Dear Editor,
Brain networks consist of several long-distance regions that interact constantly with each other. Interactions between networks suggest that different brain functions are coordinated during complex cognitive tasks [1]. A triple-network mechanism underlying cognitive control has been found across task paradigms and stimulus modalities [2]. This triple-network mechanism includes the interactions among the salience network (SN), the default mode network (DMN), and the central executive network (CEN). The core regions of the SN include the anterior insula (AI), anterior cingulate cortex, and thalamus [3]; those of the DMN are the medial prefrontal cortex, posterior cingulate cortex, and lateral parietal cortex [3]; and for the CEN, the dorsolateral prefrontal cortex, inferior parietal cortex, and precuneus are the key nodes [3]. Available evidence has shown increased activation in the CEN and SN, and decreased activation in the DMN during cognitive tasks [1, 2]. Furthermore, Sridharan et al. demonstrated that the SN initiates control signals to activate the CEN and deactivate the DMN during the resting state, providing a view of the network mechanism underlying endogenous cognitive control [1].
The network mechanism may vary under different conditions. For example, it can be influenced by important baseline conditions, such as the eyes-open (EO) and eyes-closed (EC) conditions. Riedl et al. and Xu et al. found that the SN exhibits increased glucose metabolism and altered functional connectivity of the blood oxygenation level-dependent (BOLD) signal with other areas during EO compared to EC [4, 5]. Moreover, the functional connectivity of the BOLD signal between the DMN and parts of both the SN and the CEN increases during EO [6]. However, how the triple-network mechanism is impacted by EO and EC has not been thoroughly investigated. The importance of the EO and EC conditions can be ascribed to their deep involvement in psychological experiments and psychiatric disorder studies [7]. They are commonly used as baseline conditions requiring participants to perform any cognitive task, but their spontaneous BOLD signals show distinct activity patterns. Therefore, in order to get a deeper understanding of the network mechanism underlying endogenous cognitive control, and help establish proper baseline conditions for future neuroscience experiments, it is necessary to clarify the influence of EC/EO on the three networks and their interactions.
Unlike electrophysiological methods, resting-state functional magnetic resonance imaging (fMRI) has the capacity to measure the neuronal activity in networks of interest in deep brain regions with high spatial resolution. Moreover, resting-state fMRI can capture stable coupling between networks with a sufficiently long segment of data. Therefore, based on resting-state fMRI data, we were able to detect reliable network-wise changes in correlation between EC and EO using functional connectivity (FC) analysis.
Besides FC analysis, dynamic causal modeling (DCM), a nonlinear system identification procedure, is a useful tool for inferring how the coupling among areas is influenced by experimental inputs [8], such as EC and EO. DCM is able to infer hidden neuronal activity from the recorded BOLD signal with posterior estimates of neurobiological features, such as the connection strength of synapses among neuronal populations and modulation by experimental inputs [8]. Thus, DCM basically estimates the parameters of effective connectivity at the neuronal level, which may reduce the influence of the slow temporal resolution of fMRI signals, and was used to investigate the modulation of the network mechanism by EC and EO in our experiments.
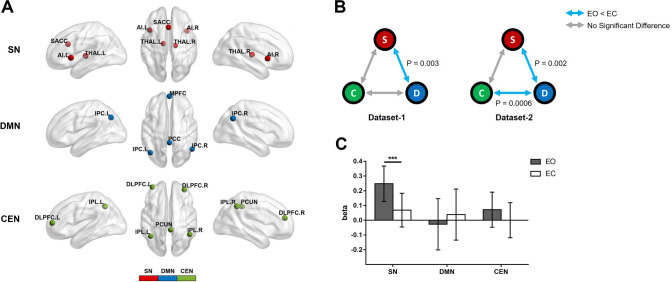
The objectives of this study were to: (1) determine the differences in the correlations among the three networks (SN, DMN, and CEN) between EC and EO; and (2) investigate how the causality between these three networks is influenced by EC and EO. In the first experiment, 22 healthy participants (12 males; average age, 20.31 years) were recruited, and their resting-state fMRI data were obtained under EO and EC conditions (resting-state dataset 1). Adopting the regions of interest (ROIs) from a previous study, the ROI-based FCs were calculated (Fig. 1A) [3]. We compared the differences of FC among the three networks between EC and EO in this dataset, and examined the change in the FC pattern in an open resting-state dataset (resting-state dataset 2, http://fcon_1000.projects.nitrc.org/indi/retro/BeijingEOEC.html) (details in supplementary material). Paired t-testing of resting-state dataset 1 showed decreased FC between the SN and the DMN during EO compared to EC (P < 0.005 Bonferroni-corrected), while that of resting-state dataset 2 suggested reduced FC in two pairs of networks: SN–DMN (P < 0.005, Bonferroni-corrected) and DMN–CEN (P < 0.001, Bonferroni-corrected) (Fig. 1B). There was no significant difference in FC between the SN and the CEN during EO and EC in the two datasets (Fig. 1B). These results were replicated with network components extracted by spatially-constrained independent component analysis (Fig. S3).
Fig. 1.
Difference of functional connectivity (FC) and beta value in the three networks between eyes open (EO) and eyes closed (EC). A Plots of the three networks rendered on the cortical surface [red , regions specific to the salience network (SN); blue , regions specific to the default mode network (DMN); green, regions specific to the central executive network (CEN); anterior insula (AI), subgenual anterior cingulate cortex (SACC), thalamus (THAL), medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), lateral parietal cortex (LPC), dorsolateral prefrontal cortex (DLPFC), inferior parietal cortex (IPL), precuneus (PCUN); L/R, left/right]. B FC differences among the three networks in the two resting datasets (color coding as in A; S, SN; D, DMN; C, CEN; blue arrows, significantly decreased FC during EO versus EC; gray arrows, no significant difference). C Paired t-test results of the beta value comparisons between EO and EC within each network in block-design experiments (***P < 0.001).
To further explore the causality changes among the three networks from EC to EO, 32 healthy participants (24 males; average age, 28.09 years) were enrolled. Each participant attended one fMRI run, which included 17 EC conditions (duration, 16 s) and 16 EO conditions (duration, 12 s). In order to find out which network was the input network modulated by the driving inputs (EC and EO) for the subsequent DCM analysis, we first estimated the coefficients (beta values) of BOLD signals for the EC and EO conditions in each network using a general linear model, and compared the beta values of the networks between the two conditions using the paired t-test (details in supplementary material). The result showed significant BOLD signal changes in the SN between the two conditions (P < 0.001, Bonferroni-corrected), while no significant difference was found in the other two networks (Fig. 1C). These results suggested that the SN would be the input network in the subsequent DCM model.
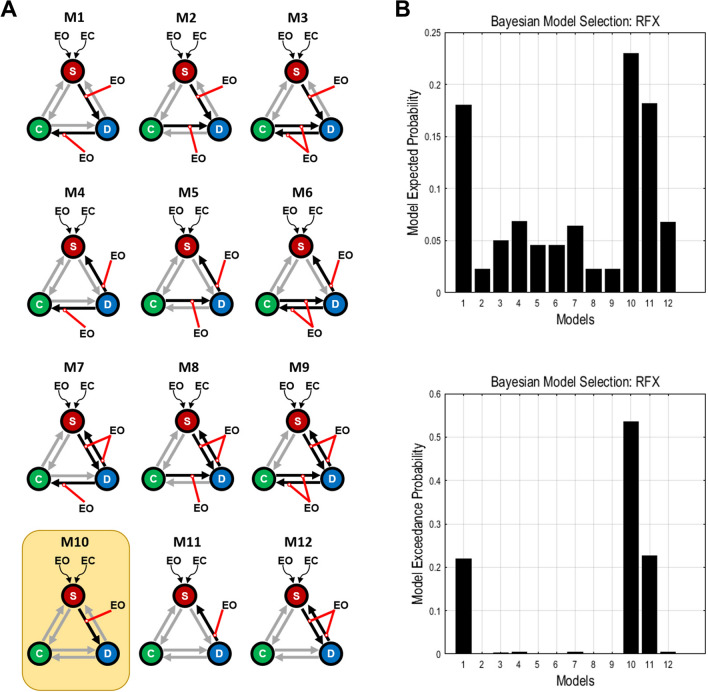
The stochastic DCM was then used to estimate the modulation of network causality by EC and EO. The stochastic DCM, an extension of the DCM, includes the random fluctuations of BOLD signals in the state equation [9]. According to a previous study, the reproducibility of the stochastic DCM is excellent as it showed consistent results across three datasets (each with 60 participants) [10]. Based on the data from the first experiment, decreased SN–DMN FC was found in both resting-state datasets, while decreased DMN–CEN FC was seen only in resting-state dataset 2. We concluded that EO might modulate the bidirectional or unidirectional connection between the SN and the DMN, and could affect the bidirectional, unidirectional, or no extrinsic connection between the DMN and the CEN. As a result, 12 hypothesized network models were constructed for each participant (Fig. 2A).
Fig. 2.
Model spaces of dynamic causal modeling (DCM) and the results of random-effect Bayesian model selection (BMS) for the block-design experiments. A Twelve hypothesized models estimated with DCM. Each model contains bidirectional connections among the three networks and two driving inputs (EC and EO) to the SN (red lines, modulatory effects of EO; black arrows, connections influenced by EO; gray arrows, unaffected connections; S, salience network; C, central executive network; D, default mode network; EO, eyes open; EC, eyes closed). B Expected probability and exceedance probability from the random-effect BMS. Model 10 was optimal, with highest expected and exceedance probability (yellow frame in A).
Random-effects Bayesian model selection (BMS), which accounts for between-subjects heterogeneity, was used to select the optimal model across all candidates [11]. In order to show that the optimal model did not stem from the specific method of model selection, we followed the approach of a previous study by using another model-selection algorithm, the fixed-effects BMS, to enhance the reproducibility of the optimal model [12]. The fixed-effects BMS, assuming the model is homogeneous across subjects, can provide complementary information to the random-effects BMS [12] (details in supplementary material). The results of random-effects BMS demonstrated that Model 10 (M10) was optimal, with the highest model exceedance probability (0.5355) (Fig. 2B), which shows that the SN receives its driving inputs from EC and EO, and EO modulates the signals transmitted from the SN to the DMN. The same optimal model was selected using the fixed-effects BMS (Fig. S4), suggesting that the model we found is reliable.
In summary, we conducted two fMRI experiments, and investigated how the interactions among the three large-scale networks were modulated by the EO and EC conditions. The results from resting-state datasets showed lower FC between the SN and the DMN in EO than EC. Furthermore, the results from the block-design dataset suggested significantly greater activation in the SN during EO than EC, and we proposed an optimal DCM model demonstrating that EO modulates the signals transmitted from the SN to the DMN.
Opening the eyes is a basic behavior to direct attention to the external world, and changes our brain from an internal to an external state [5, 6]. The SN may be responsible for processing the external information during this switch. The SN assigns saliency to various stimuli, segregating the most relevant signals among internal and external stimuli in order to guide behavior [2]. A previous study suggested that EO leads to higher regional properties (nodal degree, efficiency and betweenness centrality) in exteroceptive-related networks, including regions of the SN, to allocate more resources for exteroceptive processing compared to EC [5]. Moreover, the SN is able to affect the activity of the DMN and its interactions with other networks [2]. The DMN is associated with internal information processing [13], so the decreased FC between the SN and the DMN during EO suggests that attentional resources are directed to external processing from internal processing to a larger extent in the EO than in the EC condition.
In the block-design experiments, the increased activation in the SN during EO further suggested that the SN assigns saliency to external stimuli when our eyes are open. This result is supported by previous reports. For example, a simultaneous FDG–PET/fMRI study found an increase in glucose metabolism within the SN during EO compared to EC [4]. This result not only supports the idea that more attentional resources are allocated for external processing during EO, but also suggests that the SN may be the input network for model construction in DCM analysis.
The optimal model (M10), derived from DCM analysis, showed that EO modulates the signals from the SN to the DMN and provides a potential explanation for the decreased FC between the SN and the DMN. Previous studies have suggested that the SN initiates cognitive control signals to the DMN [1, 12]. Moreover, a special class of neurons, von Economo neurons, are exclusively found within the SN and may relay signals from the SN to other parts of the brain [14]. And an anatomical study has also shown that the structural integrity of the SN predicts the efficient regulation of activity in the DMN [15]. Taken together, it is reasonable to conclude that the SN transmits signals to the DMN. As revealed by our model, this signaling would be modulated by EO, possibly leading to decreased coupling between the SN and the DMN. This suggests that EO might disrupt the control signal from the SN to the DMN in order to assign more attentional resources to the external world.
Although the SN and the DMN have been considered to be involved in self-processing, a meta-analysis revealed that the SN, specifically the AI, is activated during self-related stimuli (not non-self-related stimuli), while the DMN, centered on cortical midline structures including the medial prefrontal cortex and posterior cingulate cortex, shows activation during both self- and non-self-related stimuli [16]. These results demonstrate that the SN plays a more special role in self-related processing than the DMN, while the DMN could be involved in more general functions, such as social cognition and monitoring the environment. Accordingly, our findings further suggest that the SN assigns self-specificity to an external stimulus, and changes the external stimulus into a self-related stimulus. However, the exact mechanism deserves further investigation.
A novel theory has recently been proposed that neural activity and mental features (e.g. self, consciousness, and perception of the passage of time) are connected into an intrinsic relationship by the dynamics of “inner time and space”, which suggests that neural dynamics are mental dynamics [17]. The dynamics of inner time and space has been proposed to be constructed by the spatiotemporal dynamics of spontaneous activity in the brain. In contrast, the dynamics of “outer time and space” describe the spatiotemporal dynamics of the outside world. Following this theory, the present study, which showed an altered dynamic interaction between two self-related networks (SN and DMN) at the neuronal level, suggests that self-processing changes along with the switch from EC to EO. We have further assumed that the spatiotemporal dynamics of the self-related mental feature would change with the dynamic interaction between the SN and the DMN. This offers a new perspective to expand our understanding of the self-processing in different conditions besides EC and EO.
In some recent studies, the SN has been divided into dorsal and ventral regions [18]. The dorsal SN, which includes the bilateral dorsal AI and part of the subgenual anterior cingulate cortex (SACC), plays an important role in attention and switching between cognitive resources, and a causal control role in the DMN and the CEN [12]. The ventral SN, consisting of the bilateral ventral AI and part of the SACC, is thought to be crucial during emotions [18]. Thus, the dorsal AI and SACC were included in the current study, whereas the ventral AI was excluded. Our results further expand the understanding of the dorsal SN by showing that the control signal from the dorsal SN to the DMN can be influenced by EO.
One issue in this study should be mentioned. Functional brain networks are usually dynamic and time-varying, even at rest. However, due to the low temporal resolution of our fMRI dataset, it was difficult to investigate the dynamic characteristics of functional brain networks in the current study. Thus, the impact of EC/EO on the dynamic interactions among the three networks could be further explored using measurements with high temporal resolution, such as magnetoencephalography or electroencephalography in further studies.
In summary, our results demonstrate that the triple-network mechanism changes once we open our eyes. Specifically, the coupling between the SN and the DMN decreased and activation of the SN increased during EO rather than EC, and EO modulated the signaling from the SN to the DMN. These findings show that the network mechanism underlying endogenous cognitive control is influenced by the eyes. Our study lays a foundation for the design of psychological experiments and the interpretation of results under different conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31771249 and 31971032), the Major Program of the National Social Science Fund of China (18ZDA293), the Basic and Applied Basic Research Foundation of Guangdong Province, China (2020A1515011250), Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence Fund (2019023), Key Realm R&D Program of Guangdong Province (2019B030335001), Key Realm R&D Program of Guangzhou (202007030005), and the National Natural Science Foundation of China (31871135).
Conflict of interest
The authors claim that there are no conflicts of interest.
Contributor Information
Xilin Zhang, Email: xlzhang@m.scnu.edu.cn.
Pengmin Qin, Email: qin.pengmin@gmail.com.
References
- 1.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin PM, Wu XH, Huang ZR, Duncan NW, Tang WJ, Wolff A, et al. How are different neural networks related to consciousness? Ann Neurol. 2015;78:594–605. doi: 10.1002/ana.24479. [DOI] [PubMed] [Google Scholar]
- 4.Riedl V, Bienkowska K, Strobel C, Tahmasian M, Grimmer T, Forster S, et al. Local activity determines functional connectivity in the resting human brain: a simultaneous FDG-PET/fMRI study. J Neurosci. 2014;34:6260–6266. doi: 10.1523/JNEUROSCI.0492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu P, Huang R, Wang J, Van Dam NT, Xie T, Dong Z, et al. Different topological organization of human brain functional networks with eyes open versus eyes closed. Neuroimage. 2014;90:246–255. doi: 10.1016/j.neuroimage.2013.12.060. [DOI] [PubMed] [Google Scholar]
- 6.Jao T, Vertes PE, Alexander-Bloch AF, Tang IN, Yu YC, Chen JH, et al. Volitional eyes opening perturbs brain dynamics and functional connectivity regardless of light input. Neuroimage. 2013;69:21–34. doi: 10.1016/j.neuroimage.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong Y, Wang C, Gao W, Xiao Q, Lu D, Jiao Q, et al. Aberrant resting-state functional connectivity in the default mode network in pediatric bipolar disorder patients with and without psychotic symptoms. Neurosci Bull. 2018;35:581–590. doi: 10.1007/s12264-018-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 9.Daunizeau J, Stephan KE, Friston KJ. Stochastic dynamic causal modelling of fMRI data: should we care about neural noise? Neuroimage. 2012;62:464–481. doi: 10.1016/j.neuroimage.2012.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal-Casas D, Balaguer-Ballester E, Gerchen MF, Iglesias S, Walter H, Heinz A, et al. Multi-site reproducibility of prefrontal-hippocampal connectivity estimates by stochastic DCM. Neuroimage. 2013;82:555–563. doi: 10.1016/j.neuroimage.2013.05.120. [DOI] [PubMed] [Google Scholar]
- 11.Rigoux L, Stephan KE, Friston KJ, Daunizeau J. Bayesian model selection for group studies—revisited. Neuroimage. 2014;84:971–985. doi: 10.1016/j.neuroimage.2013.08.065. [DOI] [PubMed] [Google Scholar]
- 12.Chand GB, Wu J, Hajjar I, Qiu D. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 2017;7:401–412. doi: 10.1089/brain.2017.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Northoff G, Wainio-Theberge S, Evers K. Is temporo-spatial dynamics the "common currency" of brain and mind? In Quest of "Spatiotemporal Neuroscience". Phys Life Rev 2019. [DOI] [PubMed]
- 18.Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.