Abstract
Introduction:
Occult hypoperfusion (OH), or global hypoperfusion with normal vital signs, is a risk factor for poor outcomes in elderly trauma patients. We hypothesized that OH is associated with worse outcomes than shock in both young and elderly trauma patients.
Methods:
A single-center cohort study of adult (≥16 years) trauma patients from 2016 to 2018 with a base excess (BE) measured on arrival was performed. Perfusion states were defined as: Shock if HR>120 or SBP<90; OH if BE<−2, HR<120, and SBP>90; and Normal for all others. Patients were stratified as young (<55 years) or elderly (≥55). Bayesian log binomial regression were utilized to assess the relationship between arrival perfusion state and mortality or serious complication.
Results:
Of 3,126 included patients, 808 were elderly. Rates of shock (33% and 31%) and OH (25% and 23%) were similar in young and elderly patients respectively. OH on arrival was associated with higher odds of mortality or serious complication than normal perfusion regardless of age group. Compared to shock, OH was associated with an OR 1.21 (95% CI 0.97–1.52, posterior probability 96%) of poor outcome in the elderly and an OR 0.52 (95% CI 0.42–0.65, posterior probability <1%) of poor outcome in younger patients. Findings were similar on sensitivity analysis excluding shock patients with BE≥−2.
Conclusions:
In elderly, but not younger, patients, OH is associated with worse outcomes than shock. Although shock parameters may need to be redefined in the elderly, more attention is necessary to the diagnosis and treatment of all hypoperfused states in this age group.
Keywords: Elderly Trauma, Occult Hypoperfusion, Shock
Precis:
In elderly trauma patients, occult hypoperfusion, defined as global hypoperfusion in the setting of normal vital signs, was associated with worse outcomes than shock. This was not observed in young patients. Improvement in time to recognition of and treatment for occult hypoperfusion may improve outcomes for elderly trauma patients.
Introduction
Individuals between 45 and 65 years of age make up nearly 30% of the United States population and represent the most rapidly growing age group. [1] It is estimated that by 2050, elderly trauma patients will make up 40% of trauma admissions. [2, 3] Advanced age is a known risk factor for poor outcomes among trauma patients, and the severity of injury often exceeds the mechanism. While the definition of advanced age in the setting of trauma has been widely debated, most studies have revealed an age-related increase in poor outcome near 55 years. [4] As patients pass this threshold, there may be multiple reasons for poor outcomes including, but not limited to, comorbidities or an age-associated decline in physiologic reserve.
Increased attention is being placed on improving the outcomes of elderly trauma patients. Quality improvement efforts have tended to focus on improvements in in-hospital care such as through multidisciplinary team care and consultation with geriatric subspecialists. [5, 6] Other efforts have focused on increased scrutiny of trauma activation criteria for elderly trauma patients in the emergency department; however, this may lead to over-triage and overutilization of resources. [7, 8]
One potential benefit of trauma team activation is the earlier recognition of the degree of physiologic derangement, such as occult hypoperfusion (OH), which is not always recognized in elderly patients. [9–12] OH is the presence of global hypoperfusion in the setting of normal vital signs, resulting in insufficient tissue perfusion and oxygenation. The presence, severity, and duration of OH is associated with increased morbidity and mortality after trauma, when compared to patients with normal perfusion on arrival. [13–16] It is unknown how poor outcomes associated with OH (“normal” vital signs in the presence of abnormal base excess or lactate) compare to the poor outcomes associated with hypoperfusion accompanied by vital sign abnormalities as in shock. Detection of OH and treatment may be delayed when compared to shock, and the resources dedicated to treating a patient with OH may be insufficient. Therefore, we hypothesized that both young and elderly patients presenting with OH would be associated with worse outcomes than overt shock.
Methods
The McGovern Medical School at UTHealth institutional review board approved this study. A retrospective cohort study was conducted at the Red Duke Trauma Instituted (RDTI) at Memorial Hermann Hospital - Texas Medical Center, a high-volume, level 1 trauma center in Houston, Texas, USA. Adult (≥16 years) trauma patients presenting between 2016 and 2018 were included. Patients admitted to the Burn Surgery service or without a base excess drawn on arrival were excluded. Demographic characteristics, injury details, and outcomes of in-hospital mortality, major complications, length of stay, and ICU length of stay were obtained from the institution’s prospectively maintained trauma registry. Patients were categorized as young if <55 years or elderly if ≥55 years. [17–19] Patients were assigned an arrival perfusion state according to arrival vitals and base excess result: shock if either SBP <90 mmHg or a HR >120 beats per minute, OH if SBP ≥90 mmHg and HR ≤120 and BE <−2, and normal for all others. [20] The Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies were followed. [21]
Outcome Measures:
The primary outcome of this study was a composite of in-hospital mortality or major complication, defined according to the National Trauma Data Bank dictionary. [22] Major complications included in the composite outcome included: unplanned intensive care unit (ICU) admission, unplanned intubation, unplanned return to operating room, acute renal failure, acute respiratory distress syndrome, or cardiac arrest. Each of the components of the primary outcome were evaluated as secondary outcomes. Additionally, unfavorable discharge disposition, defined as discharge to a location other than home, jail, or a psychiatric facility, was evaluated as a secondary outcome.
Statistical Analysis:
Statistical analyses were conducted using R version 3.53 (R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). Incidence, demographics, and outcomes were compared between arrival perfusion groups within age strata. Continuous variables were presented as medians (interquartile range, IQR). Chi-square, Wilcoxon rank-sum, and Kruskal Wallis tests were utilized to compare categorical and continuous demographic data and outcomes between arrival perfusion groups. Logistic Bayesian regression was used to estimate relative odds of the composite primary outcome between perfusion states. Similar models were created to assess each of the secondary outcomes. Clinically sound covariates were chosen a-priori: age, sex, trauma type, and injury severity score. Statistical interaction terms were evaluated in the complete cohort with frequentist models. Missing data was considered missing at random and imputed using multiple imputation chain equations. [23]
To maximize the knowledge gained from this study, Bayesian statistics were utilized for analysis. Bayesian inference utilizes a prior, or an estimated effect based on previous evidence, and likelihood, which summarizes the data from the study. The prior and likelihood are combined to produce a posterior, or estimated exposure effect, with a credible interval that conveys the magnitude and precision of the effect. A posterior may be utilized to determine the posterior probability of a specific effect, such as harm or benefit of the exposure under study. [24] Although Bayesian methods allow estimation of the probability of any magnitude of effect, harm in this study was defined as a posterior odds ratio (OR) of mortality or major complication of >1. Due to the lack of prior data regarding the comparison of patients who arrived with OH compared to shock, a weakly informative, neutral prior with an OR = 1.0 (95% credible interval 0.33–3.0) was applied.
Three sensitivity analyses were performed. First, to evaluate the possibility of misclassification of patients into the category of shock due to isolated tachycardia or hypotension, which may be related to pain or a spurious measurement, a requirement of a BE < −2 was applied to all shock patients and the analyses repeated. Second, to address alternative definitions of hypotension after trauma, the definition of shock was expanded to include patients with a SBP of 110 or less. [25] Finally, to better explore the age at which the risk of OH overcomes the risk of shock, the cohort was split at five-year increments from the age of 25 to the age of 70, and the models were re-fit using the same covariates.
Results
There were 18,331 trauma patients who presented to the RDTI between 2016 and 2018. Of those, 3,301 had a base excess measured on arrival. One-hundred seventy-five patients were excluded due to burn as the cause of injury, resulting in 3,126 patients remaining for analysis. Less than 1% of data were missing. The median age was 37 (IQR 25–55) years. The majority (74%) of the cohort was male. Participants were severely injured with a median injury severity score (ISS) of 17 (IQR 9–26). Elderly patients, when compared to young patients, were more frequently female (31% vs. 22%, p<0.001), injured by a blunt mechanism (91% vs. 64%, p<0.001), transferred from another hospital (38% vs. 24%, p<0.001), and more severely injured (median injury severity score 19 vs. 16, p<0.001). Elderly patients had a lower median arrival Glasgow Coma Scale (GCS, median 12 vs. 15, p<0.001), and heart rate (median 89 vs. 98, p<0.001), but had a higher SBP (median 129 vs. 124, p=0.009) and higher BE (−2 vs. −3, p<0.001).
The distribution of arrival perfusion states was similar between young and elderly patients. Among elderly patients, 23% arrived with OH and 31% arrived with shock. Among young patients, 25% arrived with OH and 33% arrived with shock. Elderly patients suffered major complication and death more frequently, at an 11% and 27% rate, respectively. Young patients suffered major complication and mortality at a rate of 6% and 13%, respectively.
Young patients exhibited progressive physiologic derangement from normal to OH to shock. Across this continuum, the GCS, SBP, hemoglobin, and BE declined while heart rate increased. (Table 1) Corresponding with this change, length of stay, complications and mortality increased from the normal group to the OH group to the shock group. However, elderly patients displayed a different continuum. Patients arriving with normal perfusion demonstrated superior physiology than those presenting with OH or shock, but patients with OH had a lower GCS and lower BE than patients with shock. Complications or mortality occurred least frequently in elderly patients arriving with normal perfusion and most frequently in patients arriving with OH. Elderly patients arriving with shock suffered more complications than those arriving with normal perfusion and fewer than those arriving with OH. Similarly, elderly patients with OH had a longer ICU length of stay than those arriving with either normal perfusion or shock.
Table 1:
Characteristics of Study Participants
| Young Patients | Elderly Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal (n=982) | OH (n=577) | Shock (n=759) | p | Normal (n=374) | OH (n=187) | Shock (n=247) | p | |
| Age, years | 29 (22–39) | 31 (23–41) | 31 (23–40) | 0.02 | 68 (60–77) | 64 (59–74) | 68 (61–78) | 0.007 |
| Male Sex | 811 (83%) | 436 (76%) | 568 (75%) | <0.001 | 248 (66%) | 187 (76%) | 123 (66%) | 0.03 |
| Race/Ethnicity | ||||||||
| White | 272 (28%) | 227 (30%) | 177 (31%) | 198 (53%) | 130 (53%) | 99 (53%) | ||
| Black | 323 (33%) | 170 (22%) | 124 (22%) | <0.001 | 54 (14%) | 30 (12%) | 28 (15%) | 0.97 |
| Hispanic | 112 (11%) | 125 (17%) | 108 (19%) | 41 (48%) | 24 (10%) | 20 (11%) | ||
| Asian | 16 (2%) | 11 (1%) | 8 (1%) | 8 (43%) | 5 (2%) | 2 (1%) | ||
| Blunt Mechanism of Injury | 519 (53%) | 537 (71%) | 422 (73%) | <0.001 | 346 (93%) | 218 (88%) | 169 (90%) | 0.20 |
| Transferred from Outside Hospital | 297 (30%) | 172 (23%) | 89 (15%) | <0.001 | 151 (40%) | 99 (40%) | 60 (32%) | 0.14 |
| Injury Severity Score | 10 (5–19) | 17 (10–27) | 22 (12–34) | <0.001 | 17 (10–26) | 22 (10–29) | 22 (10–32) | <0.001 |
| Arrival Glasgow Coma Scale | 15 (12–15) | 13 (3–15) | 12 (3–15) | <0.001 | 13 (3–15) | 7 (3–15) | 13 (3–15) | 0.007 |
| Arrival Systolic Blood Pressure, mmHg | 130 (118–145) | 124 (108–141) | 104 (82–131) | <0.001 | 138 (120–156) | 129 (110–150) | 88 (75–130) | <0.001 |
| Arrival Diastolic Blood Pressure, mmHg | 80 (70–90) | 76 (65–85) | 63 (50–80) | <0.001 | 79 (68–88) | 76 (67–87) | 56 (46–79) | <0.001 |
| Arrival Heart Rate | 89 (77–101) | 95 (82–107) | 129 (122–139) | <0.001 | 83 (72–97) | 88 (76–104) | 115 (85–130) | <0.001 |
| Arrival Hemoglobin | 13.9 (12.8–14.9) | 13.4 (11.9–14.5) | 13 (11.3–14.5) | <0.001 | 12.8 (11.1–14.2) | 12.5 (10.9–13.8) | 11.9 (10.4–13.5) | <0.001 |
| Arrival Base Excess, mmol/L | 0 (−1–1) | −5 (−7– −4) | −6 (−9– −2) | <0.001 | 0 (−1–2) | −5 (−7– −4) | −4 (−8 – −1) | <0.001 |
| ICU Length of Stay | 0 (0–2) | 2 (0–5) | 2 (0–7) | <0.001 | 2 (0–4) | 3 (1–8) | 2 (1–6) | 0.003 |
| Hospital Length of Stay | 4 (2–9) | 7 (3–15) | 9 (3–20) | <0.001 | 6 (2–13) | 7 (2–15) | 8 (3–16) | 0.24 |
| Any Complication | 28 (3%) | 40 (5%) | 74 (13%) | <0.001 | 34 (9%) | 39 (16%) | 18 (10%) | 0.03 |
| Unplanned ICU Admission | 14 (1%) | 20 (3%) | 21 (4%) | 0.02 | 16 (4%) | 16 (7%) | 6 (3%) | 0.25 |
| Unplanned Intubation | 4 (0.5%) | 7 (1%) | 12 (2%) | 0.006 | 4 (1%) | 6 (2%) | 3 (2%) | 0.42 |
| Unplanned Return to OR | 11 (1%) | 11 (1%) | 24 (4%) | <0.001 | 15 (4%) | 12 (5%) | 5 (3%) | 0.52 |
| Acute Renal Failure | 3 (0.5%) | 8 (1%) | 24 (4%) | <0.001 | 6 (2%) | 14 (6%) | 8 (4%) | 0.02 |
| ARDS | 2 (0.5%) | 6 (1%) | 9 (2%) | 0.01 | 2 (1%) | 4 (2%) | 3 (2%) | 0.35 |
| Cardiac Arrest | 4 (0.5%) | 7 (1%) | 17 (3%) | <0.001 | 15 (4%) | 9 (4%) | 12 (6%) | 0.32 |
| In-hospital Mortality | 26 (3%) | 69 (9%) | 94 (16%) | <0.001 | 75 (20%) | 85 (34%) | 58 (31%) | <0.001 |
| Complication or Mortality | 51 (5%) | 102 (13%) | 154 (27%) | <0.001 | 101 (27%) | 111 (45%) | 69 (37%) | <0.001 |
Continuous data presented as: median (IQR)
OH – Occult Hypoperfusion; ICU – intensive care unit; OR – Operating Room; ARDS – Acute Respiratory Distress Syndrome
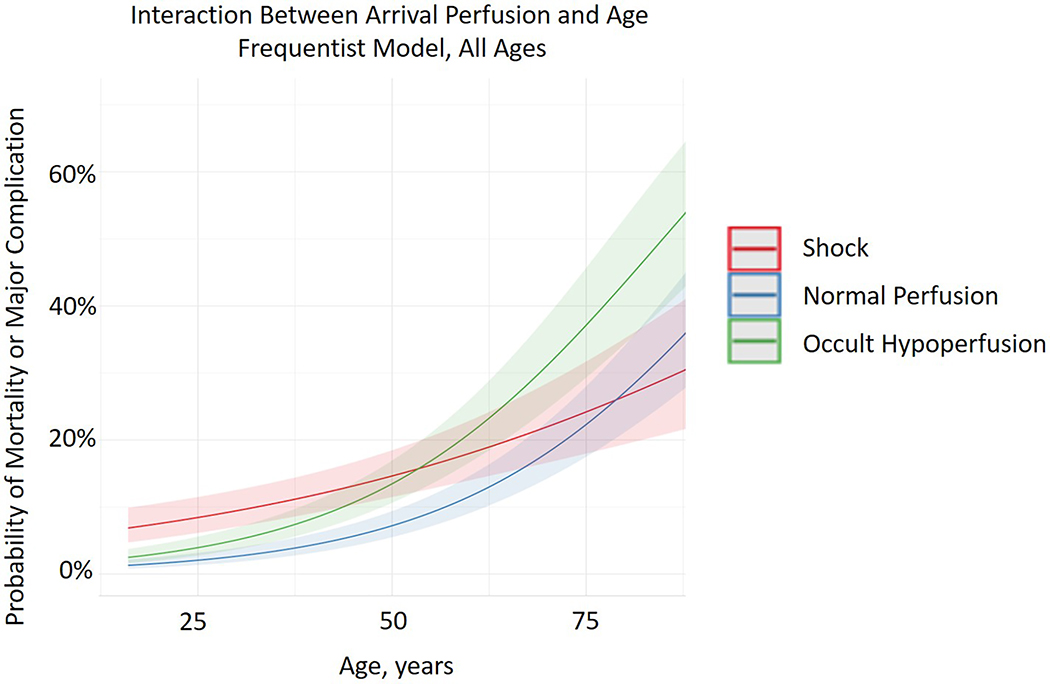
Statistical Interaction of Age and Arrival Perfusion:
On multivariable analysis of the complete cohort, age and perfusion state demonstrated a strong interaction (p<0.001). (Figure 1)
Figure 1.
Interaction between arrival perfusion and age, frequentist model, all ages.
Multivariable Analysis:
After the cohort was split by age, young patients demonstrated incremental odds of complication or mortality, with patients with normal perfusion having the lowest risk. Patients arriving with OH were at higher odds of poor outcome than patients arriving with normal perfusion but at a lower odds than patients arriving with shock. (Table 2) Elderly patients did not follow this incremental increase in odds of complication or mortality. Patients arriving with normal perfusion were, again, at the lowest odds for complication or mortality, followed by patients arriving with shock. It was patients arriving with OH demonstrated the highest odds of major complication or mortality, when compared to both normal perfusion and shock. There was a 96% posterior probability that arrival OH is associated with more major complications and mortality than shock in elderly patients.
Table 2:
Bayesian Multivariable Analysis of Arrival Perfusion and Primary Outcome
| Young (<55 years) | Elderly (≥55 years) | ||||
|---|---|---|---|---|---|
| Relative Odds of Mortality or Major Complication (95% Credible Interval) | Posterior Probability* | Relative Odds of Mortality or Major Complication (95% Credible Interval) | Posterior Probability* | ||
| Compared to Normal Perfusion | OH | 2.26 (1.68–3.05) | >99% | 1.65 (1.33–2.02) | >99% |
| Shock | 4.54 (3.42–5.95) | >99% | 1.36 (1.06–1.73) | >99% | |
| Compared to Shock | Normal | 0.22 (0.16–0.29) | <1% | 0.73 (0.57–0.93) | <1% |
| OH | 0.52 (0.42–0.65) | <1% | 1.21 (0.97–1.52) | 96% | |
Posterior probability that arrival perfusion state is associated with increased mortality or major complication
There was a high posterior probability that arrival OH is associated with more complications and mortality than shock in the elderly. (Table 3) There was a low posterior probability that arrival OH is associated with more episodes of cardiac arrest and unfavorable discharge disposition in elderly patients when compared to shock. In contrast, there was a low posterior probability that arrival OH is associated with more complications than shock in young patients.
Table 3:
Bayesian Multivariable Analyses of Arrival Perfusion and Secondary Outcomes
| Young (<55 years) | Elderly (≥55 years) | |||
|---|---|---|---|---|
| Relative Odds of Mortality or Major Complication (95% Credible Interval) | Posterior Probability* | Relative Odds of Mortality or Major Complication (95% Credible Interval) | Posterior Probability* | |
| Unplanned ICU Admission | 0.87 (0.49–1.45) | 26% | 1.55 (0.80–3.04) | 90% |
| Unplanned Intubation | 0.69 (0.35–1.41) | 15% | 1.36 (0.67–2.77) | 81% |
| Unplanned Return to OR | 0.49 (0.26–0.88) | <1% | 1.32 (0.56–3.10) | 74% |
| Acute Renal Failure | 0.43 (0.22–0.80) | <1% | 1.39 (0.70–2.80) | 83% |
| Acute Respiratory Distress Syndrome | 0.82 (0.36–1.77) | 30% | 1.18 (0.46–2.96) | 64% |
| Cardiac Arrest | 0.53 (0.26–1.05) | 3% | 0.71 (0.35–1.39) | 16% |
| Any Major Complication | 0.47 (0.33–0.66) | <1% | 1.47 (0.93–2.36) | 95% |
| Unfavorable Discharge Disposition | 0.89 (0.77–1.04) | 7% | 0.95 (0.84–1.08) | 20% |
| Mortality | 0.59 (0.44–0.79) | <1% | 1.14 (0.87–1.48) | 81% |
Posterior probability that OH is associated with increased outcome, when compared to shock
Sensitivity Analyses:
To evaluate the possibility of misclassification of patients into the shock category, patients were reclassified from shock to normal if their BE ≥−2. Of 187 elderly patients with shock, 75 (40%) of patients were reclassified to the normal group. Of 577 young patients with shock, 147 (26%) had BE ≥−2, which were also reclassified into the normal group. After reclassification, similar findings were revealed on multivariable analysis. (Table 4) However, the elderly OH and shock groups converged, with a drop in posterior probability that OH increases mortality or complication from 96% to 77% with the new classifications.
Table 4:
Sensitivity Analysis after Reclassification of Shock to Require BE <−2
| Young (<55 years) | Elderly (≥55 years) | ||||
|---|---|---|---|---|---|
| Odds Ratio for Mortality or Major Complication (95% Credible Interval) | Posterior Probability* | Odds Ratio for Mortality or Major Complication (95% Credible Interval) | Posterior Probability* | ||
| Compared to Normal Perfusion | OH | 1.87 (1.42–2.42) | >99% | 1.61 (1.31–1.95) | >99% |
| Shock | 4.19 (3.25–5.34) | >99% | 1.46 (1.11–1.87) | >99% | |
| Compared to Shock | Normal | 0.24 (0.18–0.31) | <1% | 0.68 (0.53–0.89) | <1% |
| OH | 0.47 (0.37–0.59) | <1% | 1.11 (0.86–1.43) | 77% | |
Posterior probability that arrival perfusion state is associated with increased mortality or major complication
A second reclassification of patients was undertaken to raise the SBP definition of shock to include patients with SBP <110. Of 567 elderly patients with either normal or OH perfusion on arrival, 56 (10%) of patients had a SBP <110, which were then reclassified to the shock group. Of 1,577 young patients with either normal or OH perfusion on arrrival, 162 (10%) had SBP <110, which were also reclassified into the shock group. After reclassification, there were no differences noted on multivariable analysis. Among elderly patients, there was an odds ratio of 1.20 (95% CI 0.95–1.48) and a 94% posterior probability that arrival OH was associated with more mortality or major complication than shock. (Table 5)
Table 5:
Sensitivity Analysis after Reclassification Using an SBP Threshold of 110 to Define Shock
| Young (<55 years) | Elderly (≥55 years) | ||||
|---|---|---|---|---|---|
| Odds Ratio for Mortality or Major Complication (95% Credible Interval) | Posterior Probability* | Odds Ratio for Mortality or Major Complication (95% Credible Interval) | Posterior Probability* | ||
| Compared to Normal Perfusion | OH | 2.15 (1.58–2.97) | >99% | 1.61 (1.29–2.01) | >99% |
| Shock | 4.04 (3.08–5.40) | >99% | 1.36 (1.07–1.71) | >99% | |
| Compared to Shock | Normal | 0.24 (0.18–0.32) | <1% | 0.73 (0.57–0.92) | <1% |
| OH | 0.56 (0.44–0.70) | <1% | 1.20 (0.95–1.48) | 94% | |
Posterior probability that arrival perfusion state is associated with increased mortality or major complication
Finally, to assess the age at which OH becomes more hazardous than shock, varying age cut-offs were utilized to split young and elderly. At a younger age cutoff, arrival with OH was associated with less mortality or major complications than shock. After the age of 50 years, there was a prominent increase in odds of poor outcomes associated with OH compared to shock (OR 1.18, 95% CI 0.96–1.49, posterior probability 94%).
Discussion
The current study found that OH is common across age groups, occurring in approximately one quarter of severely injured trauma patients. More importantly, OH is associated with the highest probability of poor outcomes in elderly patients, even worse than overt shock. This pattern was not observed in young trauma patients, where the highest probability of poor outcome was noted in the arrival shock group. The demonstrated interaction between age and arrival perfusion status became apparent after 50 years of age and suggests an age-dependent physiologic change in the response to trauma, which may be under-recognized or inappropriately treated. Among elderly patients, worse outcomes observed in patients who present with OH (compared to those presenting with shock) may be due to either worsened underlying physiology or lack of timely detection and prompt treatment of this hypoperfusion in the setting of normal vital signs. The findings of our study may be due to a combination of these two phenomena.
In a normal response to central blood volume loss, a vagal withdrawal results in activation of the sympathetic fibers to the heart and blood vessels, inducing increased heart rate and vasoconstriction to preserve end-organ perfusion. [26–29] An age-related decline in cardiac β- adrenergic receptor responsiveness in response to systemic hypoxia has been shown in human models of respiratory hypoxia. [30, 31] A departure from the normal compensatory mechanisms including increase in heart rate, may signal global inability or pharmacological manipulation, leaving the elderly patient unable to compensate for physiologic stress. If this is the case, identification of hypoperfusion by means other than traditional vital signs is paramount to ensuring proper and timely treatment. The addition of biomarkers to the initial assessment of perfusion may be of particular value. Lactic acid and base excess are the foremost biomarkers currently utilized to support a clinical diagnosis of hypoperfusion. [32, 33] Both of these markers are elevated due to tissue hypoxia, characterized by supply-dependent oxygen consumption and reflect a metabolic acidosis resistant to respiratory compensation. The sensitivity analysis performed in this study reveals a convergence of the odds of poor outcome associated with both OH and shock when base excess was added to the shock definition. This finding suggests that incorporation of base excess into a shock definition for the elderly may improve risk stratification. However, this reclassification did not mitigate the full effect observed, with a persistently high posterior probability that OH is associated with worse outcomes than shock. This suggests that the lack of a vital sign response, specifically the lack of a tachycardia response, in an elderly individual may indicate a global decrease in physiologic reserve and ability to recover from traumatic injury.
Contrary to our hypothesis, young patients did not exhibit the same relationship between outcomes when comparing those arriving with OH and those with shock as elderly. Our initial hypothesis was that arrival OH would be associated with worse outcomes than shock in both age groups, based on the suspicion of clinical under-recognition and under-treatment. However, young patients may be able to compensate for hypoperfusion after the initial assessment, whereas compensatory mechanisms may be altered in the elderly. This may be related to three age-related changes in the response to hypoxia or hypoperfusion: impaired vasodilation, reduced mobilization of capacitance blood from peripheral circulation, and decreased net capillary fluid absorption from extravascular tissues, which has been demonstrated by a number of experimental studies. [34–37] Elderly patients with OH may be unable to compensate for the lost volume due to trauma, whereas compensatory mechanisms are intact for young patients.
The present study also showed that the change in the poor prognosis between OH and shock became apparent at an elderly definition of 50 years old. The American Association for the Surgery of Trauma and the Committee on Trauma both consider a patient “geriatric” at the age of 65. [38] However, numerous studies have shown that detrimental changes in the response to trauma occur earlier, with some studies revealing an age effect as early as of 45 years. [39] Given these findings, efforts to improve identification and treatment of OH in elderly should be applied to patients as young as 45 to 50 years.
Differences in optimal triage between young and elderly patients has gained traction recently, with more scrutiny being placed on the application of traditional vital signs. The majority of this work has centered on pre-hospital triage and the improvement of the National Trauma Triage Protocol. Recent works have encouraged increasing the threshold to define hypotension in the elderly and the substitution of traditional vital signs with the shock index, both with proposed incremental improvement in nationwide triage of patients at high risk of poor outcome. [25, 44, 45] These findings support the use of more individualized assessment of perfusion status, based on patient-specific factors including age. However, data is limited regarding the optimal method to detect OH after hospital arrival where available triage tools are more widely available than in the pre-hospital setting. One study compared methods of detecting OH on hospital arrival and found BE measurement was superior to shock index and rate over pressure evaluation, however the findings of this study are worth reinvestigating with specific consideration of age. [20] Inferior vena cava measurements, evaluated either by point-of-care ultrasound or by computed tomography, have been found to be superior to vital signs and the shock index in the prediction of both OH and poor outcomes. [46–49] The optimal method for detecting OH in elderly trauma patients warrants further study. The present study evaluated two systolic blood pressure thresholds for shock, 90 and 110 mmHg, with almost no difference in the association between OH and shock in the elderly. This finding supports routine measurement of base excess to aid in the detection of hypoperfusion in patients as young as 50 years. Furthermore, notification systems that alert the care team with an abnormal base excess result may improve time to detection and treatment of OH for those who are screened. A shift in clinical practice from primary use of vital signs to primary use of base excess alone is not advised given that, as previously mentioned, the absence of a tachycardia response is likely clinically important and the discordance of markers of metabolic acidosis and vital signs should signal increased vigilance in the elderly.
There are limitations to the presented study. We are primarily limited by the retrospective nature of this study, which did not have lactic acid values available for analysis. Other studies have shown that lactic acid and base excess similarly reveal tissue hypoxia secondary to hypoperfusion after traumatic injury and that lactic acidosis is marginally superior in the prediction of poor outcomes including mortality and complications. [32, 50] Second, this was a single-center study, which may represent a local weakness in the recognition and treatment of OH in elderly patients after traumatic injury. Validation of these findings in a multi-center cohort is warranted. Third, we did not utilize prior medical history or frailty as a covariables in the prediction of poor outcomes. Medical history and frailty both play important roles in the response of an individual to traumatic injury, but immediate, clinical decision making is almost always done without knowledge of a patient’s history or a formal frailty assessment. Therefore, the pragmatic decision was made to not include those factors in this analysis, to increase generalizability to current practices. Finally, we are limited by selection bias, which was primarily due to the lack of base excess measurements taken on many of our patients. Base excess is routinely drawn for patients who are a highest-level trauma activation only. The data presented in the present study utilized a single time point, admission vitals and lab values. Notably, 82% of trauma patients presenting to the study center did not have a base excess measured on arrival. This highlights that laboratory values are not currently used as the primary method to screen for hypoperfusion. Furthermore, if the patient is transferred from a within-system hospital, labs would not be repeated unless otherwise clinically indicated on arrival.
Conclusions:
Among severely injured trauma patients, OH was common and occurred in one quarter of patients. In elderly, but not younger, patients, OH was associated with worse outcomes than shock. The age at which this relationship became apparent was after 50 years old. Further work to improve care in elderly trauma patients should include an assessment on the timeliness of recognition of OH and an evaluation of the optimal treatment strategies for OH. Routine measurement of base excess in the elderly and implementation of a result alert system may decrease the time to recognition and treatment of OH in the elderly.
Acknowledgments
Funding: This work was supported by the William Stamps Farish Fund, the Howell Family Foundation, the James H. “Red” Duke Professorship, and the National Institute of General Medical Sciences of the National Institutes of Health [5T32GM008792].
References
- 1.Howden LM, M.J., Age and Sex Composition: 2010. United States Census Bureau, 2011. [Google Scholar]
- 2.Hildebrand F, et al. , Impact of age on the clinical outcomes of major trauma. Eur J Trauma Emerg Surg, 2016. 42(3): p. 317–32. [DOI] [PubMed] [Google Scholar]
- 3.Hashmi A, et al. , Predictors of mortality in geriatric trauma patients: a systematic review and meta-analysis. J Trauma Acute Care Surg, 2014. 76(3): p. 894–901. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs DG, et al. , Practice management guidelines for geriatric trauma: the EAST Practice Management Guidelines Work Group. J Trauma, 2003. 54(2): p. 391–416. [DOI] [PubMed] [Google Scholar]
- 5.Losh J, et al. , Multidisciplinary Patient Management Improves Mortality in Geriatric Trauma Patients. Am Surg, 2019. 85(2): p. 230–233. [PubMed] [Google Scholar]
- 6.Olufajo OA, et al. , Integrating Geriatric Consults into Routine Care of Older Trauma Patients: One-Year Experience of a Level I Trauma Center. J Am Coll Surg, 2016. 222(6): p. 1029–35. [DOI] [PubMed] [Google Scholar]
- 7.Bardes JM, et al. , Old Age With a Traumatic Mechanism of Injury Should Be a Trauma Team Activation Criterion. J Emerg Med, 2019. 57(2): p. 151–155. [DOI] [PubMed] [Google Scholar]
- 8.Callahan ZM, et al. , Geriatric patients on antithrombotic therapy as a criterion for trauma team activation leads to over triage. Am J Surg, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Adams SD, et al. , Unique pattern of complications in elderly trauma patients at a Level I trauma center. J Trauma Acute Care Surg, 2012. 72(1): p. 112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams SD and Holcomb JB, Geriatric trauma. Curr Opin Crit Care, 2015. 21(6): p. 520–6. [DOI] [PubMed] [Google Scholar]
- 11.Joseph B, et al. , The impact of frailty on failure-to-rescue in geriatric trauma patients: A prospective study. J Trauma Acute Care Surg, 2016. 81(6): p. 1150–1155. [DOI] [PubMed] [Google Scholar]
- 12.Joseph B, et al. , Mortality after trauma laparotomy in geriatric patients. J Surg Res, 2014. 190(2): p. 662–6. [DOI] [PubMed] [Google Scholar]
- 13.Davis JW, et al. , Admission base deficit predicts transfusion requirements and risk of complications. J Trauma, 1996. 41(5): p. 769–74. [DOI] [PubMed] [Google Scholar]
- 14.Tisherman SA, et al. , Clinical practice guideline: endpoints of resuscitation. J Trauma, 2004. 57(4): p. 898–912. [DOI] [PubMed] [Google Scholar]
- 15.Neville AL, et al. , Mortality risk stratification in elderly trauma patients based on initial arterial lactate and base deficit levels. Am Surg, 2011. 77(10): p. 1337–41. [PubMed] [Google Scholar]
- 16.Martin JT, et al. , ‘Normal’ vital signs belie occult hypoperfusion in geriatric trauma patients. Am Surg, 2010. 76(1): p. 65–9. [PubMed] [Google Scholar]
- 17.Champion HR, et al. , Major trauma in geriatric patients. Am J Public Health, 1989. 79(9): p. 1278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis JW and Kaups KL, Base deficit in the elderly: a marker of severe injury and death. J Trauma, 1998. 45(5): p. 873–7. [DOI] [PubMed] [Google Scholar]
- 19.MacKenzie EJ, et al. , A national evaluation of the effect of trauma-center care on mortality. N Engl J Med, 2006. 354(4): p. 366–78. [DOI] [PubMed] [Google Scholar]
- 20.Thom O, et al. , Pilot study of the prevalence, outcomes and detection of occult hypoperfusion in trauma patients. Emerg Med J, 2010. 27(6): p. 470–2. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, et al. , The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet, 2007. 370(9596): p. 1453–7. [DOI] [PubMed] [Google Scholar]
- 22.National Trauma Data Bank Data Dictionary. 2019.
- 23.Zhang Z, Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med, 2016. 4(2): p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijeysundera DN, et al. , Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol, 2009. 62(1): p. 13–21 e5. [DOI] [PubMed] [Google Scholar]
- 25.Eastridge BJ, et al. , Hypotension begins at 110 mm Hg: redefining “hypotension” with data. J Trauma, 2007. 63(2): p. 291–7; discussion 297–9. [DOI] [PubMed] [Google Scholar]
- 26.Batchinsky AI, et al. , Sympathetic nerve activity and heart rate variability during severe hemorrhagic shock in sheep. Auton Neurosci, 2007. 136(1–2): p. 43–51. [DOI] [PubMed] [Google Scholar]
- 27.Convertino VA and Sather TM, Vasoactive neuroendocrine responses associated with tolerance to lower body negative pressure in humans. Clin Physiol, 2000. 20(3): p. 177–84. [DOI] [PubMed] [Google Scholar]
- 28.Convertino VA, Rickards CA, and Ryan KL, Autonomic mechanisms associated with heart rate and vasoconstrictor reserves. Clin Auton Res, 2012. 22(3): p. 123–30. [DOI] [PubMed] [Google Scholar]
- 29.Cooke WH and Convertino VA, Heart rate variability and spontaneous baroreflex sequences: implications for autonomic monitoring during hemorrhage. J Trauma, 2005. 58(4): p. 798–805. [DOI] [PubMed] [Google Scholar]
- 30.Lhuissier FJ, Canoui-Poitrine F, and Richalet JP, Ageing and cardiorespiratory response to hypoxia. J Physiol, 2012. 590(21): p. 5461–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christou DD and Seals DR, Decreased maximal heart rate with aging is related to reduced {beta}-adrenergic responsiveness but is largely explained by a reduction in intrinsic heart rate. J Appl Physiol (1985), 2008. 105(1): p. 24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callaway DW, et al. , Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J Trauma, 2009. 66(4): p. 1040–4. [DOI] [PubMed] [Google Scholar]
- 33.Baxter J, et al. , Do lactate levels in the emergency department predict outcome in adult trauma patients? A systematic review. J Trauma Acute Care Surg, 2016. 81(3): p. 555–66. [DOI] [PubMed] [Google Scholar]
- 34.Richards JC, et al. , Impaired peripheral vasodilation during graded systemic hypoxia in healthy older adults: role of the sympathoadrenal system. Am J Physiol Heart Circ Physiol, 2017. 312(4): p. H832–H841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donato AJ, et al. , Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol, 2009. 297(1): p. H425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindenberger M and Lanne T, Reduced defense of central blood volume during acute lower body negative pressure-induced hypovolemic circulatory stress in aging women. Shock, 2012. 37(6): p. 579–85. [DOI] [PubMed] [Google Scholar]
- 37.Olsen H, Vernersson E, and Lanne T, Cardiovascular response to acute hypovolemia in relation to age. Implications for orthostasis and hemorrhage. Am J Physiol Heart Circ Physiol, 2000. 278(1): p. H222–32. [DOI] [PubMed] [Google Scholar]
- 38.Kozar RA, et al. , Injury in the aged: Geriatric trauma care at the crossroads. J Trauma Acute Care Surg, 2015. 78(6): p. 1197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris JA Jr., et al. , Mortality in trauma patients: the interaction between host factors and severity. J Trauma, 1990. 30(12): p. 1476–82. [PubMed] [Google Scholar]
- 40.Jin G, et al. , Traumatic brain injury and hemorrhagic shock: evaluation of different resuscitation strategies in a large animal model of combined insults. Shock, 2012. 38(1): p. 49–56. [DOI] [PubMed] [Google Scholar]
- 41.Johansson PI and Ostrowski SR, Acute coagulopathy of trauma: balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med Hypotheses, 2010. 75(6): p. 564–7. [DOI] [PubMed] [Google Scholar]
- 42.Johansson PI, et al. , A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg, 2011. 254(2): p. 194–200. [DOI] [PubMed] [Google Scholar]
- 43.Yu Q, et al. , Identification of Fibrinogen as a Key Anti-Apoptotic Factor in Human Fresh Frozen Plasma for Protecting Endothelial Cells In Vitro. Shock, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown JB, et al. , Systolic blood pressure criteria in the National Trauma Triage Protocol for geriatric trauma: 110 is the new 90. J Trauma Acute Care Surg, 2015. 78(2): p. 352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haider AA, et al. , Substituting systolic blood pressure with shock index in the National Trauma Triage Protocol. J Trauma Acute Care Surg, 2016. 81(6): p. 1136–1141. [DOI] [PubMed] [Google Scholar]
- 46.Milia DJ, et al. , Clinical utility of flat inferior vena cava by axial tomography in severely injured elderly patients. J Trauma Acute Care Surg, 2013. 75(6): p. 1002–5; discussion 1005. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen A, et al. , Flat or fat? Inferior vena cava ratio is a marker for occult shock in trauma patients. J Surg Res, 2014. 192(2): p. 263–7. [DOI] [PubMed] [Google Scholar]
- 48.Akilli B, et al. , Inferior vena cava diameter as a marker of early hemorrhagic shock: a comparative study. Ulus Travma Acil Cerrahi Derg, 2010. 16(2): p. 113–8. [PubMed] [Google Scholar]
- 49.Yanagawa Y, Sakamoto T, and Okada Y, Hypovolemic shock evaluated by sonographic measurement of the inferior vena cava during resuscitation in trauma patients. J Trauma, 2007. 63(6): p. 1245–8; discussion 1248. [DOI] [PubMed] [Google Scholar]
- 50.Caputo ND, et al. , Comparing biomarkers of traumatic shock: the utility of anion gap, base excess, and serum lactate in the ED. Am J Emerg Med, 2015. 33(9): p. 1134–9. [DOI] [PubMed] [Google Scholar]