The neurovascular unit (NVU) is an essential anatomical and physiological entity for the maintenance of brain homeostasis and function; it is composed of endothelial cells, pericytes, astrocytes, neurons, vascular smooth muscle cells, microglia, and oligodendroglia [1]. The NVU regulates important brain vessel properties such as the integrity of the blood-brain barrier (BBB) and cerebral blood flow (CBF). Considerable efforts have been made to investigate the cerebral microcirculatory dysfunction in ageing and Alzheimer’s disease (AD) [2].
Pericytes are perivascular mural cells that wrap processes around and along brain capillaries. They have come into focus in recent years for their regulatory roles in cerebrovascular integrity and CBF [3, 4]. Dysfunction or ablation of pericytes results in a series of pathological changes, including reduced pericyte coverage of the capillary wall, capillary constriction, BBB leakage, white matter dysfunction, synaptic loss, neuronal degeneration, and cognitive deficits [3–5]. Pericyte degeneration leads to metabolic stress, neurovascular uncoupling, and impaired neuronal excitability [6]. Moreover, pericyte loss reduces the clearance of Aβ peptides from brain interstitial fluid and accelerates amyloid angiopathy and cerebral amyloidosis in mouse models of AD [7].
Although there is increasing evidence of a tight relationship between pericytes and neurovascular endothelial cells in BBB function, the regulatory roles of pericytes in AD and other neurodegenerative diseases remained largely unknown. Recently, two studies have revealed the emerging links between pericytes, NVUs, and neurodegeneration, in which pericytes are involved in oligomeric Aβ-induced cerebral vascular constriction and CBF reduction, and pericyte-derived pleiotrophin (PTN) is identified as a neurotrophic factor required for neuronal survival and cognitive functions [8, 9].
Using two-photon in vivo microscopy and acute brain slices to directly visualize and measure vascular changes in the brain, Attwell and colleagues from University College London have demonstrated that oligomeric Aβ alters CBF and neurovascular coupling at the very early stages of AD progression [8]. They measured the diameters of cerebral capillaries near the pericyte soma in humans and mice that had shown the pathological hallmark of AD (Aβ deposition). Their results revealed the surprising fact that, in human patients depositing Aβ and tau, the cerebral capillaries were constricted specifically at pericyte locations. In biopsies of living AD patients’ brains, the capillary diameters at pericyte locations were significantly smaller than at a distance from the pericyte soma. Moreover, increased accumulation of Aβ was associated with greater constriction of the capillaries. The same constriction was found in a mouse model of AD, in which capillaries in the cerebellum, which lacks Aβ deposition, were not constricted, suggesting that Aβ may trigger the constriction. This constriction was predicted to reduce CBF by ~50% in the AD mice, similar to the 42% decrease in the gray matter of AD patients.
To determine whether Aβ is mechanistically associated with this phenomenon, live cortical slices, resected from glioma patients who underwent surgery to remove tumors, were used to study the acute effects of Aβ. When oligomeric Aβ (a soluble species of Aβ found in AD brains that correlates better with cognitive decline than amyloid plaques) was applied to the capillaries in these slices, it evoked constriction (based on measurement of the capillary diameter near the pericyte soma), while arterioles and venules were unchanged in diameter. In a manner similar to the human brain slices, both oligomeric Aβ1–40 and Aβ1–42 evoked capillary constriction in rat brain slices after 1 h of exposure.
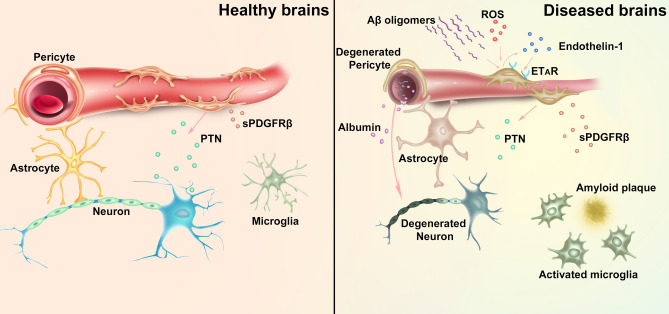
The authors went further to explore the mechanisms of how Aβ oligomers interact with pericytes to constrict cerebral capillaries. They found that pericytes, but not resident microglia or perivascular macrophages, were the main cell type that generated reactive oxygen species (ROS) after Aβ stimulation (Fig. 1). Endothelin-1, which was released after ROS stimulation, perhaps from the pericytes, acted on ETA receptors to induce pericyte constriction. Aβ-evoked pericyte constriction can be halted by blocking NADPH oxidase 4 (which is expressed in pericytes) as well as by blocking ETA receptors, or reversed by applying the vasodilator C-type natriuretic peptide, which blocks Ca2+ release from internal stores in pericytes and activates myosin light chain phosphatase, thus inhibiting constriction of capillaries by these cells.
Fig. 1.
Schematic of the roles of pericytes in neurovascular interactions in healthy and diseased brains. Left: in the healthy brain, pericytes maintain the integrity of the blood-brain barrier (BBB) and regulate cerebral blood flow. Pericytes secrete pleiotrophin (PTN) to support neuronal growth. Right: in Alzheimer’s disease, Aβ oligomers stimulate ROS generation and endothelin-1 release (possibly from pericytes), which in turn activates pericyte ETA receptors (ETAR), leading to capillary constriction and cerebral blood flow reduction. Degeneration of pericytes causes BBB breakdown, plasma leakage, and deprivation of PTN, which result in neurodegeneration. An increase of pericyte-derived soluble PDGFRβ (sPDGFRβ) in the CSF is also associated with early cognitive impairment and BBB breakdown.
In addition to capillary constriction, Zlokovic and colleagues from the University of Southern California have reported an unexpected but critical role of pericytes in regulating neuronal survival and cognitive function. Notably, they generated a pericyte-specific, double-Cre driver system, which used control by the Pdgfrb and Cspg4 promoters, two genes that encode the pericyte-expressing proteins PDGFRβ and NG2 [9]. After cross-breeding with a Cre-dependent tdTomato reporter mouse line, this triple transgenic mouse model expresses inducible fluorescent protein tdTomato only in capillary pericytes and not in smooth muscle cells on the arterioles. The same double-Cre driver system was also used to generate pericyte-specific iDTR transgenic mice to specifically ablate nearly 60% of the CD13-positive pericytes in the adult mouse brain at 3 and 15 days after diphtheria toxin injection. Reduced CBF and decreased capillary diameter, followed by tissue hypoxia and edema, were found in the pericyte-ablated mice, and loss of pericytes also led to pronounced BBB breakdown, reduced tight junctions, and increased plasma protein leakage [9].
Accelerated loss of neurons and cognitive deficits were also found after pericyte ablation. A loss of 20%–25% of NeuN-positive neurons and 35%–40% of SMI312-positive neuritic density occurred in the cortex and hippocampus of these pericyte-ablated mice. The neuronal loss was attributed to both BBB breakdown and a lack of pericyte-derived PTN, a neurotrophic factor that is only expressed in pericytes and not in other cell types in the brain [Fig. 1]. Pericyte ablation led to a >60% reduction of PTN protein in brain capillaries and ~65% reduction of PTN in the cerebrospinal fluid (CSF) at day 15 after diphtheria toxin injection. A loss of function study of PTN by siRNA silencing in mice with intact pericyte coverage revealed neuronal vulnerability to circulatory stress. Conversely, continuous infusion of PTN into the cerebral ventricles rescued the neuronal loss and attenuated the cognitive impairment in pericyte-ablated mice, implying a critical regulatory role of pericyte-secreted PTN in cognitive function in healthy and diseased brains.
Another recent work by Zlokovic and colleagues also revealed an elevated level of soluble PDGFRβ (sPDGFRβ) in the CSF of patients with early cognitive dysfunction. Changes in the sPDGFRβ level correlated with increased BBB dysfunction, independent of changes in biomarkers like Aβ and/or hyper-phosphorylated tau protein [10], indicating that sPDGFRβ could be an early biomarker for detecting BBB breakdown and human cognitive dysfunction independent of Aβ and tau accumulation.
Together, these findings highlight the novel roles of pericytes in the physiological and pathological states of the NVU. In brains affected by AD, Aβ oligomers can induce capillary constriction through pericytes and result in a long-term reduction of CBF. In the case of pericyte loss, as in the brains of AD patients or genetic ablation in mouse models, pericyte degeneration results in neurovascular uncoupling and BBB breakdown. Reduction of pericyte-derived PTN also results in neuronal loss and cognitive impairment. These new findings on pericytes in pathological conditions reaffirm their critical roles in the context of neurodegenerative disease.
Future studies in the search for disease-associated pericyte biomarkers and elucidation of the complicated properties of pericytes regarding the crosstalk of NVUs in healthy and diseased brains should provide more information on the pathological roles played by pericytes. Identifying novel pericyte biomarkers associated with AD may shed new light on potential therapeutic targets to restore the function of NVUs, as indicated by recent work that shows a high correlation of increased pericyte-derived sPDGFRβ in the CSF of patients with BBB leakage and early cognitive impairment [10]. Single-cell transcriptome analysis may also provide novel insight into the heterogeneous properties of pericytes at different stages of disease progression and the mechanistic understanding of neurovascular uncoupling in neurodegenerative diseases.
Acknowledgements
This highlight was supported by grants from the National Key R&D Program of China (2017YFC1307500 and 2017YFC1307504), the National Natural Science Foundation of China (81925031, 81972967 and 81820108026), and Science and Technology Program of Guangzhou Municipality, China (202007030001).
Conflict of interest
The authors declare no potential conflicts of interest.
References
- 1.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun BL, Li WW, Zhu C, Jin WS, Zeng F, Liu YH, et al. Clinical research on Alzheimer’s disease: Progress and perspectives. Neurosci Bull. 2018;34:1111–1118. doi: 10.1007/s12264-018-0249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24:326–337. doi: 10.1038/nm.4482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20:406–416. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 2019, 365: eaav9518. [DOI] [PMC free article] [PubMed]
- 9.Nikolakopoulou AM, Montagne A, Kisler K, Dai Z, Wang Y, Huuskonen MT, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22:1089–1098. doi: 10.1038/s41593-019-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]