Abstract
Purpose of review
The proportion of overweight and obese persons with HIV (PWH) has increased since the introduction of antiretroviral therapy (ART). We aim to summarize recent literature on risks of weight gain, discuss adipose tissue changes in HIV and obesity, and synthesize current understanding of how excess adiposity and HIV contribute to metabolic complications.
Recent findings
Recent studies have implicated contemporary ART regimens, including use of integrase strand transfer inhibitors and tenofovir alafenamide, as a contributor to weight gain, though the mechanisms are unclear. Metabolic dysregulation is linked to ectopic fat and alterations in adipose immune cell populations that accompany HIV and obesity. These factors contribute to an increasing burden of metabolic diseases in the aging HIV population.
Summary
Obesity compounds an increasing burden of metabolic disease among PWH, and understanding the role of fat partitioning and HIV- and ART-related adipose tissue dysfunction may guide prevention and treatment strategies.
Keywords: obesity, HIV, metabolic disease, weight gain, adipose tissue, inflammation
Introduction
The prevalence of HIV-associated wasting has declined with the introduction of combination antiretroviral therapy (ART). However, this has been accompanied by an increasing proportion of overweight and obese persons with HIV (PWH) [1–3]. The obesogenic environment of resource rich countries [4, 5], reversal of the catabolic state associated with uncontrolled viremia [6], and direct ART-effects contribute to the changing rates of obesity [7]. While weight gain after initiation of ART is associated with reduced risk of mortality in underweight and normal weight individuals [8], the risk for metabolic diseases including diabetes mellitus, neurocognitive impairment, liver disease, and cardiovascular disease is increased with excess adiposity [9–12]. Furthermore, weight gain in PWH confers greater risk of metabolic disease compared with HIV-negative individuals [9, 11].
Adipose tissue is a large endocrine organ composed of multiple cell types with important functions related to energy storage, metabolism, neuroendocrine signaling, and immunologic regulation [13]. HIV per se and ART promote qualitative [14, 15], quantitative [7, 16], and distributive alterations in adipose tissue [17–20]. Both HIV infection and excess adiposity are associated with systemic inflammation that may derive, in part, from changes in the adipose tissue innate and adaptive immune cell profile [21–27]. Together, HIV-specific factors and excess adiposity may explain the excess risk of metabolic diseases in PWH. Here, we review the current epidemiology of obesity and risk factors for weight gain, the current understanding of the role of adipose tissue biology in the development of metabolic diseases, and major complications associated with obesity in PWH (Figure 1).
Figure 1.
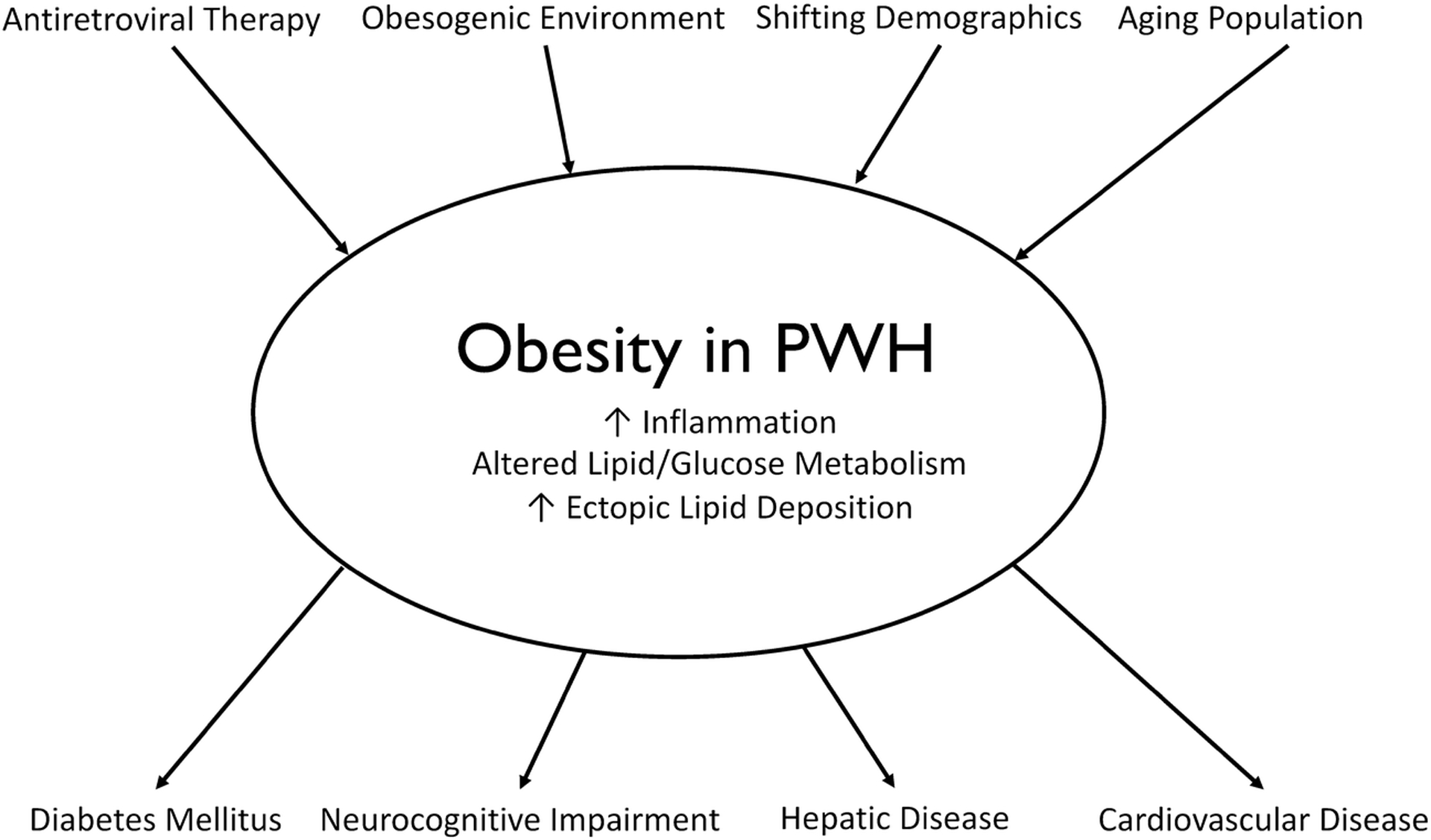
Proposed model of obesity in persons with HIV (PWH). Contemporary antiretroviral therapy agents (principally integrase strand transfer inhibitors and tenofovir alafenamide), an obesogenic environment (high-fat diet and physical inactivity), shifting demographics, and an aging population predispose to obesity. Obesity in persons with HIV results in increased inflammation, increased ectopic lipid disposition, and alterations in lipid and glucose metabolism. This contributes to metabolic complications including diabetes mellitus, neurocognitive impairment, and hepatic disease. The link between obesity as measured by body mass index and cardiovascular disease is not completely understood.
Epidemiology of Obesity in Person with HIV
The proportion of overweight (body mass index [BMI] 25.0 – 29.9 kg/m2) and obese (BMI ≥30 kg/m2) PWH has increased globally. Among PWH in a prospective US Military study, the percentage who were overweight or obese at HIV diagnosis increased from 28% between 1985–1990 to 51% between 1996–2004 [1]. In a multi-cohort analysis of over 14,000 PWH in the United States and Canada, the percentage of obese individuals at ART initiation increased from 9% to 18% between 1998 and 2010 [2]. Furthermore, 22% of individuals with normal BMI became overweight and 18% of overweight individuals became obese within 3 years after ART initiation [2]. Other studies have confirmed high prevalence and incidence of obesity in PWH [3, 28–30], and these changes parallel trends in the general population [31]. Women, minorities, and persons of lower socioeconomic status with HIV carry a disproportionate burden of obesity. Pooled analysis of three randomized clinical trials comparing 760 women with 3,041 men initiating ART found that women had an average BMI increase of 0.59 kg/m2 higher than men [32]. In a predominantly Hispanic cohort, uninsured minority PWH had a greater prevalence of obesity and greater risk of weight gain compared with Caucasians or insured minorities with HIV [33]. The prevalence of obesity in African American women with HIV is greater than in African American women without HIV in one study [31]. Taken together, obesity prevalence has increased dramatically since the start of the HIV epidemic, with a disproportionate burden among women and minorities.
Anthropometric Parameters
BMI is an anthropometric measurement commonly used in the clinical and research setting, in part due to ease of calculation, high reproducibility, and lack of a need for specialized equipment. Higher BMI is associated with cardiometabolic diseases in the general population including diabetes and cardiovascular disease [34, 35]. However, BMI poorly discriminates between lean body mass and fat body mass, which can be influenced by sex, age, and race/ethnicity [36]. Anthropometric indices of central adiposity estimate the visceral adipose tissue (VAT) compartment and can predict risk of cardiometabolic complications better than BMI [37, 38], particularly in PWH, who more commonly have VAT expansion than HIV-negative individuals with a similar BMI [39, 40]. However, these measurements are imperfect and cannot assess the relative contributions of subcutaneous adipose tissue (SAT) and VAT to waist circumference [41]. Magnetic resonance imaging and computed tomography (CT) remain the gold standard for quantifying SAT and VAT [42], but are principally used for research purposes. Dual-energy X-ray absorptiometry can quantify total trunk fat mass, but software for estimating VAT often provides an underestimate compared to CT imaging in PWH [43]. Recently, CT was used to characterize adipose density as a surrogate marker for adipocyte size and fibrosis in PWH before and after ART [44], but at present adipose tissue biopsy is the standard practice for assessing adipose tissue morphology. In summary, several anthropometric indices are available that characterize adiposity in PWH, but measurements of central adiposity are most strongly associated with risk of metabolic disease. CT imaging offers superior quantification of adipose tissue compartments and is increasingly utilized in research settings.
Risk Factors Associated with Weight Gain in Persons with HIV
Weight gain is common among PWH initiating ART and is associated with reduced mortality for individuals who are initially underweight or normal weight [8]. While a shared obesogenic environment likely explains the similar trends in obesity observed between PWH and the general population [31], the initiation of ART likely contributes to weight gain through a variety of mechanisms. HIV-associated wasting, or the weight loss accompanying advanced CD4+ T cell depletion, is characterized by anorexia secondary to effects of elevated inflammatory markers on the hypothalamus [45], increased basal metabolic requirements that can exceed 30% of baseline during secondary infections [6, 46], and a catabolic state resulting from increased protein turnover [47]. The initiation of ART reverses this catabolic state, reduces circulating inflammatory markers, and can improve appetite and nutrient absorption [48]. Lower baseline CD4+ T cell count and higher HIV RNA viral load are associated with more weight gain after ART initiation [2, 8, 28, 32, 49]. Excess caloric intake, a high-fat diet, and reduced physical activity also promote weight gain and obesity [4, 5].
Many studies have examined the role of ART medications in weight gain among PWH. Older nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) have not been associated with differential weight gain [1, 7, 28, 49, 50], but studies of protease inhibitors (PIs) have been mixed. In an ACTG trial, 269 PWH were randomized to an NRTI backbone and either efavirenz (EFV) or atazanavir-ritonavir (ATV/r), and ATV/r was associated with greater BMI gain with a trend towards increased VAT compared with EFV at 96 weeks [51].
Integrase strand transfer inhibitors (INSTI; e.g., raltegravir [RAL], elvitegravir [EVG], dolutegravir [DTG] and bictegravir [BIC]) are a more recently available class of ART medications with a good tolerability profile and genetic barrier to HIV drug resistance [52, 53]. INSTI-based ART regimens are now the recommended first-line treatment for most PWH [54], but several recent studies, primarily from single sites or cohorts, report greater weight gain among persons receiving INSTI-based ART regimens for initial therapy as compared to PI and NNRTI-based regimens. In a cohort from Brazil, PWH on RAL-based regimens were 7-fold more likely to become obese (BMI ≥ 30 kg/m2) compared to those receiving NNRTI- or PI-based regimens [49]. In other observational studies, INSTI-based regimens generally, and particularly DTG-based ART regimens [55–58], were associated with greater weight gain.
In the prospective AIDS Clinical Trial Group (ACTG) study A5257, the use of RAL with tenofovir disoproxil fumarate and emtricitabine (TDF/FTC) as an initial ART regimen was associated with greater increases in waist circumference and increased odds of a >10% weight gain at 96 weeks, compared to ART regimens including ritonavir-boosted darunavir or ritonavir-boosted atazanavir, each combined with TDF/FTC [59]. In Cameroon, the NAMSAL study randomized 613 PWH to either tenofovir-lamivudine with DTG or EFV and found that individuals randomized to the DTG-containing regimen gained significantly more weight (5 kg vs 3 kg) at 48 weeks with the most weight gain observed among women [60]. Similarly, a recent pooled analysis of 8 phase III clinical trials involving 5680 ART-naïve participants found that 17.3% of participants had ≥ 10% weight gain from baseline, and weight gain was greater with INSTIs (3.24 kg) compared to NNRTI (1.93 kg) and PI (1.72 kg) [7].
The use of tenofovir alafenamide (TAF), approved in the US in November 2015, is rapidly increasing given the lower incidence of adverse bone and renal effects compared to TDF. Recent data from randomized trials and smaller observational studies indicate that TAF use may predispose to weight gain independent of concomitant INSTI use [16, 61]. In the ADVANCE study, ART-naïve South African women randomized to DTG/TAF/FTC gained an average of 6.4 kg at 48 weeks of treatment, as compared to 3.2 kg on DTG/TDF/FTC and 1.7 kg on EFV/TDF/FTC [16]. For men, weight gain at 48 weeks was 4.7 kg, 3.0 kg, and 0.5 kg, respectively, on the same regimens. Over the 48 weeks, weight continued to increase in both men and women. Similarly, a pooled analysis of randomized controlled clinical trials found higher weight gain over 96 weeks among those starting TAF-containing regiments as compared to other NRTIs. At 96 weeks, mean weight gains by NRTI were: TAF, 4.25 kg; abacavir (ABC), 3.08 kg; TDF, 2.07 kg; and AZT 0.39 kg after adjusting for age, race, sex, baseline clinical factors, and additional agents in the regimen [7].
The mechanisms contributing to weight gain on ART are incompletely understood and could reflect off target effects of the medications or a greater efficacy and suppression of viral reservoirs that reduces the metabolic cost of HIV infection [62]. Several studies are forthcoming that will shed light on whether metabolic abnormalities are independent of weight gain, whether genetic polymorphisms underlie risk of weight gain, and whether changing to another regimen can reverse weight gain.
Adipose Tissue Distribution and Deposition is Altered in HIV
HIV per se and ART medications influence partitioning and distribution of the adipose tissue compartment that can predispose to metabolic complications. As discussed previously, HIV-associated wasting was characterized by anorexia, increased protein turnover, and higher resting energy expenditure, mediated in part by persistent inflammation in the setting of uncontrolled viremia. SAT from untreated PWH demonstrates mitochondrial DNA depletion and reduced function of several enzymes important for aerobic metabolism relative to HIV-negative persons [17]. Viral proteins including HIV viral protein R (Vpr), trans-activator of transcription protein (Tat), and negative regulatory factor (Nef) can mediate changes in adipocyte function through alteration in expression of adiponectin, lipoprotein lipase, glucose transporter type 4 (GLUT4), and peroxisome proliferator-activated receptor gamma [63–65].
Early ART regimens incorporating thymidine analogues affected adipose distribution with lipoatrophy of the face, limbs, and buttocks and lipohypertrophy of visceral, cervical, and dorsocervical areas [18–20, 66, 67]. Importantly, PWH on ART had increased total body fat in the trunk and lower percentage in the limbs compared with HIV-negative despite absence of clinically apparent lipodystrophy [68], and these changes persist even after changing ART [18]. Impaired energy storage in the SAT compartment is hypothesized to result in expansion of VAT and other ectopic adipose deposits [69], which may occur because VAT is less susceptible to toxic effects of ART and HIV than the SAT compartment [70, 71]. Persons with lipodystrophy have a higher rate of total lipolysis as measured using intravenous infusions of stable isotopes of glycerol and palmitate, and increased free fatty acid levels compared with HIV-negative [72]. In addition, PWH have reduced postprandial clearance and storage of plasma triacylglycerols [73], and increased rate of intrahepatic and intra-adipocyte fatty acid reesterfication [74]. Finally, de novo hepatic lipogenesis is increased 3 to 4 fold in HIV-associated wasting compared with controls [75]. Taken together, accelerated lipolysis, increased de novo hepatic lipogenesis, and reduced clearance of plasma free fatty acids likely contribute to increased ectopic fat deposition in the liver, epicardium, and skeletal muscle that can increase the risk of metabolic diseases [76–81]. Increases in ectopic adipose tissue compartments may accompany weight gain in PWH [16], though longitudinal studies with CT imaging are needed.
Adipose Tissue is Characterized by Increased Inflammation in HIV and Obesity
The stromal vascular fraction of adipose tissue contains innate and adaptive immune cells that modulate adipocyte energy storage, function, and inflammation. Macrophages accumulate in adipose tissue with progressive weight gain in HIV-negative individuals [22]. In the setting of obesity, both adipocytes and macrophages secrete higher levels of monocyte chemoattractant protein 1 (MCP-1), which drives local proliferation of macrophage populations [24], and the polarization towards an inflammatory subtype (M1) characterized by higher levels of TNF-α, IL-6, IL-12, IL-23, and inducible nitric oxide synthase [23]. Macrophage-derived cytokines interfere with adipocyte insulin signaling through downregulation of insulin receptor substrate 1, GLUT4, and phosphoinositide 3-kinase p85alpha [82–84]. Studies of macrophages in adipose tissue of PWH are limited and have conflicting results. One study of SAT showed increasing macrophage density and inflammatory cytokines were associated with peripheral lipoatrophy compared with HIV-negative [85]. A different study of gluteal fold adipose tissue from PWH with BMI between 18 and 35 kg/m2 showed similar macrophage density compared to matched HIV-negative controls but higher IL-6, IL-8, IL-12p40, and MIP-1α [15]. Taken together, macrophage adipose tissue infiltration increases with obesity, but not necessarily with HIV infection.
In contrast to mixed results on macrophage density, several recent studies report a profound shift in adipose tissue T cell profile towards a CD8+ cell predominance in both HIV and simian immunodeficiency virus (SIV, a model of retrovirus infection that approximates HIV) [27, 86, 87], an intriguing finding given the marked CD8+ T cell infiltration in obesity and the higher rates of metabolic disease in PWH [88–91]. SAT CD8+ T cells from PWH demonstrate increased antigen receptor clonality [87], a finding also reported in animal models of obesity [92]. While this finding of increased clonality could refect local T cell expansion, other studies report a lack of Ki-67 expression on SIV-infected macaque adipose tissue CD8+ T cells and high CD57 expression, a marker of senescence or late differentiation and an indicator of reduced replicative capacity, both of which may reflect minimal in situ proliferation [93–95]. A recent study found that despite profound CD8+ enrichment in SAT from PWH compared to controls without HIV, the distribution of CD4+ and CD8+ subsets (naïve, central memory, effector memory [TEM], and effector memory RA+ [TEMRA]) was similar, suggesting the accumulation of CD8+ cells occurs in a relatively stochastic manner, as opposed to accumulation of one broad subset [96]. These findings suggest the changes in adipose tissue gene expression with HIV infection are not due to the preferential accumulation of a specific CD8+ T cell phenotype. Furthermore, the same study found the SAT of diabetic PWH to be markedly enriched for CD4+ TEM and TEMRA cells co-expressing CD57, CX3CR1, and GPR56, a phenotype reported to have anti-viral specificity, compared to non-diabetic PWH [97–100]. This finding was notable as CD4+ T cells are an important regulator of insulin resistance and tissue inflammation [26], but in PWH they can also serve as a reservoir for HIV persistence, even in virologically suppressed persons [27]. The latent HIV reservoir in adipose tissue CD4+ T cells likely contributes to tissue inflammation and is an important area to consider in HIV cure research.
Obesity Contributes to Comorbidities
Diabetes Mellitus
The estimated incidence of diabetes in PWH ranges between 3.1 and 14 cases per 1000 patient years [21, 88–90, 101]. In one study of men, the incidence of diabetes in PWH was over 4-fold higher than in HIV-negative after adjusting for age and BMI [88]. In addition, the incidence of prediabetes, or individuals who are at risk for developing diabetes, is even higher with an estimated 125 cases per 1000 patient years in a recent meta-analysis [102]. Traditional risk factors for diabetes among PWH shared with the general population include older age, increasing BMI and central adiposity, family history of diabetes, and African American or Hispanic origin [12, 102–105]. Specific to HIV, lower baseline CD4+ T cell count and older NRTIs and PIs contribute to additional risk of diabetes [88, 103]. Furthermore, the risk of diabetes with weight gain is greater than in HIV-negative individuals [9]; for each 5 pounds of weight gained, the risk of incident diabetes increases 14% in PWH compared with just 8% in HIV-negative individuals [9]. Some of this elevated risk may be related to higher circulating inflammatory markers [21, 106]. In the SMART and ESPRIT clinical trials, higher IL-6 and high-sensitivity c-reactive protein (hsCRP) at enrollment were associated with incident diabetes, and each doubling of IL-6 was associated with around a 30% increased risk [21]. Virological suppression with ART does not completely normalize systemic inflammation [107], and weight gain with ART may further offset reduction in inflammation [108]. Finally, obesity itself is a pro-inflammatory condition, and is associated with increased circulating inflammatory markers in both PWH and HIV-negative [109, 110]. As observed in the general population, diabetic PWH have significantly higher risk of cardiovascular disease, chronic kidney disease, and mortality compared to non-diabetics [105], highlighting the importance of addressing metabolic disease risk factors in the HIV population. However, how obesity mediates excess risk of insulin resistance in PWH is incompletely understood. In one study the prevalence of metabolically healthy obese PWH was similar to the prevalence in obese HIV-negative individuals, but was lower in non-obese PWH compared with non-obese HIV-negative individuals [111]. Another study found that obese PWH had worse cardiovascular parameters but no difference in insulin sensitivity compared with obese HIV-negative individuals [110]. Taken together, obesity in PWH appears to have a greater impact on cardiovascular parameters than insulin resistance and non-traditional risk factors likely have an important role in the development of diabetes.
Neurocognitive Impairment
The prevalence of neurocognitive impairment (NCI) is common in PWH even after the introduction of effective ART, suggesting additional factors aside from viral replication contribute to cognitive dysfunction [112]. In a sub-study of the CHARTER cohort, the prevalence of NCI was 40% and increased central adiposity, as measured by waist circumference, was associated with a higher risk of NCI [10]. Another study confirmed the risk of increased NCI with higher waist circumference, likely mediated through elevated systemic inflammation as reflected by IL-6 and cerebrospinal fluid soluble CD40L (a marker of microglial and macrophage activation) concentrations [113]. Moreover, greater VAT measured by CT was associated with more volume loss of the superior temporal gyrus, insula, and right caudate nucleus and was the strongest predictor of volume changes even after accounting for HIV serostatus [114]. The presence of metabolic disease was independently associated with greater global neurocognitive deficits among PWH, principally the domains of learning, fine motor skills, and executive function, which was not observed among HIV-negative controls [115]. Moreover, diabetes and elevated triglycerides, two aspects of metabolic disease exacerbated by obesity, were strongly associated with increased NCI in the PWH [115]. In summary, evidence suggests that central adiposity and diabetes are major risk factors for NCI among PWH and intervention may prevent or delay cognitive decline.
Cardiovascular Disease
PWH are at increased risk for cardiovascular disease (CVD) including myocardial infarction, stroke, and atherosclerosis [116–119]. In a recent systematic review, the pooled risk ratio for CVD was over 2-fold greater in PWH compared with HIV-negative individuals, and the global burden of CVD in PWH increased between 1990 and 2015 [119]. While traditional risk factors such as male gender, older age, diabetes, hypertension, and race are associated with CVD [117], PWH have elevated risk relative to HIV-negative even after adjusting for demographic characteristics, Framingham risk factors, comorbidities, and viral suppression [120]. ART exposure [116], systemic inflammation [121], reduced arterial elasticity [122], and endothelial dysfunction [123], may contribute to excess risk of CVD in PWH. In the SMART trial, PWH with the highest quartile of IL-6 had a hazard ratio of 4.65 for CVD compared with individuals in the lowest quartile, and this was independent of other predictors of CVD [121]. Central adiposity is associated with other predictors of cardiovascular disease in PWH [39], and ectopic adipose deposition has been associated with CVD in both PWH and HIV-negative [76, 77, 124]. However, the role of obesity, defined solely by BMI, in modifying risk of CVD is unclear. In the D:A:D study, individuals were longitudinally followed and compared to individuals with normal BMI, obese individuals had a 1.31 (confidence interval 1.03–1.67) relative risk of CVD [12]. A recent study compared non-obese and obese PWH and found that while obese individuals had higher levels of circulating inflammatory markers including IL-6, hsCRP, and TNF-α receptor 1, both groups had similar levels of intercellular adhesion molecule 1 and carotid intima-media thickening, suggesting a limited role of adiposity-mediated inflammation in endovascular activation and excess CVD burden [110]. While obesity is a proinflammatory condition and a contributor to hypertension and insulin resistance, important risk factors for CVD, further studies are necessary to understand the relationship of excess adiposity, inflammation, and risk of CVD in PWH.
Liver Disease
Liver disease is the leading cause of non-AIDS related mortality in PWH [125]. PWH carry a disproportionate burden of non-alcoholic fatty liver disease (NAFLD) with an estimated prevalence of 35% [126], compared to 25% in the general population [127]. NAFLD represents a spectrum of disease that ranges from simple steatosis to steatohepatitis to fibrosis, and can eventually lead to hepatic cirrhosis and hepatocellular carcinoma [128]. PWH with NAFLD are more likely to develop steatohepatitis and hepatic fibrosis than HIV-negative persons with NAFLD [129, 130]. In addition, HIV-associated NAFLD is associated with increased risk of extrahepatic disease including diabetes and CVD [131, 132]. Higher BMI is a risk factor for HIV-associated NAFLD and measurements of central adiposity are strongly associated with NAFLD and may be useful as a screening tool [126, 133, 134]. Interestingly, PWH with NAFLD had lower BMI and higher physical activity level compared to HIV-negative patients with NAFLD [11].
PWH have been shown to have increased lipolysis and hepatic re-esterification of fatty acids [72, 74], increased de novo lipogenesis [75] and decreased ability to clear very-low-density lipoprotein (VLDL) triglyceride [135]. Furthermore, changes in hepatic expression of PPARgamma and sterol regulatory element-binding protein (SREBP)-1 have been shown to be associated with steatohepatitis and lipodystrophy in PWH [136]. Exposure to certain ART agents has also been shown to be a risk factor for the development of NAFLD in PWH [137–141]. Collectively, the changes in lipid and glucose metabolism in PLWH lead to increased ectopic lipid deposition in the liver and other tissues which contribute to the activation of inflammatory pathways associated with metabolic dysregulation, inflammation and fibrosis in the liver [128, 142–144].
Weight Loss Interventions
Medical Weight Loss Interventions
Behavioral interventions through caloric restriction and physical exercise are effective in promoting weight loss in PWH, including adipose tissue loss in subcutaneous and visceral compartments [145–152]. These interventions had mixed efficacy on improving metabolic parameters [146, 147], but a recent study evaluating diet-induced weight-loss demonstrated improved insulin sensitivity in women with HIV to the same extent as women without HIV [152]. Several oral agents are FDA approved for weight loss including orlistat, lorcaserin, phentermine-topiramate, liraglutide, and naltrexone-bupropion, but limited data are available on use in PWH. Tesamorelin is a synthetic growth hormone-releasing hormone analogue FDA approved for reduction of central adiposity associated with lipodystrophy that has been shown to reduce VAT and liver fat and may improve metabolic parameters in those who have VAT reduction [153, 154]. As discussed in a previous section, there are currently no studies that have examined if changing ART regimen affects weight gain associated with contemporary ART.
Surgical Weight Loss Interventions
Bariatric surgery remains the most effective intervention for durable weight loss among obese members of the general population, with significant improvement in associated co-morbidities including hypertension, obstructive sleep apnea, diabetes, and hyperlipidemia [155]. VAT loss occurs postoperatively and is greatest in individuals with baseline diabetes [156]. In one study of obese diabetic patients, 50% of those who underwent bariatric surgery maintained diabetes remission at 5 years compared to none in the medical intervention group [157]. Evidence of efficacy is limited in PWH, but retrospective studies suggest surgery is effective and safe [158–160]. A retrospective study of 11 PWH who underwent bariatric surgery matched 1:5 with HIV-negative participants showed a similar reduction in weight and no excess morbidity or mortality [161], and mortality in PWH was not increased after bariatric surgery in a study using a large national database [162]. However, alteration of the gastrointestinal tract could reduce absorption of ART medications. One study of PWH undergoing sleeve gastrectomy assessed pharmacokinetics of ART regimens and found that raltegravir and atazanavir concentrations were below standard values, and 4 individuals had detectable viral loads after surgery, suggesting decreased absorption [163]. In summary, bariatric surgery is an effective intervention for durable weight loss and improvement of co-morbidities, but more studies are required to evaluate potential pharmacokinetic effects.
Conclusions
The prevalence and incidence of obesity in PWH has increased over the past two decades, likely reflective of trends in the population at large, improved survival, and the introduction of new ART agents. The rise in obesity has been accompanied by an increasing burden of metabolic diseases including insulin resistance, neurocognitive impairment, and hepatic disease, though the effects on cardiovascular disease are less clear. Understanding the effects of ART and HIV on fat partitioning and adipose tissue metabolic function may lead to therapeutic interventions that prevent and manage metabolic complications in PWH.
Source of funding:
This work was supported by the National Institutes of Health grants T32AI00747426, K12HL143956, and R01DK112262.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
Samuel Bailin, Curtis Gabriel, Celestine Wanjalla, and John Koethe declare that they have no conflicts of interest.
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Crum-Cianflone N, Roediger MP, Eberly L, Headd M, Marconi V, Ganesan A et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5(4):e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. •.Koethe JR, Jenkins CA, Lau B, Shepherd BE, Justice AC, Tate JP et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32(1):50–8. doi: 10.1089/aid.2015.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported trends in weight gain and obesity over 12 years among PLWH in North America.
- 3.Tate T, Willig AL, Willig JH, Raper JL, Moneyham L, Kempf MC et al. HIV infection and obesity: where did all the wasting go? Antivir Ther. 2012;17(7):1281–9. doi: 10.3851/IMP2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez D, Kalichman S, Cherry C, Kalichman M, Washington C, Grebler T. Dietary intake and overweight and obesity among persons living with HIV in Atlanta Georgia. AIDS Care. 2017;29(6):767–71. doi: 10.1080/09540121.2016.1238441. [DOI] [PubMed] [Google Scholar]
- 5.Vancampfort D, Mugisha J, De Hert M, Probst M, Firth J, Gorczynski P et al. Global physical activity levels among people living with HIV: a systematic review and meta-analysis. Disabil Rehabil. 2018;40(4):388–97. doi: 10.1080/09638288.2016.1260645. [DOI] [PubMed] [Google Scholar]
- 6.Melchior JC, Salmon D, Rigaud D, Leport C, Bouvet E, Detruchis P et al. Resting energy expenditure is increased in stable, malnourished HIV-infected patients. Am J Clin Nutr. 1991;53(2):437–41. doi: 10.1093/ajcn/53.2.437. [DOI] [PubMed] [Google Scholar]
- 7. ••.Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]; This pooled analysis of 8 phase III randomized clinical trials found greater weight gain among ART-naïve persons starting dolutegravir, bictegravir, and TAF-containing regimens.
- 8.Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60(12):1852–9. doi: 10.1093/cid/civ192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. •.Herrin M, Tate JP, Akgun KM, Butt AA, Crothers K, Freiberg MS et al. Weight Gain and Incident Diabetes Among HIV-Infected Veterans Initiating Antiretroviral Therapy Compared With Uninfected Individuals. J Acquir Immune Defic Syndr. 2016;73(2):228–36. doi: 10.1097/QAI.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study in the Veterans Aging Cohort Study found weight gain was associated with greater risk of incident diabetes in PWH compared to HIV-negative individuals.
- 10.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78(7):485–92. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammed SS, Aghdassi E, Salit IE, Avand G, Sherman M, Guindi M et al. HIV-positive patients with nonalcoholic fatty liver disease have a lower body mass index and are more physically active than HIV-negative patients. J Acquir Immune Defic Syndr. 2007;45(4):432–8. doi: 10.1097/QAI.0b013e318074efe3. [DOI] [PubMed] [Google Scholar]
- 12.Achhra AC, Sabin C, Ryom L, Hatleberg C, Antonella d’Aminio M, de Wit S et al. Body Mass Index and the Risk of Serious Non-AIDS Events and All-Cause Mortality in Treated HIV-Positive Individuals: D: A: D Cohort Analysis. J Acquir Immune Defic Syndr. 2018;78(5):579–88. doi: 10.1097/QAI.0000000000001722. [DOI] [PubMed] [Google Scholar]
- 13.Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol. 2016;231(3):R77–R99. doi: 10.1530/JOE-16-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza Dantas Oliveira SH, de Souza Aarao TL, da Silva Barbosa L, Souza Lisboa PG, Tavares Dutra CD, Margalho Sousa L et al. Immunohistochemical analysis of the expression of TNF-alpha, TGF-beta, and caspase-3 in subcutaneous tissue of patients with HIV lipodystrophy syndrome. Microb Pathog. 2014;67–68:41–7. doi: 10.1016/j.micpath.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Shikuma CM, Gangcuangco LM, Killebrew DA, Libutti DE, Chow DC, Nakamoto BK et al. The role of HIV and monocytes/macrophages in adipose tissue biology. J Acquir Immune Defic Syndr. 2014;65(2):151–9. doi: 10.1097/01.qai.0000435599.27727.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ••.Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019;381(9):803–15. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]; A large randomized controlled trial that found dolutegravir-containing regimens were associated with greater weight gain than efavirenz, and regimens with both tenofovir alafenamide and dolutegravir were associated with the greatest weight gain.
- 17.Giralt M, Domingo P, Guallar JP, Rodriguez de la Concepcion ML, Alegre M, Domingo JC et al. HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV-1/HAART-associated lipodystrophy. Antivir Ther. 2006;11(6):729–40. [PubMed] [Google Scholar]
- 18.Grunfeld C, Saag M, Cofrancesco J Jr., Lewis CE, Kronmal R, Heymsfield S et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. AIDS. 2010;24(11):1717–26. doi: 10.1097/QAD.0b013e32833ac7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter VM, Hoy JF, Bailey M, Colman PG, Nyulasi I, Mijch AM. The prevalence of lipodystrophy in an ambulant HIV-infected population: it all depends on the definition. HIV Med. 2001;2(3):174–80. [DOI] [PubMed] [Google Scholar]
- 20.Saves M, Raffi F, Capeau J, Rozenbaum W, Ragnaud JM, Perronne C et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34(10):1396–405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 21.Betene ADC, De Wit S, Neuhaus J, Palfreeman A, Pepe R, Pankow JS et al. Interleukin-6, high sensitivity C-reactive protein, and the development of type 2 diabetes among HIV-positive patients taking antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;67(5):538–46. doi: 10.1097/QAI.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49(4):744–7. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 23.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19(1):162–71. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 26.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS. 2015;29(6):667–74. doi: 10.1097/QAD.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasse B, Iff M, Ledergerber B, Calmy A, Schmid P, Hauser C et al. Obesity Trends and Body Mass Index Changes After Starting Antiretroviral Treatment: The Swiss HIV Cohort Study. Open Forum Infect Dis. 2014;1(2):ofu040. doi: 10.1093/ofid/ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erlandson KM, Taejaroenkul S, Smeaton L, Gupta A, Singini IL, Lama JR et al. A Randomized Comparison of Anthropomorphic Changes With Preferred and Alternative Efavirenz-Based Antiretroviral Regimens in Diverse Multinational Settings. Open Forum Infect Dis. 2015;2(3):ofv095. doi: 10.1093/ofid/ofv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilozue C, Howe B, Shaw S, Haigh K, Hussey J, Price DA et al. Obesity in the HIV-infected population in Northeast England: a particular issue in Black-African women. Int J STD AIDS. 2017;28(3):284–9. doi: 10.1177/0956462416649131. [DOI] [PubMed] [Google Scholar]
- 31.Amorosa V, Synnestvedt M, Gross R, Friedman H, MacGregor RR, Gudonis D et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39(5):557–61. [PubMed] [Google Scholar]
- 32.Bares SH, Smeaton LM, Xu A, Godfrey C, McComsey GA. HIV-Infected Women Gain More Weight than HIV-Infected Men Following the Initiation of Antiretroviral Therapy. J Womens Health (Larchmt). 2018;27(9):1162–9. doi: 10.1089/jwh.2017.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor BS, Liang Y, Garduno LS, Walter EA, Gerardi MB, Anstead GM et al. High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr. 2014;65(2):e33–40. doi: 10.1097/QAI.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flint AJ, Rexrode KM, Hu FB, Glynn RJ, Caspard H, Manson JE et al. Body mass index, waist circumference, and risk of coronary heart disease: a prospective study among men and women. Obes Res Clin Pract. 2010;4(3):e171–e81. doi: 10.1016/j.orcp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bays HE, Chapman RH, Grandy S, Group SI. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61(5):737–47. doi: 10.1111/j.1742-1241.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuttall FQ. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr Today. 2015;50(3):117–28. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 38.Savva SC, Lamnisos D, Kafatos AG. Predicting cardiometabolic risk: waist-to-height ratio or BMI. A meta-analysis. Diabetes Metab Syndr Obes. 2013;6:403–19. doi: 10.2147/DMSO.S34220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beraldo RA, Meliscki GC, Silva BR, Navarro AM, Bollela VR, Schmidt A et al. Anthropometric measures of central adiposity are highly concordant with predictors of cardiovascular disease risk in HIV patients. Am J Clin Nutr. 2018;107(6):883–93. doi: 10.1093/ajcn/nqy049. [DOI] [PubMed] [Google Scholar]
- 40.Dimala CA, Ngu RC, Kadia BM, Tianyi FL, Choukem SP. Markers of adiposity in HIV/AIDS patients: Agreement between waist circumference, waist-to-hip ratio, waist-to-height ratio and body mass index. PLoS One. 2018;13(3):e0194653. doi: 10.1371/journal.pone.0194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring). 2011;19(2):402–8. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85(5):1197–202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 43.Fourman LT, Kileel EM, Hubbard J, Holmes T, Anderson EJ, Looby SE et al. Comparison of visceral fat measurement by dual-energy X-ray absorptiometry to computed tomography in HIV and non-HIV. Nutr Diabetes. 2019;9(1):6. doi: 10.1038/s41387-019-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lake JE, Moser C, Johnston L, Magyar C, Nelson SD, Erlandson KM et al. CT Fat Density Accurately Reflects Histologic Fat Quality in Adults With HIV On and Off Antiretroviral Therapy. J Clin Endocrinol Metab. 2019;104(10):4857–64. doi: 10.1210/jc.2018-02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macallan DC, Noble C, Baldwin C, Jebb SA, Prentice AM, Coward WA et al. Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med. 1995;333(2):83–8. doi: 10.1056/NEJM199507133330202. [DOI] [PubMed] [Google Scholar]
- 46.Grunfeld C, Pang M, Shimizu L, Shigenaga JK, Jensen P, Feingold KR. Resting energy expenditure, caloric intake, and short-term weight change in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Am J Clin Nutr. 1992;55(2):455–60. doi: 10.1093/ajcn/55.2.455. [DOI] [PubMed] [Google Scholar]
- 47.Macallan DC, McNurlan MA, Milne E, Calder AG, Garlick PJ, Griffin GE. Whole-body protein turnover from leucine kinetics and the response to nutrition in human immunodeficiency virus infection. Am J Clin Nutr. 1995;61(4):818–26. doi: 10.1093/ajcn/61.4.818. [DOI] [PubMed] [Google Scholar]
- 48.Borges AH, O’Connor JL, Phillips AN, Ronsholt FF, Pett S, Vjecha MJ et al. Factors Associated With Plasma IL-6 Levels During HIV Infection. J Infect Dis. 2015;212(4):585–95. doi: 10.1093/infdis/jiv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakal DR, Coelho LE, Luz PM, Clark JL, De Boni RB, Cardoso SW et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother. 2018;73(8):2177–85. doi: 10.1093/jac/dky145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shlay JC, Bartsch G, Peng G, Wang J, Grunfeld C, Gibert CL et al. Long-term body composition and metabolic changes in antiretroviral naive persons randomized to protease inhibitor-, nonnucleoside reverse transcriptase inhibitor-, or protease inhibitor plus nonnucleoside reverse transcriptase inhibitor-based strategy. J Acquir Immune Defic Syndr. 2007;44(5):506–17. doi: 10.1097/QAI.0b013e31804216cf. [DOI] [PubMed] [Google Scholar]
- 51.McComsey GA, Kitch D, Sax PE, Tebas P, Tierney C, Jahed NC et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53(2):185–96. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Podany AT, Scarsi KK, Fletcher CV. Comparative Clinical Pharmacokinetics and Pharmacodynamics of HIV-1 Integrase Strand Transfer Inhibitors. Clin Pharmacokinet. 2017;56(1):25–40. doi: 10.1007/s40262-016-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park TE, Mohamed A, Kalabalik J, Sharma R. Review of integrase strand transfer inhibitors for the treatment of human immunodeficiency virus infection. Expert Rev Anti Infect Ther. 2015;13(10):1195–212. doi: 10.1586/14787210.2015.1075393. [DOI] [PubMed] [Google Scholar]
- 54.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed [insert date]. [Google Scholar]
- 55.Bourgi K, Rebeiro PF, Turner M, Castilho JL, Hulgan T, Raffanti SP et al. Greater Weight Gain in Treatment Naive Persons Starting Dolutegravir-Based Antiretroviral Therapy. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menard A, Meddeb L, Tissot-Dupont H, Ravaux I, Dhiver C, Mokhtari S et al. Dolutegravir and weight gain: an unexpected bothering side effect? Aids. 2017;31(10):1499–500. [DOI] [PubMed] [Google Scholar]
- 57.Rizzardo S, Lanzafame M, Lattuada E, Luise D, Vincenzi M, Tacconelli E et al. Dolutegravir monotherapy and body weight gain in antiretroviral naive patients. AIDS. 2019;33(10):1673–4. doi: 10.1097/QAD.0000000000002245. [DOI] [PubMed] [Google Scholar]
- 58.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–31. doi: 10.1097/QAI.0000000000001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhagwat P, Ofotokun I, McComsey GA, Brown TT, Moser C, Sugar CA et al. Changes in Waist Circumference in HIV-Infected Individuals Initiating a Raltegravir or Protease Inhibitor Regimen: Effects of Sex and Race. Open Forum Infect Dis. 2018;5(11):ofy201. doi: 10.1093/ofid/ofy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. •.Group NAS, Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, Eymard-Duvernay S, Leroy S et al. Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N Engl J Med. 2019;381(9):816–26. doi: 10.1056/NEJMoa1904340. [DOI] [PubMed] [Google Scholar]; This recent randomized controlled trial found a dolutegravir-containing regimen was associated with greater weight gain than efavirenz-containing regimen.
- 61.Gomez M, Seybold U, Roider J, Harter G, Bogner JR. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015–2017. Infection. 2019;47(1):95–102. doi: 10.1007/s15010-018-1227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couturier J, Winchester LC, Suliburk JW, Wilkerson GK, Podany AT, Agarwal N et al. Adipocytes impair efficacy of antiretroviral therapy. Antiviral Res. 2018;154:140–8. doi: 10.1016/j.antiviral.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diaz-Delfin J, Domingo P, Wabitsch M, Giralt M, Villarroya F. HIV-1 Tat protein impairs adipogenesis and induces the expression and secretion of proinflammatory cytokines in human SGBS adipocytes. Antivir Ther. 2012;17(3):529–40. doi: 10.3851/IMP2021. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal N, Iyer D, Patel SG, Sekhar RV, Phillips TM, Schubert U et al. HIV-1 Vpr induces adipose dysfunction in vivo through reciprocal effects on PPAR/GR co-regulation. Sci Transl Med. 2013;5(213):213ra164. doi: 10.1126/scitranslmed.3007148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otake K, Omoto S, Yamamoto T, Okuyama H, Okada H, Okada N et al. HIV-1 Nef protein in the nucleus influences adipogenesis as well as viral transcription through the peroxisome proliferator-activated receptors. AIDS. 2004;18(2):189–98. doi: 10.1097/00002030-200401230-00007. [DOI] [PubMed] [Google Scholar]
- 66.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40(2):121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Price J, Hoy J, Ridley E, Nyulasi I, Paul E, Woolley I. Changes in the prevalence of lipodystrophy, metabolic syndrome and cardiovascular disease risk in HIV-infected men. Sex Health. 2015;12(3):240–8. doi: 10.1071/SH14084. [DOI] [PubMed] [Google Scholar]
- 68.Kosmiski L, Kuritzkes D, Hamilton J, Sharp T, Lichtenstien K, Hill J et al. Fat distribution is altered in HIV-infected men without clinical evidence of the HIV lipodystrophy syndrome. HIV Med. 2003;4(3):235–40. [DOI] [PubMed] [Google Scholar]
- 69.Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012;205 Suppl 3:S383–90. doi: 10.1093/infdis/jis205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leroyer S, Vatier C, Kadiri S, Quette J, Chapron C, Capeau J et al. Glyceroneogenesis is inhibited through HIV protease inhibitor-induced inflammation in human subcutaneous but not visceral adipose tissue. J Lipid Res. 2011;52(2):207–20. doi: 10.1194/jlr.M000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gallego-Escuredo JM, Villarroya J, Domingo P, Targarona EM, Alegre M, Domingo JC et al. Differentially altered molecular signature of visceral adipose tissue in HIV-1-associated lipodystrophy. J Acquir Immune Defic Syndr. 2013;64(2):142–8. doi: 10.1097/QAI.0b013e31829bdb67. [DOI] [PubMed] [Google Scholar]
- 72.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among human immunodeficiency virus-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51(9):1143–7. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 73.Sekhar RV, Jahoor F, Pownall HJ, Rehman K, Gaubatz J, Iyer D et al. Severely dysregulated disposal of postprandial triacylglycerols exacerbates hypertriacylglycerolemia in HIV lipodystrophy syndrome. Am J Clin Nutr. 2005;81(6):1405–10. doi: 10.1093/ajcn/81.6.1405. [DOI] [PubMed] [Google Scholar]
- 74.Sekhar RV, Jahoor F, White AC, Pownall HJ, Visnegarwala F, Rodriguez-Barradas MC et al. Metabolic basis of HIV-lipodystrophy syndrome. Am J Physiol Endocrinol Metab. 2002;283(2):E332–7. doi: 10.1152/ajpendo.00058.2002. [DOI] [PubMed] [Google Scholar]
- 75.Hellerstein MK, Grunfeld C, Wu K, Christiansen M, Kaempfer S, Kletke C et al. Increased de novo hepatic lipogenesis in human immunodeficiency virus infection. J Clin Endocrinol Metab. 1993;76(3):559–65. doi: 10.1210/jcem.76.3.8445011. [DOI] [PubMed] [Google Scholar]
- 76.Orlando G, Guaraldi G, Zona S, Carli F, Bagni P, Menozzi M et al. Ectopic fat is linked to prior cardiovascular events in men with HIV. J Acquir Immune Defic Syndr. 2012;59(5):494–7. doi: 10.1097/QAI.0b013e31824c8397. [DOI] [PubMed] [Google Scholar]
- 77.Guaraldi G, Scaglioni R, Zona S, Orlando G, Carli F, Ligabue G et al. Epicardial adipose tissue is an independent marker of cardiovascular risk in HIV-infected patients. AIDS. 2011;25(9):1199–205. doi: 10.1097/QAD.0b013e3283474b9f. [DOI] [PubMed] [Google Scholar]
- 78.Iantorno M, Soleimanifard S, Schar M, Brown TT, Bonanno G, Barditch-Crovo P et al. Regional coronary endothelial dysfunction is related to the degree of local epicardial fat in people with HIV. Atherosclerosis. 2018;278:7–14. doi: 10.1016/j.atherosclerosis.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gan SK, Samaras K, Thompson CH, Kraegen EW, Carr A, Cooper DA et al. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 2002;51(11):3163–9. doi: 10.2337/diabetes.51.11.3163. [DOI] [PubMed] [Google Scholar]
- 80.Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127(1):43–54. doi: 10.1172/JCI88880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reeds DN, Yarasheski KE, Fontana L, Cade WT, Laciny E, DeMoss A et al. Alterations in liver, muscle, and adipose tissue insulin sensitivity in men with HIV infection and dyslipidemia. Am J Physiol Endocrinol Metab. 2006;290(1):E47–E53. doi: 10.1152/ajpendo.00236.2005. [DOI] [PubMed] [Google Scholar]
- 82.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271(5249):665–8. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 83.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292(1):E166–74. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao D, Madi M, Ding C, Fok M, Steele T, Ford C et al. Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol Metab. 2014;307(3):E289–304. doi: 10.1152/ajpendo.00430.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jan V, Cervera P, Maachi M, Baudrimont M, Kim M, Vidal H et al. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther. 2004;9(4):555–64. [PubMed] [Google Scholar]
- 86.Damouche A, Pourcher G, Pourcher V, Benoist S, Busson E, Lataillade JJ et al. High proportion of PD-1-expressing CD4(+) T cells in adipose tissue constitutes an immunomodulatory microenvironment that may support HIV persistence. Eur J Immunol. 2017;47(12):2113–23. doi: 10.1002/eji.201747060. [DOI] [PubMed] [Google Scholar]
- 87.Koethe JR, McDonnell W, Kennedy A, Abana CO, Pilkinton M, Setliff I et al. Adipose Tissue is Enriched for Activated and Late-Differentiated CD8+ T Cells and Shows Distinct CD8+ Receptor Usage, Compared With Blood in HIV-Infected Persons. J Acquir Immune Defic Syndr. 2018;77(2):e14–e21. doi: 10.1097/QAI.0000000000001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 89.Capeau J, Bouteloup V, Katlama C, Bastard JP, Guiyedi V, Salmon-Ceron D et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26(3):303–14. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 90.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31(6):1224–9. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bastard JP, Couffignal C, Fellahi S, Bard JM, Mentre F, Salmon D et al. Diabetes and dyslipidaemia are associated with oxidative stress independently of inflammation in long-term antiretroviral-treated HIV-infected patients. Diabetes Metab. 2019. doi: 10.1016/j.diabet.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 92.McDonnell WJ, Koethe JR, Mallal SA, Pilkinton MA, Kirabo A, Ameka MK et al. High CD8 T-Cell Receptor Clonality and Altered CDR3 Properties Are Associated With Elevated Isolevuglandins in Adipose Tissue During Diet-Induced Obesity. Diabetes. 2018;67(11):2361–76. doi: 10.2337/db18-0040db18-0040 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Damouche A, Lazure T, Avettand-Fenoel V, Huot N, Dejucq-Rainsford N, Satie AP et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS pathogens. 2015;11(9):e1005153. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87(1):107–16. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 95.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175(12):8415–23. [DOI] [PubMed] [Google Scholar]
- 96.Wanjalla CN, McDonnell WJ, Barnett L, Simmons JD, Furch BD, Lima MC et al. Adipose Tissue in Persons With HIV Is Enriched for CD4(+) T Effector Memory and T Effector Memory RA(+) Cells, Which Show Higher CD69 Expression and CD57, CX3CR1, GPR56 Co-expression With Increasing Glucose Intolerance. Front Immunol. 2019;10:408. doi: 10.3389/fimmu.2019.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gordon CL, Lee LN, Swadling L, Hutchings C, Zinser M, Highton AJ et al. Induction and Maintenance of CX3CR1-Intermediate Peripheral Memory CD8(+) T Cells by Persistent Viruses and Vaccines. Cell Rep. 2018;23(3):768–82. doi: 10.1016/j.celrep.2018.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168(12):6173–80. [DOI] [PubMed] [Google Scholar]
- 99.Pachnio A, Ciaurriz M, Begum J, Lal N, Zuo J, Beggs A et al. Cytomegalovirus Infection Leads to Development of High Frequencies of Cytotoxic Virus-Specific CD4+ T Cells Targeted to Vascular Endothelium. PLoS Pathog. 2016;12(9):e1005832. doi: 10.1371/journal.ppat.1005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng YM, van de Garde MD, Cheng KF, Baars PA, Remmerswaal EB, van Lier RA et al. Specific expression of GPR56 by human cytotoxic lymphocytes. J Leukoc Biol. 2011;90(4):735–40. doi: 10.1189/jlb.0211092. [DOI] [PubMed] [Google Scholar]
- 101.McMahon CN, Petoumenos K, Hesse K, Carr A, Cooper DA, Samaras K. High rates of incident diabetes and prediabetes are evident in men with treated HIV followed for 11 years. AIDS. 2018;32(4):451–9. doi: 10.1097/QAD.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 102.Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and Risk Factors for Prediabetes and Diabetes Mellitus Among HIV-infected Adults on Antiretroviral Therapy: A Systematic Review and Meta-analysis. Epidemiology. 2018;29(3):431–41. doi: 10.1097/EDE.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 103.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–34. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duncan AD, Goff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: A cross-sectional study. PLoS One. 2018;13(3):e0194199. doi: 10.1371/journal.pone.0194199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Putcharoen O, Wattanachanya L, Sophonphan J, Siwamogsatham S, Sapsirisavat V, Gatechompol S et al. New-onset diabetes in HIV-treated adults: predictors, long-term renal and cardiovascular outcomes. AIDS. 2017;31(11):1535–43. doi: 10.1097/QAD.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 106.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33(10):2244–9. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neuhaus J, Jacobs DR Jr., Baker JV, Calmy A, Duprez D, La Rosa A et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mave V, Erlandson KM, Gupte N, Balagopal A, Asmuth DM, Campbell TB et al. Inflammation and Change in Body Weight With Antiretroviral Therapy Initiation in a Multinational Cohort of HIV-Infected Adults. J Infect Dis. 2016;214(1):65–72. doi: 10.1093/infdis/jiw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745–51. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 110.Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. AIDS. 2016;30(1):83–91. doi: 10.1097/QAD.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lake JE, Li X, Palella FJ Jr., Erlandson KM, Wiley D, Kingsley L et al. Metabolic health across the BMI spectrum in HIV-infected and HIV-uninfected men. AIDS. 2018;32(1):49–57. doi: 10.1097/QAD.0000000000001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sattler FR, He J, Letendre S, Wilson C, Sanders C, Heaton R et al. Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J Acquir Immune Defic Syndr. 2015;68(3):281–8. doi: 10.1097/QAI.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lake JE, Popov M, Post WS, Palella FJ, Sacktor N, Miller EN et al. Visceral fat is associated with brain structure independent of human immunodeficiency virus infection status. J Neurovirol. 2017;23(3):385–93. doi: 10.1007/s13365-016-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu B, Pasipanodya E, Montoya JL, Moore RC, Gianella S, McCutchan A et al. Metabolic Syndrome and Neurocognitive Deficits in HIV Infection. J Acquir Immune Defic Syndr. 2019;81(1):95–101. doi: 10.1097/QAI.0000000000001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 117.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV. Circulation. 2018;138(11):1100–12. doi: 10.1161/CIRCULATIONAHA.117.033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baker JV, Duprez D, Rapkin J, Hullsiek KH, Quick H, Grimm R et al. Untreated HIV infection and large and small artery elasticity. J Acquir Immune Defic Syndr. 2009;52(1):25–31. doi: 10.1097/qai.0b013e3181b02e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iantorno M, Schar M, Soleimanifard S, Brown TT, Moore R, Barditch-Crovo P et al. Coronary artery endothelial dysfunction is present in HIV-positive individuals without significant coronary artery disease. AIDS. 2017;31(9):1281–9. doi: 10.1097/QAD.0000000000001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30(7):850–6. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 126. •.Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS. 2017;31(11):1621–32. doi: 10.1097/QAD.0000000000001504. [DOI] [PubMed] [Google Scholar]; This meta-analysis of five studies reported prevalence and associated risk factors for steatosis, NASH and fibrosis among PWH.
- 127.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 128.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377(21):2063–72. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 129.Lui G, Wong VW, Wong GL, Chu WC, Wong CK, Yung IM et al. Liver fibrosis and fatty liver in Asian HIV-infected patients. Aliment Pharmacol Ther. 2016;44(4):411–21. doi: 10.1111/apt.13702. [DOI] [PubMed] [Google Scholar]
- 130.Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther. 2015;41(4):368–78. doi: 10.1111/apt.13052. [DOI] [PubMed] [Google Scholar]
- 131.Crum-Cianflone N, Krause D, Wessman D, Medina S, Stepenosky J, Brandt C et al. Fatty liver disease is associated with underlying cardiovascular disease in HIV-infected persons(*). HIV Med. 2011;12(8):463–71. doi: 10.1111/j.1468-1293.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zizza A, Guido M, Tumolo MR, De Donno A, Bagordo F, Grima P. Atherosclerosis is associated with a higher risk of hepatic steatosis in HIV-infected patients. J Prev Med Hyg. 2017;58(3):E219–E24. [PMC free article] [PubMed] [Google Scholar]
- 133.Mohr R, Boesecke C, Dold L, Schierwagen R, Schwarze-Zander C, Wasmuth JC et al. Return-to-health effect of modern combined antiretroviral therapy potentially predisposes HIV patients to hepatic steatosis. Medicine (Baltimore). 2018;97(17):e0462. doi: 10.1097/MD.0000000000010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dewar C, Anstee QM, Price DA, Payne B. Central obesity and nonalcoholic fatty liver disease in people living with HIV: a case for targeted screening? HIV Med. 2019;20(1):e1–e2. doi: 10.1111/hiv.12674. [DOI] [PubMed] [Google Scholar]
- 135.Reeds DN, Mittendorfer B, Patterson BW, Powderly WG, Yarasheski KE, Klein S. Alterations in lipid kinetics in men with HIV-dyslipidemia. Am J Physiol Endocrinol Metab. 2003;285(3):E490–7. doi: 10.1152/ajpendo.00118.2003. [DOI] [PubMed] [Google Scholar]
- 136.Lemoine M, Barbu V, Girard PM, Kim M, Bastard JP, Wendum D et al. Altered hepatic expression of SREBP-1 and PPARgamma is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS. 2006;20(3):387–95. doi: 10.1097/01.aids.0000206503.01536.11. [DOI] [PubMed] [Google Scholar]
- 137.Moreno-Torres A, Domingo P, Pujol J, Blanco-Vaca F, Arroyo JA, Sambeat MA. Liver triglyceride content in HIV-1-infected patients on combination antiretroviral therapy studied with 1H-MR spectroscopy. Antivir Ther. 2007;12(2):195–203. [PubMed] [Google Scholar]
- 138.Price JC, Seaberg EC, Latanich R, Budoff MJ, Kingsley LA, Palella FJ et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol. 2014;109(5):695–704. doi: 10.1038/ajg.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akhtar MA, Mathieson K, Arey B, Post J, Prevette R, Hillier A et al. Hepatic histopathology and clinical characteristics associated with antiretroviral therapy in HIV patients without viral hepatitis. Eur J Gastroenterol Hepatol. 2008;20(12):1194–204. doi: 10.1097/MEG.0b013e328305b9e0. [DOI] [PubMed] [Google Scholar]
- 140.Guaraldi G, Squillace N, Stentarelli C, Orlando G, D’Amico R, Ligabue G et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47(2):250–7. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 141.Perazzo H, Cardoso SW, Yanavich C, Nunes EP, Morata M, Gorni N et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J Int AIDS Soc. 2018;21(11):e25201. doi: 10.1002/jia2.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eckert C, Klein N, Kornek M, Lukacs-Kornek V. The complex myeloid network of the liver with diverse functional capacity at steady state and in inflammation. Front Immunol. 2015;6:179. doi: 10.3389/fimmu.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61(3):1066–79. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sakaguchi S, Takahashi S, Sasaki T, Kumagai T, Nagata K. Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet. 2011;26(1):30–46. [DOI] [PubMed] [Google Scholar]
- 145.Roubenoff R, Weiss L, McDermott A, Heflin T, Cloutier GJ, Wood M et al. A pilot study of exercise training to reduce trunk fat in adults with HIV-associated fat redistribution. AIDS. 1999;13(11):1373–5. doi: 10.1097/00002030-199907300-00015. [DOI] [PubMed] [Google Scholar]
- 146.Engelson ES, Agin D, Kenya S, Werber-Zion G, Luty B, Albu JB et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55(10):1327–36. doi: 10.1016/j.metabol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 147.Fitch KV, Anderson EJ, Hubbard JL, Carpenter SJ, Waddell WR, Caliendo AM et al. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS. 2006;20(14):1843–50. doi: 10.1097/01.aids.0000244203.95758.db. [DOI] [PubMed] [Google Scholar]
- 148.Thoni GJ, Fedou C, Brun JF, Fabre J, Renard E, Reynes J et al. Reduction of fat accumulation and lipid disorders by individualized light aerobic training in human immunodeficiency virus infected patients with lipodystrophy and/or dyslipidemia. Diabetes Metab. 2002;28(5):397–404. [PubMed] [Google Scholar]
- 149.Webel AR, Moore SM, Longenecker CT, Currie J, Horvat Davey C, Perazzo J et al. Randomized Controlled Trial of the SystemCHANGE Intervention on Behaviors Related to Cardiovascular Risk in HIV+ Adults. J Acquir Immune Defic Syndr. 2018;78(1):23–33. doi: 10.1097/QAI.0000000000001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guariglia DA, Pedro RE, Deminice R, Rosa FT, Peres SB, Franzoi De Moraes SM. Effect of combined training on body composition and metabolic variables in people living with HIV: A randomized clinical trial. Cytokine. 2018;111:505–10. doi: 10.1016/j.cyto.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 151.Becofsky K, Wing EJ, McCaffery J, Boudreau M, Wing RR. A Randomized Controlled Trial of a Behavioral Weight Loss Program for Human Immunodeficiency Virus-Infected Patients. Clin Infect Dis. 2017;65(1):154–7. doi: 10.1093/cid/cix238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. •.Reeds DN, Pietka TA, Yarasheski KE, Cade WT, Patterson BW, Okunade A et al. HIV infection does not prevent the metabolic benefits of diet-induced weight loss in women with obesity. Obesity (Silver Spring). 2017;25(4):682–8. doi: 10.1002/oby.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compared diet-induced weight loss and metabolic parameters of women with HIV compared with women without HIV. Weight loss improved insulin sensitivity to the same extent in both groups.
- 153.Stanley TL, Falutz J, Marsolais C, Morin J, Soulban G, Mamputu JC et al. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin Infect Dis. 2012;54(11):1642–51. doi: 10.1093/cid/cis251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312(4):380–9. doi: 10.1001/jama.2014.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 156.Favre L, Marino L, Roth A, Acierno J Jr., Hans D, Demartines N et al. The Reduction of Visceral Adipose Tissue after Roux-en-Y Gastric Bypass Is more Pronounced in Patients with Impaired Glucose Metabolism. Obes Surg. 2018;28(12):4006–13. doi: 10.1007/s11695-018-3455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 158.Akbari K, Som R, Sampson M, Abbas SH, Ramus J, Jones G. The Effect of Bariatric Surgery on Patients with HIV Infection: a Literature Review. Obes Surg. 2018;28(8):2550–9. doi: 10.1007/s11695-018-3319-4. [DOI] [PubMed] [Google Scholar]
- 159.Selke H, Norris S, Osterholzer D, Fife KH, DeRose B, Gupta SK. Bariatric surgery outcomes in HIV-infected subjects: a case series. AIDS Patient Care STDS. 2010;24(9):545–50. doi: 10.1089/apc.2010.0132. [DOI] [PubMed] [Google Scholar]
- 160.Pourcher G, Peytavin G, Schneider L, Gallien S, Force G, Pourcher V. Bariatric surgery in HIV patients: experience of an Obesity Reference Center in France. Surg Obes Relat Dis. 2017;13(12):1990–6. doi: 10.1016/j.soard.2017.09.514. [DOI] [PubMed] [Google Scholar]
- 161.Sharma G, Strong AT, Boules M, Tu C, Szomstein S, Rosenthal R et al. Comparative Outcomes of Bariatric Surgery in Patients With and Without Human Immunodeficiency Virus. Obes Surg. 2018;28(4):1070–9. doi: 10.1007/s11695-017-2996-8. [DOI] [PubMed] [Google Scholar]
- 162.Sharma P, McCarty TR, Ngu JN, O’Donnell M, Njei B. Impact of bariatric surgery in patients with HIV infection: a nationwide inpatient sample analysis, 2004–2014. AIDS. 2018;32(14):1959–65. doi: 10.1097/QAD.0000000000001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Amouyal C, Buyse M, Lucas-Martini L, Hirt D, Genser L, Torcivia A et al. Sleeve Gastrectomy in Morbidly Obese HIV Patients: Focus on Anti-retroviral Treatment Absorption After Surgery. Obes Surg. 2018;28(9):2886–93. doi: 10.1007/s11695-018-3308-7. [DOI] [PubMed] [Google Scholar]


