraction able to be maintenance by clonal expansion in absence of activation (Lee et al., 2017); however, they can revert to a viral productive state upon stimulation (Crooks et al., 2015) (Finzi et al., 1999) (Chun et al., 1997).(Chun et al., 1997; Finzi et al., 1999; Crooks et al., 2015).
Fig 4.
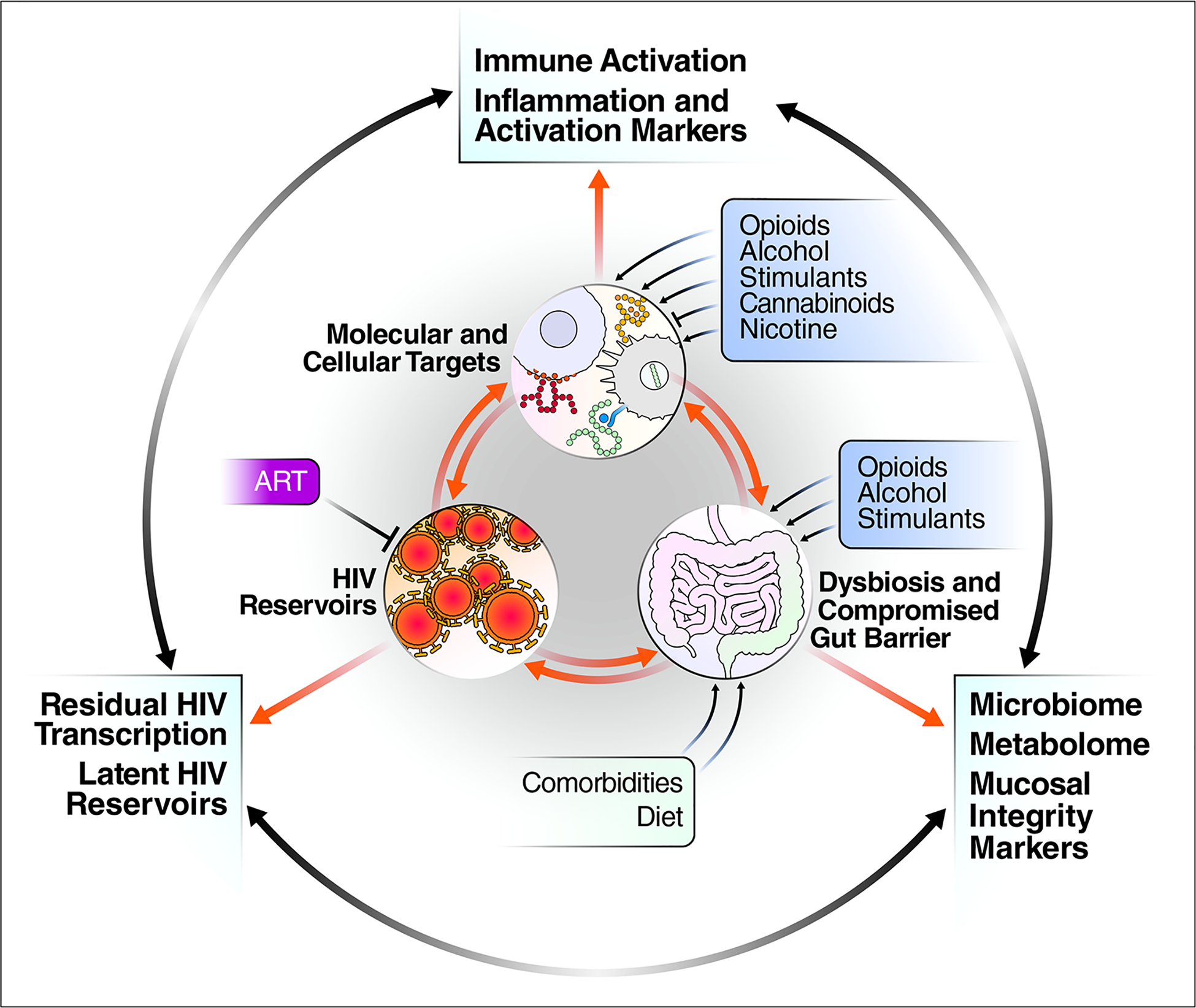
Conceptual model representing the interaction between substance abuse affecting immune activation, dysbiosis (compromised gut barrier) and persistent HIV after ART suppression. Common approaches used to monitor these three areas are listed in outer periphery of figure.
Supporting an effect of opioid-mediated activation on HIV latency, recent observations indicate that ART suppressed PWIDs have lower level of viral reservoir (Bachmann et al., 2019). Specifically, the authors performed a longitudinal study in 84 ART suppressed PWIDs and reported a smaller HIV reservoir size (as measured by total HIV DNA) as compared to ART suppressed controls 1.5 years after initiation of ART. While authors ruled out Hepatitis C and Interferon therapy as confounders, they did not establish the mechanism for the lower HIV DNA levels apart from a potential survival bias (PWID started ART after a longer time of infection thus may have lower viral loads). We would postulate that opioid-mediated activation and the potential for latency reactivation may have fueled the decline as well (i.e.: through “burnout” of latently infected cells). Apart from the evidence of higher myeloid activation, the role of MOR-mediated direct effects on T-cell chromatin and epigenetic silencing [e.g. through modulation of histone deacetylases (HDAC), Histone Lysine Methyltransferase (HKMT) and Polycomb Repressive Complex 2 (PCR2)], levels of negative (e.g. NELF) or positive transcription factors [e.g. the NFkB / positive transcription elongation factor (P-TEF)b / 7SK small nuclear ribonucleoprotein (snRNP) transcriptional complex), or negative regulation of RNA processing and transport (e.g. miRNAs) may also impact reservoir formation and persistence, as well as increased reactivation from latency. While mechanism of latency and its reversal (outside the scope of this manuscript) have been published in recent years (Cary et al., 2016; Mbonye and Karn, 2017; Khoury et al., 2018), it also remains to be determined if PWIDs receiving MOUD have more or less efficient reactivation of HIV transcription in the presence of current “shock & kill strategies” with latency reserving agents (e.g. HDAC inhibitors, PKC agonists, etc.). Even if the decrease described by Bachmann et al (Bachmann et al., 2019) was in part due to viral reactivation while on continuous ART, long-term opioid use does not result in HIV eradication, indicating the presence of other, competing effects that contribute to HIV persistence in PWIDs. In addition, poor ART adherence, including prolonged interruptions, has recently been associated with higher proviral DNA levels [measured by Intact proviral DNA assay (IPDA)] in ART treated PWID (Kirk et al., 2020), suggesting that, in addition to pre-treatment baselines, ART adherence should be carefully recorded and factored in the analysis of future studies evaluating changes in persistent HIV measures.
Future Directions: Opioids and HIV Reservoirs Dynamics
There is an overall need to foster original studies on opioids and HIV reservoir dynamics, as highlighted by priority areas yet to be explored such as:
Establishing the relative importance of regulatory mechanisms (see table 1) in promoting HIV residual transcription in the presence of ART (i.e., as a source of activation) and/or replication in opioid users addressing potential differences in modality, frequency and duration of opioid use.
Characterizing the contribution of microbial translocation in mucosal tissues to tissue-based viral reservoirs in opioid users, with a focus on GALT - in relation to microbiome alterations.
Characterizing the role of the CNS as a preferential HIV reservoir site in opioid users - with a focus on resident and infiltrating immune mediators, as well as their relationship with HIV-associated neurocognitive disorders (HAND).
Establishing the specific role of MOR agonists (particularly methadone and buprenorphine, due to their predominant clinical use, but also immunosuppressive vs. non-immunosuppressive opioid analgesics) and modalities of exposure (e.g. acute, vs. Chronic, oral vs. parenteral, etc.) on the intestinal microbiome, and their role in sustaining immune activation and HIV reservoirs in HIV-infected, ART suppressed individuals.
Determining the impact of MOR engagement during ART on immune activation/exhaustion, and on the retention of reservoir levels.
Determining the role of MOR interactions in contributing or antagonizing leading HIV cure-directed strategies based on latency reversal and/or redirecting cell-mediated responses against latently infected cells (eg., ADCC, T-cell CAR, etc.).
Based on what we have already learned on the systemic effects of opioid use on immune, viral and target organ systems (CNS, GI tract), it is clear that HIV reservoir dynamics as well as the prospects of achieving stable remission or eradication in this population will depend on achieving greater clarity on the dynamic interplay between the MOR, immune activation and systemic viral reservoir dynamics after ART suppression.
Abbreviations used in this manuscript
| Abbreviation | Definition | What is it? |
|---|---|---|
| 7SK snRNP | 7SK small nuclear ribonucleoprotein | Combine with other proteins (e.g. NF-kB) to form transcriptional complexes |
| AIDS | Acquired immune deficiency syndrome | The disease caused by HIV |
| AP-1 | Activator Protein 1 | Transcription factor, is part of the early response to cellular activation |
| APOBEC | Apolipoprotein B mRNA editing enzyme | Proteins with anti-HIV activity, induced by IFN stimulation |
| ART | Anti-retroviral therapy | Antiretroviral drugs, usually used in combination |
| cAMP | Cyclic adenosine monophosphate | Second-messenger in numerous signaling pathways |
| CCL | C-C Motif Chemokine Ligand (e.g.: CCL5) | Chemokine, some with pro-inflammatory effect |
| CCR | C-C type chemokine receptor (e.g.: CCR5, and HIV coreceptor) | Cellular receptor for CC chemokines, also co-receptor for HIV |
| CD | Cluster differentiation | Term used to indicate monoclonal antibodies that react with the same molecule/set of molecules |
| CNS | Central nervous system | Brain and spinal cord |
| CREB | cAMP response element-binding protein | Transcription factor activated by camp |
| CRP | C-reactive protein | An acute-phase reactant, marker of inflammation in the serum |
| CSF | Cerebrospinal fluid | Liquid present in the CNS cavities and meningeal spaces |
| CXCR | C-X-C type chemokine receptor (e.g.: CXCR4, an HIV co-receptor) | Cellular receptor for CXC chemokines |
| DOR | Delta opioid receptor | Enkefalins receptor |
| ENS | Enteric nervous system | Division of the peripheral nervous system (PNS) that can control gastrointestinal motility independently of central nervous system (CNS) input |
| GALT | Gut-associated immune tissue | Lymphoid tissue associated with the intestinal mucosa |
| GI | Gastrointestinal | Pertaining to the digestive tract |
| Glut1 | Glucose transporter 1 | Protein facilitating the transport of glucose across the cell membrane |
| HAND | HIV-associated neurocognitive disorders | Neurocognitive and neurological disorders associated with HIV infection |
| HDAC | Histone deacetylase | Chromatin modifier, involved in epigenetic regulation and silencing of genes |
| HIV | Human immunodeficiency virus | An RNA retrovirus that causes acquired immune deficiency syndrome (AIDS) in humans |
| HKMT | Histone lysine methyltransferase | Chromatin modifier, involved in epigenetic regulation and silencing of genes |
| IFN | Interferon | Group of secreted pro-inflammatory molecules with potent antiviral effects |
| IL- | Interleukin (e.g. IL-2, IL-6) | Small protein with biological/immunological effects |
| IP10 | Interferon (IFN)-γ inducible protein 10 (a.k.a. CXCL10) | Proinflammatory chemokine induced by interferons |
| IPDA | Intact proviral DNA assay | PCR-based method to assess intact cell-associated HIV |
| Ki67 | Kiel-67 antigen | A marker of cell proliferation |
| LBP | LPS-binding protein | Endogenous molecule that binds and inactivates bacterial LPS |
| LPS | Lipopolysaccaride | Lipopolysaccaride expressed on the cell wall of Gram-negative bacteria |
| LTR | Long-terminal repeat | Non-coding sequences in HIV RNA with regulatory function |
| MCP-1 | Monocyte chemoattractant protein 1 | Pro-inflammatory chemokine |
| miRNA | Micro-RNA | Small RNA molecules able to interfere with protein synthesis. Some have anti-HIV effects |
| MLCK | Myosin light chain kinase | Enzyme responsible for muscle contraction |
| MOR | Mu opiod receptor | One of the receptors for natural and synthetic opioids |
| MOUDs | Medications for opioid use disorder | Medications used to treat opioid use disorder, including methadone, naltrexone, and buprenorphine |
| NFAT | Nuclear factor of activated T cells | Transcription factor, is part of the early response to stimulation of T-cell receptor and other pathways |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells | Transcription factor, induces the transcription of various pro-inflammatory molecules |
| NK | Natural Killer | Innate immunity cells involved in first-line response to pathogens |
| OUD | Opioid use disorder | Problematic pattern of opioid use leading to clinically significant impairment or distress (DSM-5) |
| PBMC | Peripheral blood mononuclear cells | White blood cells including lymphocytes and monocytes, but not granulocytes or erythrocytes |
| PCR2 | Polycomb repressive complex 2 methyltransferase | Chromatin modifier, involved in epigenetic regulation and silencing of genes |
| PD1 | Programmed cell death protein 1 | A marker of cellular activation and exhaustion |
| PKC | Protein Kinase C | Signaling molecules, mediates cell activation |
| P-TEF B | Positive transcription elongation factor B | Combine with other proteins (e.g. Nf-kb) to form transcriptional complexes |
| PWID | Person who inject drugs | A person, often with OUD, who injects drugs, usually opioids |
| RANTES | Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted | Pro-inflammatory chemokine with anti-HIV effects |
| sCD | Soluble CD (e.g., sCD14, sCD163) | Soluble variety of a molecule normally expressed on a cell membrane, originated by enzymatic cleavage or shedding |
| SIV | Simian immunodeficiency virus | Retrovirus similar to HIV, infecting non-human primates |
| T helper type 1 | Th-1 | Adaptive (memory) immune cells involved in long-term memory and cell-mediated responses to pathogens |
| TLR | TOLL-like receptor | Receptors sensing, among others, pathogen structures such as lipopolysacccarides or nucleic acids |
| TNF-α | Tumor necrosis factor-alpha | Pro-inflammatory molecule |
| Treg | Regulatory T cells | T cell subset responsible for the modulation/dampening of immune responses |
| TRIM | Tripartite motif-containing protein | Proteins with anti-HIV activity, induced by IFN stimulation |
Funding:
This work was supported by grants to LJM (NIH: 1UM1Al126620, R01 DA048728, R01 DA049666), Robert I. Jacobs Fund of the Philadelphia Foundation, Ken Nimblett and The Summerhill Trust, the Kean Family Professorship, and the Wistar Institute.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest:
Authors have no conflict of Interest.
References
- (2014) Taking Control: Pathways to Drug Policies That Work. In: Global Commission on Drug Policy. [Google Scholar]
- Abdel-Mohsen M et al. (2018) CD32 is expressed on cells with transcriptionally active HIV but does not enrich for HIV DNA in resting T cells. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulle S, Hagberg L, Svennerholm B, Fuchs D, Gisslen M (2002) Continuing intrathecal immunoactivation despite two years of effective antiretroviral therapy against HIV-1 infection. Aids 16:2145–2149. [DOI] [PubMed] [Google Scholar]
- Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A, Sikaroodi M, Bajaj JS (2017) Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther 45:319–331. [DOI] [PubMed] [Google Scholar]
- Akbarali HI, Dewey WL (2019) Gastrointestinal motility, dysbiosis and opioid-induced tolerance: is there a link? Nat Rev Gastroenterol Hepatol 16:323–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hashimi M, Scott SW, Thompson JP, Lambert DG (2013) Opioids and immune modulation: more questions than answers. Br J Anaesth 111:80–88. [DOI] [PubMed] [Google Scholar]
- Allam O, Samarani S, Mehraj V, Jenabian MA, Tremblay C, Routy JP, Amre D, Ahmad A (2018) HIV induces production of IL-18 from intestinal epithelial cells that increases intestinal permeability and microbial translocation. PLoS One 13:e0194185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum AJ, Reilly LC, Gonzalez JS, Richardson MA, Leveroni CL, Safren SA (2009) The impact of neuropsychological functioning on adherence to HAART in HIV-infected substance abuse patients. AIDS Patient Care STDS 23:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann N et al. (2019) Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun 10:3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandera A, De Benedetto I, Bozzi G, Gori A (2018) Altered gut microbiome composition in HIV infection: causes, effects and potential intervention. Curr Opin HIV AIDS 13:73–80. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR (2011) Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol 17:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, Dauer P, Chen C, Dalluge J, Johnson T, Roy S (2016) Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 9:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard JP, Soulie C, Fellahi S, Haim-Boukobza S, Simon A, Katlama C, Calvez V, Marcelin AG, Capeau J (2012) Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther. [DOI] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P (1998) HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain 121 (Pt 11):2043–2052. [DOI] [PubMed] [Google Scholar]
- Bilecki W, Wawrzczak-Bargiela A, Przewlocki R (2004) Activation of AP-1 and CRE-dependent gene expression via mu-opioid receptor. J Neurochem 90:874–882. [DOI] [PubMed] [Google Scholar]
- Borner C, Kraus J (2013) Inhibition of NF-kappaB by opioids in T cells. J Immunol 191:4640–4647. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12:1365–1371. [DOI] [PubMed] [Google Scholar]
- Brown JN, Ortiz GM, Angel TE, Jacobs JM, Gritsenko M, Chan EY, Purdy DE, Murnane RD, Larsen K, Palermo RE, Shukla AK, Clauss TR, Katze MG, McCune JM, Smith RD (2012) Morphine produces immunosuppressive effects in nonhuman primates at the proteomic and cellular levels. Mol Cell Proteomics 11:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant HU, Bernton EW, Holaday JW (1988) Morphine pellet-induced immunomodulation in mice: temporal relationships. J Pharmacol Exp Ther 245:913–920. [PubMed] [Google Scholar]
- Burdo TH, Katner SN, Taffe MA, Fox HS (2006) Neuroimmunity, drugs of abuse, and neuroAIDS. J Neuroimmune Pharmacol 1:41–49. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S (2011a) Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 204:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC (2011b) Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 204:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd D, Murray J, Safdieh G, Morgello S (2012) Impact of opiate addiction on neuroinflammation in HIV. J Neurovirol 18:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Lembo A, Katzka DA (2017) Opioids in Gastroenterology: Treating Adverse Effects and Creating Therapeutic Benefits. Clin Gastroenterol Hepatol 15:1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canan C, Alexander GC, Moore R, Murimi I, Chander G, Lau B (2019) Medicaid trends in prescription opioid and non-opioid use by HIV status. Drug Alcohol Depend 197:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Cherenack EM, Roach ME, Riley ED, Oni O, Dilworth SE, Shoptaw S, Hunt P, Roy S, Pallikkuth S, Pahwa S (2018) Substance-associated elevations in monocyte activation among methamphetamine users with treated HIV infection. AIDS 32:767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary DC, Fujinaga K, Peterlin BM (2016) Molecular mechanisms of HIV latency. J Clin Invest 126:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra LC, Kumar V, Torben W, Vande Stouwe C, Winsauer P, Amedee A, Molina PE, Mohan M (2015) Chronic administration of Delta9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques. J Virol 89:1168–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ (2002) Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol 168:4796–4801. [DOI] [PubMed] [Google Scholar]
- Cheido MA, Gevorgyan MM, Zhukova EN (2014) Comparative evaluation of opioid-induced changes in immune reactivity of CBA mice. Bull Exp Biol Med 156:363–365. [DOI] [PubMed] [Google Scholar]
- Cheung SC, Chattopadhyay SK, Morse HC 3rd, Pitha PM (1991) Expression of defective virus and cytokine genes in murine AIDS. J Virol 65:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF (1997) Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188. [DOI] [PubMed] [Google Scholar]
- Cornwell WD, Lewis MG, Fan X, Rappaport J, Rogers TJ (2013) Effect of chronic morphine administration on circulating T cell population dynamics in rhesus macaques. J Neuroimmunol 265:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks AM, Bateson R, Cope AB, Dahl NP, Griggs MK, Kuruc JD, Gay CL, Eron JJ, Margolis DM, Bosch RJ, Archin NM (2015) Precise Quantitation of the Latent HIV-1 Reservoir: Implications for Eradication Strategies. J Infect Dis 212:1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave RS, Khalili K (2010) Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system. J Cell Biochem 110:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SM, Kibbie J, Lee EJ, Guo K, Santiago ML, Austin GL, Gianella S, Landay AL, Donovan AM, Frank DN, Mc CM, Wilson CC (2017) Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS 31:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnadieu-Rigole H, Pansu N, Mura T, Pelletier S, Alarcon R, Gamon L, Perney P, Apparailly F, Lavigne JP, Dunyach-Remy C (2018) Beneficial Effect of Alcohol Withdrawal on Gut Permeability and Microbial Translocation in Patients with Alcohol Use Disorder. Alcohol Clin Exp Res 42:32–40. [DOI] [PubMed] [Google Scholar]
- Edelman EJ, Gordon KS, Tate JP, Becker WC, Bryant K, Crothers K, Gaither JR, Gibert CL, Gordon AJ, Marshall B, Rodriguez-Barradas MC, Samet JH, Skanderson M, Justice AC, Fiellin DA (2016) The impact of prescribed opioids on CD4 cell count recovery among HIV-infected patients newly initiating antiretroviral therapy. HIV Med 17:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Gordon KS, Crothers K, Akgun K, Bryant KJ, Becker WC, Gaither JR, Gibert CL, Gordon AJ, Marshall BDL, Rodriguez-Barradas MC, Samet JH, Justice AC, Tate JP, Fiellin DA (2019) Association of Prescribed Opioids With Increased Risk of Community-Acquired Pneumonia Among Patients With and Without HIV. JAMA Intern Med 179:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M (2007) Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis 196:1779–1783. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF (2008a) CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 Tat-exposed mice. J Neuroimmune Pharmacol 3:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF (2005) Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia 50:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, Hauser KF (2008b) Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS One 3:e4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A (2015) The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol Res 37:223–236. [PMC free article] [PubMed] [Google Scholar]
- Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA (2006) Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol 120:163–170. [DOI] [PubMed] [Google Scholar]
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF (1999) Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 7:528–541. [DOI] [PubMed] [Google Scholar]
- French MA, King MS, Tschampa JM, da Silva BA, Landay AL (2009) Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 200:1212–1215. [DOI] [PubMed] [Google Scholar]
- Galligan JJ (2015) HIV, opiates, and enteric neuron dysfunction. Neurogastroenterol Motil 27:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JB, Cardoso MG, Dos-Santos MC (2012) Opioids and the immune system: clinical relevance. Rev Bras Anestesiol 62:709–718. [DOI] [PubMed] [Google Scholar]
- Gessi S, Borea PA, Bencivenni S, Fazzi D, Varani K, Merighi S (2016) The activation of mu-opioid receptor potentiates LPS-induced NF-kB promoting an inflammatory phenotype in microglia. FEBS Lett 590:2813–2826. [DOI] [PubMed] [Google Scholar]
- Gill AJ, Kolson DL (2014) Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in HAND persistence. Curr HIV/AIDS Rep 11:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Quatraro A, D’Onofrio F (1985) Endogenous opiates, heroin addiction, and non-insulin-dependent diabetes. Lancet 2:769–770. [DOI] [PubMed] [Google Scholar]
- Goicoechea M, Smith DM, Liu L, May S, Tenorio AR, Ignacio CC, Landay A, Haubrich R (2006) Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis 194:29–37. [DOI] [PubMed] [Google Scholar]
- Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ (2002) Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med 50:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel C, Kutzler M, Rogers TJ (2011) Opioid-induced chemokine expression requires NF-kappaB activity: the role of PKCzeta. J Leukoc Biol 89:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE (2012) Opiate drug use and the pathophysiology of neuroAIDS. Curr HIV Res 10:435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, Nath A, Bruce-Keller AJ, Knapp PE (2005) Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res 8:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Landay AL, Jaworowski A, Crowe SM (2012) HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. Aids 26:843–853. [DOI] [PubMed] [Google Scholar]
- Henderson LJ, Reoma LB, Kovacs JA, Nath A (2020) Advances toward Curing HIV-1 Infection in Tissue Reservoirs. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman OCB E; Gabriel J; Kettelhut A; Kosco JC; Labbato D; Rodgers TO; Smith CA; Funderburg N; McComsey GA (2020) Impact of intravenous heroin and HIV on gut integrity and immune activation. In: Conference on Retroviruses and Opportunistic Infecitons Boston, MA. [Google Scholar]
- Holzer P (2004) Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett 361:192–195. [DOI] [PubMed] [Google Scholar]
- Holzer P (2008) New approaches to the treatment of opioid-induced constipation. Eur Rev Med Pharmacol Sci 12 Suppl 1:119–127. [PMC free article] [PubMed] [Google Scholar]
- Hunt PW (2012) HIV and Inflammation: Mechanisms and Consequences. Curr HIV/AIDS Rep 9:139–147. [DOI] [PubMed] [Google Scholar]
- Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG (2003) T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 187:1534–1543. [DOI] [PubMed] [Google Scholar]
- Jaureguiberry-Bravo M, Lopez L, Berman JW (2018) Frontline Science: Buprenorphine decreases CCL2-mediated migration of CD14(+) CD16(+) monocytes. J Leukoc Biol 104:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis TT, Cleary JP Jr., Gok B, Henderson AJ, Martin NG, Yajima M, Nelson EC, Cheng CS (2020) Single cell transcriptomics reveals opioid usage evokes widespread suppression of antiviral gene program. Nat Commun 11:2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari M, Sohrabi M, Zamani F (2013) The Useage of Opioids and their Adverse Effects in Gastrointestinal Practice: A Review. Middle East J Dig Dis 5:5–16. [PMC free article] [PubMed] [Google Scholar]
- Khoury G, Darcis G, Lee MY, Bouchat S, Van Driessche B, Purcell DFJ, Van Lint C (2018) The Molecular Biology of HIV Latency. Adv Exp Med Biol 1075:187–212. [DOI] [PubMed] [Google Scholar]
- Kirk GD, Mehta SH, Astemborski J, Ritter JD, Laird G, Siliciano R (2020) Nonstructured treatment interruptions contribute to latent HIV-1 reservoir in PWID. In: Conference on Retroviruses and Opportunistic Infections Boston, MA. [Google Scholar]
- Koay WLA, Siems LV, Persaud D (2018) The microbiome and HIV persistence: implications for viral remission and cure. Curr Opin HIV AIDS 13:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A (2004) Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol 78:11425–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bastard Q, Al-Ghalith GA, Gregoire M, Chapelet G, Javaudin F, Dailly E, Batard E, Knights D, Montassier E (2018) Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Aliment Pharmacol Ther 47:332–345. [DOI] [PubMed] [Google Scholar]
- Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, Gripshover B, Salata RA, Taege A, Lisgaris M, McComsey GA, Kirchner E, Baum J, Shive C, Asaad R, Kalayjian RC, Sieg SF, Rodriguez B (2011) Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 204:1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GQ, Orlova-Fink N, Einkauf K, Chowdhury FZ, Sun X, Harrington S, Kuo HH, Hua S, Chen HR, Ouyang Z, Reddy K, Dong K, Ndung’u T, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M (2017) Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J Clin Invest 127:2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrand CR, Paris JJ, Jones AM, Masuda QN, Halquist MS, Kim WK, Knapp PE, Kashuba ADM, Hauser KF, McRae M (2019) HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity. J Neurovirol 25:560–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Merrill JD, Mooney K, Song L, Wang X, Guo CJ, Savani RC, Metzger DS, Douglas SD, Ho WZ (2003) Morphine enhances HIV infection of neonatal macrophages. Pediatr Res 54:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan JA, Rugeles MT, Taborda NA (2019) Contribution of the Microbiota to Intestinal Homeostasis and its Role in the Pathogenesis of HIV-1 Infection. Curr HIV Res 17:13–25. [DOI] [PubMed] [Google Scholar]
- Martin-Kleiner I, Balog T, Gabrilovac J (2006) Signal transduction induced by opioids in immune cells: a review. Neuroimmunomodulation 13:1–7. [DOI] [PubMed] [Google Scholar]
- Martin JL, Koodie L, Krishnan AG, Charboneau R, Barke RA, Roy S (2010) Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am J Pathol 176:786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonye U, Karn J (2017) The Molecular Basis for Human Immunodeficiency Virus Latency. Annu Rev Virol 4:261–285. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A (2010) Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol 67:699–714. [DOI] [PubMed] [Google Scholar]
- McLane VD, Cao L, Willis CL (2014) Morphine increases hippocampal viral load and suppresses frontal lobe CCL5 expression in the LP-BM5 AIDS model. J Neuroimmunol 269:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil AJ (1997) Bayes estimates for immunological progression rates in HIV disease. Stat Med 16:2555–2572. [DOI] [PubMed] [Google Scholar]
- Mehandru S, Deren S, Kang SY, Banfield A, Garg A, Garmon D, LaMar M, Evering TH, Markowitz M (2015) Behavioural, Mucosal and Systemic Immune Parameters in HIV-infected and Uninfected Injection Drug Users. J Addict Res Ther 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerink H, Wisaksana R, Iskandar S, den Heijer M, van der Ven AJ, Alisjahbana B, van Crevel R (2014) Injecting drug use is associated with a more rapid CD4 cell decline among treatment naive HIV-positive patients in Indonesia. J Int AIDS Soc 17:18844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Sindberg GM, Roy S (2015) Disruption of gut homeostasis by opioids accelerates HIV disease progression. Front Microbiol 6:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S (2013) Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 8:e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Banerjee S, Zhang L, Sindberg G, Moidunny S, Li B, Robbins DJ, Girotra M, Segura B, Ramakrishnan S, Roy S (2019) Opioids Impair Intestinal Epithelial Repair in HIV-Infected Humanized Mice. Front Immunol 10:2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, Valcour V, Young MA, Crystal H, Anastos K, Aouizerat BE, Milam J, Maki PM (2013) HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr 63:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Kahler CW, Cioe PA, Tucker L, Monti PM, Mayer KH, Ramratnam B (2016) Alcohol use predicts elevation in inflammatory marker soluble CD14 in men living with HIV. AIDS Care 28:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz E, Albrecht RA, Aden B, Beeder AB, Yuan J, Garcia-Sastre A, Edlin BR, Salvatore M (2016) Active opioid use does not attenuate the humoral responses to inactivated influenza vaccine. Vaccine 34:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Lissner S, Bassotti G, Coffin B, Drewes AM, Breivik H, Eisenberg E, Emmanuel A, Laroche F, Meissner W, Morlion B (2017) Opioid-Induced Constipation and Bowel Dysfunction: A Clinical Guideline. Pain Med 18:1837–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysels DJ, Sullivan MA (2010) The relationship between opioid and sugar intake: review of evidence and clinical applications. J Opioid Manag 6:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanjako D, Ssewanyana I, Mayanja-Kizza H, Kiragga A, Colebunders R, Manabe YC, Nabatanzi R, Kamya MR, Cao H (2011) High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis 11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J, Jacobs DR Jr., Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD (2010) Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Roy S (2013) Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 45:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novati S, Sacchi P, Cima S, Zuccaro V, Columpsi P, Pagani L, Filice G, Bruno R (2015) General issues on microbial translocation in HIV-infected patients. Eur Rev Med Pharmacol Sci 19:866–878. [PubMed] [Google Scholar]
- Palmer CS, Ostrowski M, Gouillou M, Tsai L, Yu D, Zhou J, Henstridge DC, Maisa A, Hearps AC, Lewin SR, Landay A, Jaworowski A, McCune JM, Crowe SM (2014) Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS 28:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro SC, Azzoni L, Joseph J, Fair MG, Sierra-Madero JG, Rassool MS, Sanne I, Montaner LJ (2016) Antiretroviral therapy in HIV-1-infected individuals with CD4 count below 100 cells/mm3 results in differential recovery of monocyte activation. J Leukoc Biol 100:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Sarkar S, Chang SL (2012) Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend 124:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PK, Sharp B, Gekker G, Brummitt C, Keane WF (1987) Opioid-mediated suppression of cultured peripheral blood mononuclear cell respiratory burst activity. J Immunol 138:3907–3912. [PubMed] [Google Scholar]
- Pirastu R, Fais R, Messina M, Bini V, Spiga S, Falconieri D, Diana M (2006) Impaired decision-making in opiate-dependent subjects: effect of pharmacological therapies. Drug Alcohol Depend 83:163–168. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL (2011) The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention CfDCa (2020) HIV Surveillance Report, 2018 (Updated). In: HIV Surveillance Report. [Google Scholar]
- Purohit V, Rapaka RS, Rutter J, Shurtleff D (2012) Do opioids activate latent HIV-1 by down-regulating anti-HIV microRNAs? J Neuroimmune Pharmacol 7:519–523. [DOI] [PubMed] [Google Scholar]
- Rapeli P, Fabritius C, Kalska H, Alho H (2011) Cognitive functioning in opioid-dependent patients treated with buprenorphine, methadone, and other psychoactive medications: stability and correlates. BMC Clin Pharmacol 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapeli P, Fabritius C, Alho H, Salaspuro M, Wahlbeck K, Kalska H (2007) Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon S (2019) The US opioid epidemic is driving a spike in infectious diseases. Nature 571:15–16. [DOI] [PubMed] [Google Scholar]
- Reddy PV, Pilakka-Kanthikeel S, Saxena SK, Saiyed Z, Nair MP (2012) Interactive Effects of Morphine on HIV Infection: Role in HIV-Associated Neurocognitive Disorder. AIDS Res Treat 2012:953678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JL, Law WC, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Mammen MJ, Yong KT, Hui R, Prasad PN, Schwartz SA (2012) Morphine and galectin-1 modulate HIV-1 infection of human monocyte-derived macrophages. J Immunol 188:3757–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MD, Henriquez JE, Blevins LK, Bach A, Crawford RB, Kaminski NE (2020) Targeting Cannabinoid Receptor 2 on Peripheral Leukocytes to Attenuate Inflammatory Mechanisms Implicated in HIV-Associated Neurocognitive Disorder. J Neuroimmune Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DF, Barnes PJ (1989) Opioid inhibition of neurally mediated mucus secretion in human bronchi. Lancet 1:930–932. [DOI] [PubMed] [Google Scholar]
- Ronan MV, Herzig SJ (2016) Hospitalizations Related To Opioid Abuse/Dependence And Associated Serious Infections Increased Sharply, 2002–12. Health Aff (Millwood) 35:832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, Kirchner VA, Koodie L, Ma J, Meng J, Barke RA (2011) Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol 6:442–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote P, Franchi S, Panerai AE (2012) Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 18:6034–6042. [DOI] [PubMed] [Google Scholar]
- Sachdeva N, Asthana V, Brewer TH, Garcia D, Asthana D (2008) Impaired restoration of plasmacytoid dendritic cells in HIV-1-infected patients with poor CD4 T cell reconstitution is associated with decrease in capacity to produce IFN-alpha but not proinflammatory cytokines. J Immunol 181:2887–2897. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Carrigan KA, Ijames SG, Lysle DT (2004) Morphine-induced alterations of immune status are blocked by the dopamine D2-like receptor agonist 7-OH-DPAT. J Neuroimmunol 148:54–62. [DOI] [PubMed] [Google Scholar]
- Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL (2019) Trends in Drug Use-Associated Infective Endocarditis and Heart Valve Surgery, 2007 to 2017: A Study of Statewide Discharge Data. Ann Intern Med 170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Hock B, Kagerer S, Lehnert R, Limmer C, Kuefner H (2005) Less impairment on one portion of a driving-relevant psychomotor battery in buprenorphine-maintained than in methadone-maintained patients: results of a randomized clinical trial. J Clin Psychopharmacol 25:490–493. [DOI] [PubMed] [Google Scholar]
- Soyka M, Limmer C, Lehnert R, Koller G, Martin G, Kufner H, Kagerer S, Haberthur A (2011) A comparison of cognitive function in patients under maintenance treatment with heroin, methadone, or buprenorphine and healthy controls: an open pilot study. Am J Drug Alcohol Abuse 37:497–508. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ (2003) Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology 309:99–107. [DOI] [PubMed] [Google Scholar]
- Stimson GV, Cook C, Bridge J, Rio-Navarro J, Lines R, Barrett D (2010) Three cents a day is not enough: resourcing HIV-related harm reduction on a global basis. In.
- Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR (2011) Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res 1399:96–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazza M, Pirrone V, Wigdahl B, Dampier W, Lin W, Feng R, Maubert ME, Weksler B, Romero IA, Couraud PO, Nonnemacher MR (2016) Prolonged Morphine Exposure Induces Increased Firm Adhesion in an in Vitro Model of the Blood-Brain Barrier. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood ML, Nguyen T, Uebelhoer LS, Kunkel LE, Korthuis PT, Lancioni CL (2020) Altered monocyte phenotype and dysregulated innate cytokine responses among people living with HIV and opioid-use disorder. AIDS 34:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez H, Connick E, Smith KY, Lederman MM, Bosch RJ, Kim RS, St Clair M, Kuritzkes DR, Kessler H, Fox L, Blanchard-Vargas M, Landay A, Team ACTGP (2002) Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. Aids 16:1859–1866. [DOI] [PubMed] [Google Scholar]
- Vallecillo G, Robles MJ, Torrens M, Samos P, Roquer A, Martires PK, Sanvisens A, Muga R, Pedro-Botet J (2018) Metabolic syndrome among individuals with heroin use disorders on methadone therapy: Prevalence, characteristics, and related factors. Subst Abus 39:46–51. [DOI] [PubMed] [Google Scholar]
- Visconti AJ, Sell J, Greenblatt AD (2019) Primary Care for Persons Who Inject Drugs. Am Fam Physician 99:109–116. [PubMed] [Google Scholar]
- Volkow ND, Montaner J (2011) The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Aff (Millwood) 30:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C, Kane AV (2014) Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J Stud Alcohol Drugs 75:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Roy S (2017) Gut Homeostasis, Microbial Dysbiosis, and Opioids. Toxicol Pathol 45:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Loh HH, Roy S (2003) Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem 278:37622–37631. [DOI] [PubMed] [Google Scholar]
- Wang MR, Wu DD, Luo F, Zhong CJ, Wang X, Zhu N, Wu YJ, Hu HT, Feng Y, Wang X, Xiong HR, Hou W (2020) Methadone Inhibits Viral Restriction Factors and Facilitates HIV Infection in Macrophages. Front Immunol 11:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ (2011) Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol 178:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma TC, Li JL, Zhou Y, Geller EB, Adler MW, Peng JS, Zhou W, Zhou DJ, Ho WZ (2015) Heroin inhibits HIV-restriction miRNAs and enhances HIV infection of macrophages. Front Microbiol 6:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Ye L, Li J, Song L, Fulambarkar N, Ho W (2012) Morphine suppresses IFN signaling pathway and enhances AIDS virus infection. PLoS One 7:e31167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner LD, Retuerto M, Hager CL, El Kamari V, Shan L, Sattar A, Kulkarni M, Funderburg N, Ghannoum MA, Dirajlal-Fargo S, McComsey GA (2019) Fungal Translocation Is Associated with Immune Activation and Systemic Inflammation in Treated HIV. AIDS Res Hum Retroviruses 35:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese AD, Griffin MR, Schaffner W, Stein CM, Greevy RA, Mitchel EF, Grijalva CG (2019) Long-acting Opioid Use and the Risk of Serious Infections: A Retrospective Cohort Study. Clin Infect Dis 68:1862–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif S, Saubamea B, Cisternino S, Marie-Claire C, Dauchy S, Scherrmann JM, Decleves X (2008) Effect of chronic exposure to morphine on the rat blood-brain barrier: focus on the P-glycoprotein. J Neurochem 107:647–657. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF, O’Connor M, Roizen MF, Moss J (1998) Effects of low-dose morphine on gastric emptying in healthy volunteers. J Clin Pharmacol 38:1017–1020. [DOI] [PubMed] [Google Scholar]
- Zaki NG, Osman A, Moustafa H, Saad AH (2006) Alterations of immune functions in heroin addicts. Egypt J Immunol 13:153–171. [PubMed] [Google Scholar]
- Zevin AS, McKinnon L, Burgener A, Klatt NR (2016) Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS 11:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EY, Xiong J, Parker BL, Chen AY, Fields PE, Ma X, Qiu J, Yankee TM (2011) Depletion and recovery of lymphoid subsets following morphine administration. Br J Pharmacol 164:1829–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YH, Jeang KT, Tokunaga K (2012) Host restriction factors in retroviral infection: promises in virus-host interaction. Retrovirology 9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JW, Liu FL, Mu D, Deng DY, Zheng YT (2016) Increased expression and dysregulated association of restriction factors and type I interferon in HIV, HCV mono- and co-infected patients. J Med Virol 88:987–995. [DOI] [PubMed] [Google Scholar]
- Zhu JW, Liu FL, Mu D, Deng DY, Zheng YT (2017) Heroin use is associated with lower levels of restriction factors and type I interferon expression and facilitates HIV-1 replication. Microbes Infect 19:288–294. [DOI] [PubMed] [Google Scholar]


