Dear Editor,
The pathophysiological mechanisms underlying mood disorders including major depressive disorder (MDD) remain to be fully characterized. Iron is a key component in the development of the central nervous system and iron deficiency has been linked to impairments of mood and cognition [1]. Recent studies have reported an inverse association between iron uptake and the risk of depression, as MDD patients have a reduced serum concentration of iron [2]. Moreover, few investigations have monitored the symptoms of MDD patients with iron supplementation. Evidence for effects of increased iron on cognitive disorders is scarce, although treatment of small group of psychiatric patients with an iron chelator apparently led to clinical improvement [3].
The function of the brain is to a large extent defined by blood circulation and the movements of cerebrospinal and interstitial fluids (CSF and ISF). The glymphatic system is responsible for the brain-wide clearance of waste protein through a paravascular pathway and CSF–ISF exchange [4]. Operation of the glymphatic system is supported by perivascular astroglial endfeet [5]. Our previous studies demonstrated that chronic unpredictable mild stress (CUMS), a well-established model for inducing depressive-like phenotypes in rodents, partially inhibits the glymphatic system, impairing the clearance of endogenous and exogenous amyloid beta-42 from the cortex and hippocampus [6]. Exposure to CUMS does not affect motor functions, learning, or memory in mice [6]. However, it remains unknown whether iron overload impairs the operation of the glymphatic system.
It is well known that iron is a microelement essential for body growth and development and is also required for cellular metabolism. Iron can cross the blood-brain barrier (BBB) and binds to the transferrin receptors (TFRs) of endothelial cells through transferrin [7]. Iron is released into the brain parenchyma from vascular endothelial cells and is taken up by astrocytes and neurons [8]. Facing excessive iron concentrations, neurons up-regulate the expression of TFRs and ferroportin, the latter exporting iron back to the extracellular space [9]. However, if the iron accumulation exceeds the neuronal metabolic capacity for protein storage, neurotoxicity and death are induced [8]. Little is known about the expression of both proteins in MDD patients or in mouse models of depression, hence we investigated how iron affects depressive phenotypes and the operation of the glymphatic system in the CUMS mouse model.
First, we found that iron overload aggravated the circulatory disturbance of the glymphatic system induced by CUMS. Experimental animals were divided into four groups (i) controls; (ii) iron-overloaded (Iron); (iii) exposed to CUMS (CUMS); and (iv) exposed to CUMS and iron overload (CUMS + Iron). An excessive iron load was induced by intraperitoneal injection of iron dextran for 6 days (see supplementary methods). The accumulation of iron in the brain tissue was measured by Perl’s staining. As shown in Fig. S1A–E, compared to the control group, the staining ratios of Perl’s-positive iron deposits in the Iron group were increased to 545.43% ± 54.00% (P < 0.001, n = 6) in the cortex and 462.56% ± 68.27% (P < 0.001, n = 6) in the hippocampus; in the CUMS + Iron group, the staining ratios were increased to 1310.54% ± 108.93% (P < 0.001, n = 6) in cortex and 980.21% ± 161.97% (P < 0.001, n = 6) in the hippocampus. We found no iron deposits in mice exposed to CUMS alone.
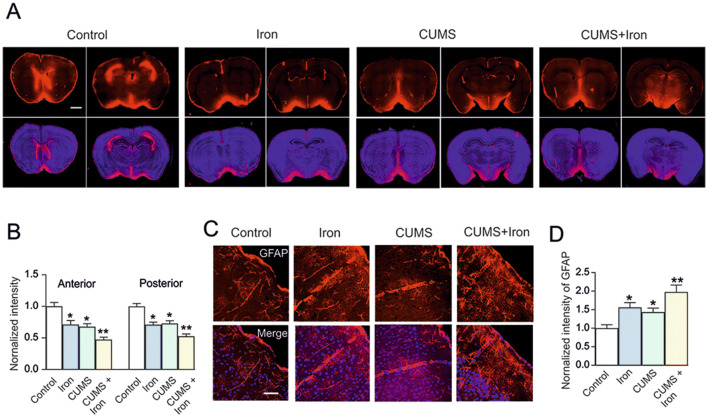
The activity of the glymphatic system was assessed by measuring the penetration of the red fluorescent tracer, OA555 (Fig. 1A). Compared with anterior slices from the control group, the penetration of OA555 was decreased to 71.33% ± 6.17% (P = 0.001, n = 6) in the Iron group, to 67.83% ± 4.79% (P < 0.001, n = 6) in the CUMS group, and to 47.33% ± 3.69% (P < 0.001, n = 6) in the CUMS + Iron group (Fig. 1B). Compared with posterior slices from the control group, the penetration of OA555 was decreased to 71.12% ± 3.80% (P < 0.001, n = 6) in the Iron group, to 72.89% ± 4.31% (P < 0.001, n = 6) in the CUMS group, and to 52.70% ± 3.49% (P < 0.001, n = 6) in the CUMS + Iron group (Fig. 1B). Furthermore, the penetration of tracer in the CUMS + Iron group was significantly different from both the Iron and CUMS groups (Fig. 1B).
Fig. 1.
Excess iron aggravates malfunction of the glymphatic system in mice exposed to CUMS. A, B Mice with or without exposure to CUMS for 6 weeks, in the last week were randomly selected to be injected with dextran or iron dextran for 6 days, then a fluorescent tracer (OA555, 45 kDa) was injected intracisternally. A Representative images showing penetration of the fluorescence tracer into the brain; OA555 (red) and DAPI (blue) are imaged simultaneously in anterior and posterior slices (scale bar, 1 mm). B Thirty minutes after injection, the animals were perfusion-fixed and the whole-slice fluorescence was calculated. The fluorescence intensities of OA555 normalized to the intensity of the control group, were assessed in anterior and posterior slices. C Immunocytochemistry of GFAP (red) and staining with DAPI (blue) in cortex (scale bar, 50 μm). D GFAP immunolabeling intensity normalized to the intensity in the control group. Data are presented as the mean ± SEM, n = 6. *P < 0.05 versus controls; **P < 0.05 vs any other group.
Cortical astrocytic morphology was visualized by immunostaining with antibodies against glial fibrillary acidic protein (GFAP) (Fig. 1C). Compared with controls, iron increased the fluorescence intensity of GFAP to 156.29% ± 12.58% (P = 0.007, n = 6), CUMS enhanced it to 143.33% ± 10.78% (P = 0.031, n = 6), and in the CUMS + Iron group it was elevated to 198.03% ± 18.23% (P < 0.001, n = 6) (Fig. 1D). The fluorescence intensity of GFAP in the CUMS + iron group was significantly different from both the Iron and the CUMS groups (Fig. 1D).
Subseqeunetly, we found that CUMS increased the expression of TFRs induced by iron overload (Fig. 2). We assessed the expression of TFRs in the cortex and hippocampus by immunocytochemistry (Fig. 2). In the cortex, compared to controls, the fluorescence intensity of TFR in the Iron group was increased to 159.93% ± 16.41% (P = 0.009, n = 6), and in the CUMS + Iron group it was enhanced to 216.03% ± 15.89% (P < 0.001, n = 6; Fig. 2C). Similar changes were found in the hippocampus: in the Iron group the TFR intensity was increased to 256.36% ± 14.81% (P < 0.001, n = 6), while in the CUMS + Iron group it was increased to 365.09% ± 30.76% (P < 0.001, n = 6; Fig. 2C). There was also a significant difference in TFR levels between the Iron and CUMS + Iron groups, both in cortex and hippocampus. However, exposure to CUMS alone did not change the intensity of TFR staining (Fig. 2C).
Fig. 2.

Exposure to CUMS promotes neuronal expression of TFR stimulated by iron overload. A, B Representative images of immunohistochemistry of TFR (red) stained with MAP2 (cyan), GFAP (green), and DAPI (blue) in cortex (A) and hippocampus (B) (mice with or without exposure to CUMS for 6 weeks, in the last week were randomly selected to be injected with dextran or iron dextran for 6 days). C TFR immunolabeling intensity normalized to the intensity in the control group in cortex and hippocampus (scale bar, 50 μm). Data are presented as the mean ± SEM, n = 6. *P < 0.05 vs control; **P < 0.05 vs any other group.
Meanwhile, CUMS increased the neuronal apoptosis induced by iron overload. The neuronal apoptosis in cortex and hippocampus was visualized with TUNEL assays (Fig. S2A, C). Compared with the control group, in the Iron group the neuronal apoptosis was increased to 169.25% ± 23.58% (P = 0.013, n = 6) in cortex and to 173.82% ± 12.14% (P = 0.003, n = 6) in the hippocampus, and in the CUMS + Iron group it increased to 222.66% ± 21.12% (P < 0.001, n = 6) in cortex and to 232.08% ± 24.57% (P < 0.001, n = 6) in the hippocampus (Fig. S2B, D). There was a significant difference in the apoptosis ratio between the Iron and CUMS + Iron groups (Fig. S2B, D). However, exposure to CUMS alone did not affect the apoptosis (Fig. S2B, D).
To further investigate the effects of iron overload on the behavioral phenotype of mice subjected to chronic stress, we ran depressive, locomotor, and cognitive behavioral tests. In the sucrose preference test, iron overload reduced the ratio of sucrose intake to 91.73% ± 3.01% of control (P = 0.154, n = 6), but the difference was not significant (Fig. S3A). In the CUMS group, the preference was decreased to 72.80% ± 4.52% (P < 0.001, n = 6), and in the CUMS + Iron group, it was reduced to 56.72% ± 2.57% (P < 0.001, n = 6) (Fig. S3A). Sucrose preference was significantly different (P = 0.009; n = 6) between the CUMS and CUMS + Iron groups. In the tail-suspension test (TST) and forced-swimming test (FST), the behavioral manifestations demonstrated similar changes. Compared with the control group, iron overload insignificantly increased the immobility time to 154.07% ± 28.23% (P = 0.251, n = 6) in the TST (Fig. S3B) and to 116.79% ± 12.13% (P = 0.354, n = 6) in the FST (Fig. S3C). In the CUMS group the immobility time increased to 212.26% ± 22.76% (P = 0.023, n = 6) in the TST (Fig. S3B) and to 144.61% ± 6.28% (P = 0.020, n = 6) in the FST (Fig. S3C). In the CUMS + Iron group the immobility time was further increased to 331.31% ± 59.32% (P < 0.001, n = 6) in the TST (Fig. S3B) and to 184.60% ± 8.25% (P < 0.001, n = 6) in the FST (Fig. S3C). Again, the difference in immobility time in both the TST and FST between the CUMS and CUMS + Iron groups was significant (P = 0.024, n = 6 and P = 0.035, n = 6, respectively).
In the pole test, exposure to CUMS did not change the movement time from the pole top to the floor (T-LA time) compared to the control group (Fig. S3D). In the Iron group however, the T-LA time was significantly increased to 142.87% ± 9.37% of the control (P = 0.025, n = 6), and in the CUMS + Iron group, was further increased to 224.51% ± 30.81% (P < 0.001, n = 6; Fig. S3D).
In the Morris water maze test, the time spent in the target quadrant and the escape latency were measured. Compared with the control group, exposure to CUMS did not affect these times. In the Iron group, the time spent in the target quadrant was decreased to 78.18% ± 7.08% of control (P = 0.031, n = 6; Fig. S3E) and the escape latency was increased to 128.69% ± 10.20% of control (P = 0.033, n = 6; Fig. S3F). In the CUMS + Iron group the time spent in the target quadrant was decreased to 56.11% ± 4.63% (P < 0.001, n = 6; Fig. S3E) while the escape latency was increased to 170.62% ± 10.55% (P < 0.001, n = 6; Fig. S3F). The times in the CUMS + Iron group were also significantly different from those in the Iron group (P = 0.039, n = 6 and P = 0.004, n = 6; Fig. S3E, F).
According to the above results, the excessive accumulation of iron in the brains of mice inhibits the functional activity of the glymphatic system. In this respect, the action of iron was similar to CUMS, which also triggers malfunction of the glymphatic system [11]. When CUMS was paired with iron overload, glymphatic function was suppressed even further, which arguably compromised the clearance of iron from the brain parenchyma. In response to iron overload, the expression of TFRs in neurons was up-regulated, and CUMS additionally enhanced TFR expression. At the same time, iron overload increased neuronal apoptosis, and this was increased even further by exposure to CUMS. At the behavioral level, iron overload exacerbated the depressive-like phenotype of stressed mice, and added motor and cognitive impairments that do not occur in mice exposed to CUMS alone.
Operation of the glymphatic system is supported by astrocytes, the principal homoeostatic cells of the central nervous system [5, 10]. In particular, polarized expression of the water channel aquaporin 4 (AQP4) in astroglial perivascular endfeet is required for the normal function of the glymphatic system [5, 6, 10]. We found that accumulation of iron in the brain parenchyma triggered reactive astrogliosis in the cortex and hippocampus, as indicated by an increase in GFAP expression. Again, pairing iron overload with CUMS further increased GFAP expression, suggesting stronger reactivity of astrocytes.
Excess iron uptake can induce cellular death and this phenomenon is always considered to be a risk for neurodegenerative diseases, such as Alzheimer’s disease or Parkinson’s disease [11]. The accumulation of iron is reported to be associated with up-regulation of ferritin expression in the substantia nigra of aged animals [12]. The level of iron transport proteins holds promise as markers of MDD, especially in patients with suicidal tendencies [13]. In this study, we found that the pairing of iron with CUMS was accompanied by an elevation of TFR expression in neurons, as well as by increased neuronal apoptosis arguably induced by iron ion (Fe2+/Fe3+) dyshomeostasis. In consequence, Fe3+ transport into neurons could be increased through up-regulated TFRs. However, exposure to CUMS alone did not affect the neuronal levels of TFRs, neither did it influence neuronal apoptosis. At the same time, iron accumulation paired with chronic stress promoted new pathological phenotypes manifested by motor and cognitive impairments that did not occur in mice exposed to CUMS alone. In addition, the depressive-like behaviors and/or anhedonia induced by CUMS were significantly exacerbated by iron overload. In the clinical setting, not all MDD patients have cognitive or motor deficits [14], although depressive disorders are risk factors for the occurrence of neurodegenerative diseases [15]. Under exposure to CUMS, the circulatory functions of the AQP4-dependent glymphatic system are impaired and this is paralleled with anhedonia and depressive behaviours [6]. In addition, mice exposed to CUMS show decreased efficiency of exchange between the subarachnoid CSF and brain parenchyma, and the circulation of a paravascular fluorescent tracer is suppressed in anhedonic mice [6].
Our results demonstrate that excess iron aggravates malfunction of the glymphatic system, thus exacerbating the depressive and cognitive behavioral phenotypes induced by chronic stress. Increased iron intake could add to the pathophysiological evolution of MDD and hence we suggest that excess iron intake has to be considered as an additional risk factor for cognitive impairment in patients with depression. Meanwhile, iron chelation may be a potential therapeutic strategy for MDD patients with high serum iron.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81871852, 81200935, 81671867 and 81971794), the Liaoning Revitalization Talents Program (XLYC1807137), the Scientific Research Foundation for Returned Scholars of the Ministry of Education of China (20151098), and the Natural Science Foundation of Liaoning Province, China (20170541030).
Conflict of interest
The authors have no conflicts of interest to disclose.
Footnotes
Shanshan Liang and Yan Lu have contributed equally to this work.
Contributor Information
Alexei Verkhratsky, Email: Alexej.Verkhratsky@manchester.ac.uk.
Xu Wu, Email: xwu@cmu.edu.cn.
Baoman Li, Email: bmli@cmu.edu.cn.
References
- 1.Lomagno KA, Hu F, Riddell LJ, Booth AO, Szymlek-Gay EA, Nowson CA, et al. Increasing iron and zinc in pre-menopausal women and its effects on mood and cognition: a systematic review. Nutrients. 2014;6:5117–5141. doi: 10.3390/nu6115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam MR, Islam MR, Shalahuddin Qusar MMA, Islam MS, Kabir MH, Mustafizur Rahman GKM, et al. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: a case–control study. BMC Psychiatry. 2018;18:94. doi: 10.1186/s12888-018-1685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutler P. Iron overload and psychiatric illness. Can J Psychiatry. 1994;39:8–11. doi: 10.1177/070674379403900104. [DOI] [PubMed] [Google Scholar]
- 4.Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44:S93–S95. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia M, Yang L, Sun G, Qi S, Li B. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. Psychopharmacology (Berl) 2017;234:365–379. doi: 10.1007/s00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- 7.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 8.Leitner DF, Connor JR. Functional roles of transferrin in the brain. Biochim Biophys Acta. 2012;1820:393–402. doi: 10.1016/j.bbagen.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Boserup MW, Lichota J, Haile D, Moos T. Heterogenous distribution of ferroportin-containing neurons in mouse brain. Biometals. 2011;24:357–375. doi: 10.1007/s10534-010-9405-2. [DOI] [PubMed] [Google Scholar]
- 10.Nedergaard M. Garbage truck of the brain Science. Neuroscience. 2013;340:1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song N, Xie J. Iron, dopamine, and α-synuclein interactions in at-risk dopaminergic neurons in Parkinson’s disease. Neurosci Bull. 2018;34:382–384. doi: 10.1007/s12264-018-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker T, Michaelides C, Ekonomou A, Geraki K, Parkes HG, Suessmilch M, et al. Dissociation between iron accumulation and ferritin upregulation in the aged substantia nigra: attenuation by dietary restriction. Aging (Albany NY) 2016;8:2488–2508. doi: 10.18632/aging.101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean B, Tsatsanis A, Lam LQ, Scarr E, Duce JA. Changes in cortical protein markers of iron transport with gender, major depressive disorder and suicide. World J Biol Psychiatry. 2020;21:119–126. doi: 10.1080/15622975.2018.1555377. [DOI] [PubMed] [Google Scholar]
- 14.Knight MJ, Lyrtzis E, Baune BT. The association of cognitive deficits with mental and physical quality of life in major depressive disorder. Compr Psychiatry. 2019;97:152147. doi: 10.1016/j.comppsych.2019.152147. [DOI] [PubMed] [Google Scholar]
- 15.Miebach L, Wolfsgruber S, Frommann I, Fließbach K, Jessen F, Buckley R, et al. Cognitive complaints in memory clinic patients and in depressive patients: an interpretative phenomenological analysis. Gerontologist. 2019;59:290–302. doi: 10.1093/geront/gnx208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.