Abstract
On March 11, 2020, the World Health Organization declared its assessment of coronavirus disease 2019 (COVID‐19) as a global pandemic. However, specific anti‐severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) drugs are still under development, and patients are managed by multiple complementary treatments. We performed a retrospective analysis to compare and evaluate the effect of low molecular weight heparin (LMWH) treatment on disease progression. For this purpose, the clinical records and laboratory indicators were extracted from electronic medical records of 42 patients with COVID‐19 (21 of whom were treated with LMWH, and 21 without LMWH) hospitalized (Union Hospital of Huazhong University of Science and Technology) from February 1 to March 15, 2020. Changes in the percentage of lymphocytes before and after LMWH treatment were significantly different from those in the control group (P = 0.011). Likewise, changes in the levels of D‐dimer and fibrinogen degradation products in the LMWH group before and after treatment were significantly different from those in the control group (P = 0.035). Remarkably, IL‐6 levels were significantly reduced after LMWH treatment (P = 0.006), indicating that, besides other beneficial properties, LMWH may exert an anti‐inflammatory effect and attenuate in part the “cytokine storm” induced by the virus. Our results support the use of LMWH as a potential therapeutic drug for the treatment of COVID‐19, paving the way for a subsequent well‐controlled clinical study.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Our results strongly suggest low molecular weight heparin (LMWH) as an effective strategy in a therapeutic or combination therapy against coronavirus disease 2019 (COVID‐19).
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ LMWH exerts an anti‐inflammatory effect by means of reducing IL‐6 and increasing lymphocyte%. We, therefore, favor the use of LMWH as a potential therapeutic drug for the treatment of COVID‐19.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ A new therapeutic approach for COVID‐19 was proposed based on the non‐anticoagulant properties of LMWH.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ In view of the COVID‐19 pandemic, our study will be of pronounced interest to a broad spectrum of clinicians and scientists of several disciplines focusing on translational and basic aspects related to COVID‐19 and virology in general.
On March 11, 2020, the World Health Organization (WHO) declared its assessment of coronavirus disease 2019 (COVID‐19) as a global pandemic. Severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) is characterized by a long incubation period, high infectivity, and multiple routes of transmission. 1 , 2 However, no effective medicines are currently available, so patients are treated symptomatically. A better understanding of the mechanisms of pathological changes will help to screen potential drugs out of the currently available medications.
Several clinical studies revealed that cytokine storms are important mechanisms underlying disease exacerbation and death of patients with COVID‐19. 3 , 4 , 5 Particularly, IL‐6 levels in severely ill patients were significantly higher than in mild cases. 6 IL‐6 is one of the core cytokines, 7 contributing to many of the key symptoms of cytokine storm, such as vascular leakage, activation of the complement, and coagulation cascades, inducing disseminated intravascular coagulation. 8 , 9 Reducing the levels of IL‐6 and decreasing its activity may prevent or even reverse the cytokine storm syndrome, 10 thereby improving the condition of patients with COVID‐19.
Substantial studies have reported that low molecular weight heparin (LMWH) has various non‐anticoagulant properties that play an anti‐inflammatory role by reducing the release of IL‐6. 11 , 12 , 13 However, the anti‐inflammatory effects of LMWH in COVID‐19 are currently unknown. By analyzing the effect of LMWH in patients with COVID‐19, our retrospective cohort study demonstrates, for the first time, the significant beneficial effect of LMWH in controlling cytokine storm and delaying disease progression (Figure 1 ).
Figure 1.
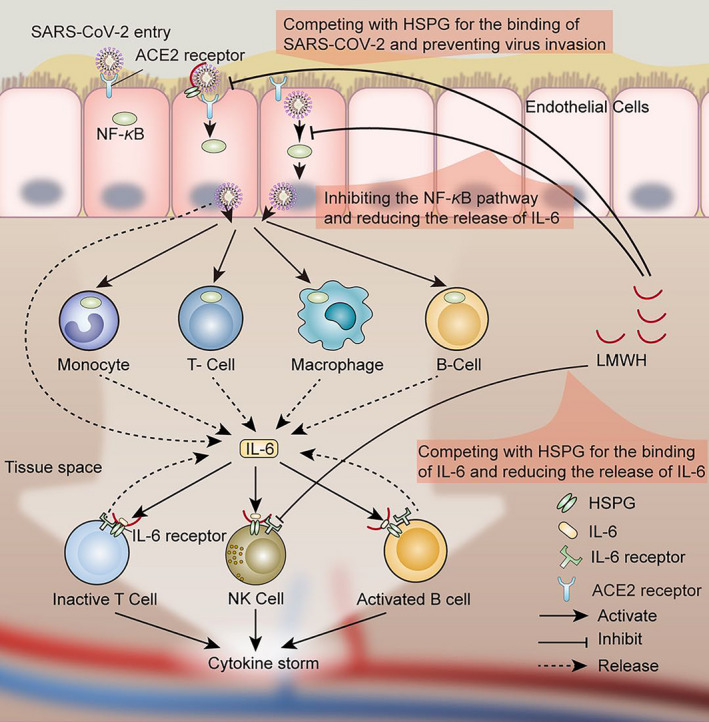
Possible mechanism of anti‐inflammatory effects of low molecular weight heparin (LMWH) in patients with coronavirus disease 2019 (COVID‐19). Under conventional antiviral treatment regimens, LMWH improves hypercoagulability, inhibits IL‐6 release, and attenuates IL‐6 biological activity. It has potential antiviral effects and helps delay or block inflammatory cytokine storms. LMWH can increases the lymphocyte% in the patients. The multiple effects of LMWH encourages its application for the treatment of patients with COVID‐19. HSPG, heparin sulfate proteoglycan; SARS‐CoV‐2, severe acute respiratory syndrome‐coronavirus 2.
METHODS
Research subjects
To investigate the therapeutic effect of LMWH on COVID‐19, we conducted a retrospective cohort study. All cases in this study were located at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, Hubei Province, China), a designated treatment hospital for patients with COVID‐19. This study was approved by the institutional review board of the hospital. In total, we retrospectively collected the electronic medical records of 42 patients with COVID‐19, the admission data for these patients were from February 1, 2020, to March 15, 2020 (Figure 2 shows the case inclusion flowchart) of which 21 patients underwent LMWH treatment (defined as the LMWH group, Table 1 presents the LMWH medication), and 21 did not (defined as the control group), during hospitalization. As a designated hospital for the treatment of patients with COVID‐19, our hospital received 850 patients during February 1, 2020, to March 15, 2020. After March 15, our hospital no longer undertook the treatment of patients with COVID‐19. Case screening was performed after all patients were discharged from the hospital. Among these, 548 of nonsevere patients (diagnosed according to the New Coronavirus Pneumonia Diagnosis Program (7th edition) published by the National Health Commission of China) were excluded. Of the 302 severe patient group, 145 received LMWH of which 124 were excluded, as indicated in Figure 2 . At this point, we went back to the 157 patients who were not treated with LMWH and decided to enroll the “first in list” 21 patients who were found suitable given the exclusion criteria that are presented in Figure 2 . Notably, 32 patients who were not treated with LMWH were eligible for the study, but only the first 21 patients were actually included without any matching attempts or adherence to specific criteria.
Figure 2.
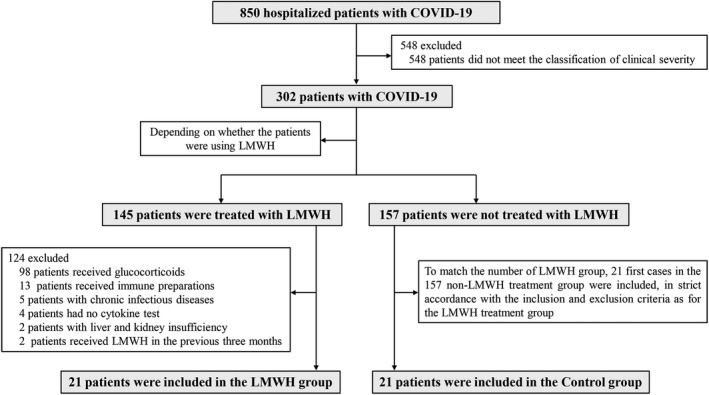
Flow chart for the inclusion and exclusion of patients with coronavirus disease 2019 (COVID‐19). Based on strict inclusion and exclusion criteria, 42 patients with COVID‐19 treated at the hospital between February 1, 2020, and March 15, 2020, were selected for the study, of which 21 underwent low molecular weight heparin (LMWH) treatment (LMWH group) and 21 did not (control group) during hospitalization.
Table 1.
LMWH use in treating conditions of the 21 patients with COVID‐19
| LMWH group (n = 21) | Treatment with LMWH | Days of treatment |
|---|---|---|
| P1 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 10 |
| P2 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 10 |
| P3 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 14 |
| P4 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 13 |
| P5 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 17 |
| P6 | Nadroparin calcium injection 4100Axa IU q.d. i.h. | 9 |
| P7 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 2 |
| P8 | LMWH sodium injection 5000 IU once i.h. | 1 |
| P9 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 16 |
| P10 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 19 |
| P11 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 14 |
| P12 | Enoxaparin sodium injection 2000Axa IU q.d. i.h. | 19 |
| P13 | Enoxaparin sodium injection 2000Axa IU q.d. i.h. | 22 |
| P14 | Nadroparin calcium injection 4100Axa IU q.d. i.h. | 11 |
| P15 | Nadroparin calcium injection 4100Axa IU q.d. i.h. | 13 |
| P16 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 8 |
| P17 | Nadroparin calcium injection 4100Axa IU q.d. i.h. | 19 |
| P18 | LMWH sodium injection 5000 IU q.d. i.h. | 8 |
| P19 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 8 |
| P20 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 7 |
| P21 | Enoxaparin sodium injection 4000Axa IU q.d. i.h. | 10 |
Details of the dose, frequency, route of administration, and days of use of LMWH in the LMWH group.
COVID‐19, coronavirus disease 2019; LMWH, low molecular weight heparin.
Inclusion criteria were: (i) patients were diagnosed as having COVID‐19 according to the New Coronavirus Pneumonia Diagnosis Program (7th edition) published by the National Health Commission of China, including any of the following: novel coronavirus nucleic acid was positive by real‐time polymerase chain reaction fluorescence; the virus gene is highly homologous to a novel coronavirus; serum novel coronavirus specific lgM antibody and lgG antibody were positive, or serum novel coronavirus‐specific lgG antibody changed from negative to positive, or the recovery period was four times higher than the acute phase; (ii) the clinical classification was severe, including any of the following: shortness of breath, respiratory rate ≥ 30 bpm; blood oxygen saturation ≤ 93% (at rest); PaO2/FiO2 ≤ 300 mm Hg; pulmonary inflammation that progresses significantly within 24–48 hours > 50%; (iii) age ≥ 18 years old; (iv) no history of bronchiectasis, bronchial asthma, or other respiratory diseases; and (v) no immunosuppressant or glucocorticoid use during treatment.
Exclusion criteria: (i) patients with severe systemic diseases and other acute or chronic infectious diseases; (ii) patients with liver and kidney insufficiency or congenital heart disease; (iii) patients who had been treated with LMWH in the previous 3 months; (iv) patients with a history of mental illness; (v) pregnant or lactating women; (vi) patients clinically classified as critically ill or housed in the intensive care unit; and (vii) patients allergic to LMWH or contraindicated for LMWH.
Data collection
The basic information, complete blood count, coagulation profile, inflammatory cytokines, and serum biochemical indicators (including liver function, kidney function, lactate dehydrogenase, C‐reactive protein (CRP) and electrolytes) of 42 patients with COVID‐19 were retrospectively analyzed. Two researchers also independently reviewed the data collection forms to double‐check the data collected.
Statistical analysis
Data analysis was performed using SPSS 22.0 statistical software. Data are expressed as mean ± SD. GraphPad version 6.0 software was used for plotting. Differences between groups were evaluated using the unpaired two‐sided Student’s t‐test for continuous measurement data, and the χ2 test for count data. The Kruskal–Wallis nonparametric test was used for the comparisons between independent groups, and paired analysis was performed within groups (related samples). Differences of P < 0.05 were considered statistically significant.
RESULTS
General characteristics of patients with COVID‐19
As shown in Table 2 , the LMWH group consisted of 13 men and 8 women aged between 42 and 91 years (median age = 69.0 years), and the control group consisted of 14 men and 7 women aged between 40 and 84 years (median age = 69.0 years). There were no significant differences in comorbidities, onset symptoms, and antiviral treatment between the two groups. These results indicate that the general characteristics of the two groups of patients were consistent and comparable.
Table 2.
General characteristics of all the included patients with COVID‐19
| Characteristics | LMWH group (n = 21) | Control (n = 21) | P value |
|---|---|---|---|
| Age, years | 69.0 (42.0–91.0) | 69.0 (40.0–84.0) | 0.54 |
| Sex | – | – | 0.75 |
| Female | 8 (38%) | 7 (33%) | – |
| Male | 13 (62%) | 14 (67%) | – |
| Comorbidity | 13 (62%) | 8 (38%) | 0.12 |
| Hypertension | 8 (38%) | 5 (24%) | 0.32 |
| Diabetes | 6 (29%) | 2 (10%) | 0.12 |
| Cardiovascular disease | 5 (24%) | 2 (10%) | 0.21 |
| Chronic obstructive lung disease | 0 | 0 | NA |
| Carcinoma | 0 | 1 (5%) | 0.31 |
| Chronic kidney disease | 0 | 0 | NA |
| Other | 4 (19%) | 1 (5%) | 0.15 |
| Signs and symptoms | |||
| Fever (temperature ≥ 37.3°C) | 15 (71%) | 13 (62%) | 0.51 |
| Cough | 9 (43%) | 7 (33%) | 0.53 |
| Sputum | 6 (29%) | 4 (19%) | 0.47 |
| Chest distress or asthma | 11 (52%) | 8 (38%) | 0.35 |
| Myalgia | 2 (10%) | 3 (14%) | 0.63 |
| Fatigue | 8 (38%) | 5 (24%) | 0.32 |
| Anorexia | 6 (29%) | 5 (24%) | 0.73 |
| Diarrhea | 2 (10%) | 1 (5%) | 0.55 |
| Nausea or vomiting | 2 (10%) | 1 (5%) | 0.55 |
| Respiratory rate ≥ 30 breaths per minute | 0 | 0 | NA |
| Pulse ≥ 125 beats per minute | 0 | 0 | NA |
| Systolic blood pressure < 90 mm Hg | 0 | 0 | NA |
| Antiviral therapy | |||
| Arbidol | 18 (86%) | 20 (95%) | 0.29 |
| Recombinant human interferon α2B (aerosol inhalation) | 6 (29%) | 6 (29%) | 1.00 |
| Ribavirin | 2 (10%) | 0 | 0.15 |
| Lopinavir/ritonavir | 2 (10%) | 0 | 0.15 |
| Traditional Chinese medicine decoction | 11 (52%) | 9 (43%) | 0.54 |
| Disease progression | |||
| Improved | 21 (100%) | 21 (100%) | NA |
| Invariable | 0 | 0 | NA |
| Deteriorative | 0 | 0 | NA |
| Time from hospitalization to virus shedding after the onset of the COVID‐19, days | 20.0 (11.0–31.0) | 19.0 (12.0–30.0) | 0.46 |
| Hospital length of stay, days | 29.0 (17.0–42.0) | 27.0 (24.0–31.0) | 0.41 |
Data are the median (IQR) or n (%). P values comparing the LMWH group and control group are from χ 2 test or unpaired two‐sided Student’s t‐test.
COVID‐19, coronavirus disease 2019; LMWH, low molecular weight heparin; NA, not applicable.
There were no significant differences in age, sex, comorbidities, onset symptoms, time from hospitalization to virus shedding, length of hospital stay, antiviral treatment, and disease progression between the two groups.
LMWH has no effect on the duration of conversion to negative and the length of patient hospitalization
As shown in Table 2 , the number of days to convert virus to negative (time from admission to virus shedding) was 20.0 days (interquartile range (IQR) 11.0–31.0 days) in the LMWH group and 19.0 days (IQR 12.0–30.0 days) in the control group (P = 0.46); the difference between the two groups was not significant. Similarly, the length of hospital stay was 29.0 days (IQR 17.0–42.0 days) in the LMWH group and 27.0 days (IQR 24.0–31.0) in the control group (P = 0.41); the difference between the two groups was not significant. Notably, all patients in the LMWH group and the control group showed overall improvement after treatment.
Effect of LMWH on cytokine levels in patients with COVID‐19
As shown in Figure 3 a there was no significant difference in IL‐6 levels between the LMWH and control groups before treatment (47.47 ± 58.86, 63.27 ± 96.27, respectively, P = 0.950). In contrast, after LMWH treatment, the levels of IL‐6 in the LMWH group were significantly lower compared with those in the control group (15.76 ± 25.71, 78.24 ± 142.41, respectively, P = 0.00039). Similarly, the changes in IL‐6 levels in the LMWH group before and after LMWH treatment were significantly different from those in the control group (−31.71 ± 65.97, 14.96 ± 151.09, respectively, P = 0.031). However, there were no significant differences in the levels of IL‐2, TNF‐α, IL‐4, IL‐10, and IFN‐γ between the LMWH treated and untreated groups (Figure 3 b–f).
Figure 3.
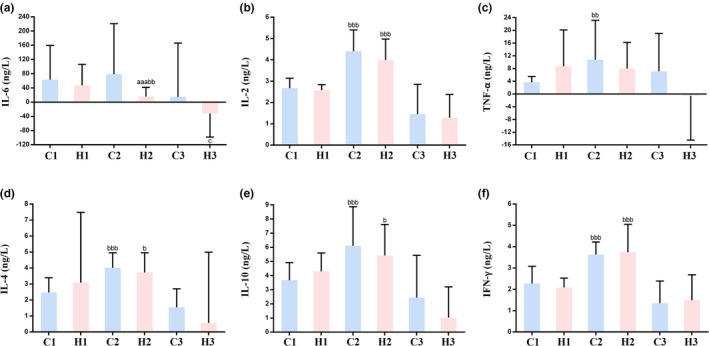
Effect of low molecular weight heparin (LMWH) on inflammatory cytokines in the included patients with coronavirus disease 2019 (COVID‐19). (a–f) IL‐6 (a), IL‐2 (b), TNF‐α (c), IL‐4 (d), IL‐10 (e), and IFN‐γ (f) levels in the two groups of patients with COVID‐19. Data are expressed as mean ± SD (n = 21). C1 vs. H1 or C2 vs. H2, a P < 0.05, aa P < 0.01, aaa P < 0.001; C1 vs. C2 or H1 vs. H2, b P < 0.05, bb P < 0.01, bbb P < 0.001; C3 vs. H3, c P < 0.05, cc P < 0.01, ccc P < 0.001. (C1: control group, indices at admission; C2: control group, indices at discharge; C3: control group, changes in indices during hospitalization; H1: LMWH group, indices before LMWH treatment; H2: LMWH group, indices after LMWH treatment; H3: LMWH group, changes in indices before and after LMWH treatment).
Effect of LMWH on blood routine characteristics
As shown in Figure 4 a–d, there was no significant difference in red blood cells, white blood cells (WBCs), monocyte%, and neutrophil% levels between the two groups. Figure 4 e reveals no significant difference in lymphocyte% (LYM%) between the LMWH and control groups before LMWH treatment (18.84 ± 8.24, 22.42 ± 8.74, respectively, P = 0.144). However, the changes in LYM% in patients of the LMWH group before and after LMWH treatment were significantly different from those in the control group (11.10 ± 9.50, 3.08 ± 9.66, respectively, P = 0.011).
Figure 4.
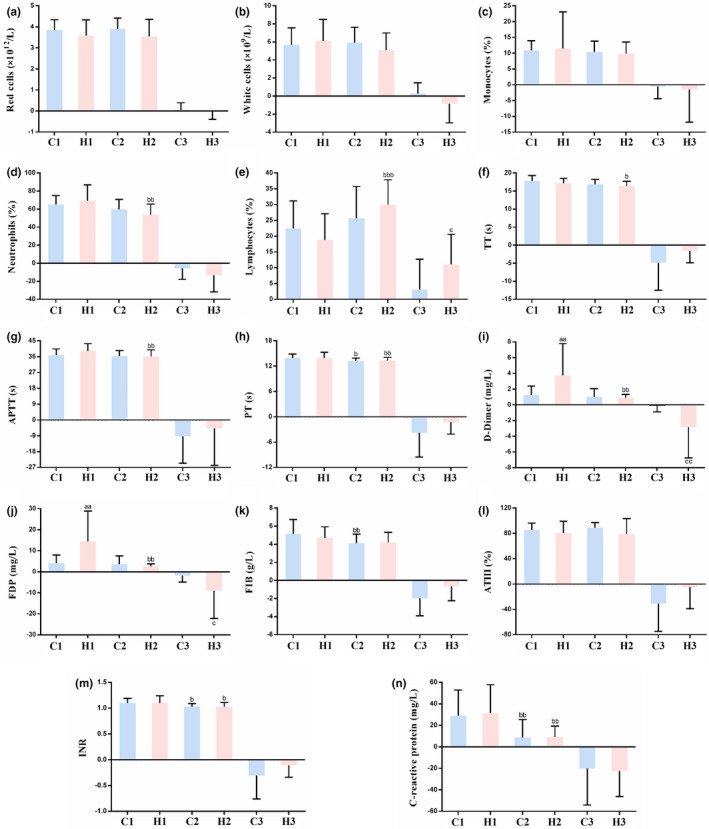
Effect of low molecular weight heparin (LMWH) on complete blood count, coagulation profile, and C‐reactive protein (CRP) in the included patients with coronavirus disease 2019 (COVID‐19). (a–n) Red blood cells (a), white blood cells (b), monocytes% (c), neutrophils% (d), lymphocytes% (e), thrombin time (TT, f), activated partial thromboplastin time (APTT, g), prothrombin time (PT, h), D‐dimer (i), fibrinogen degradation products (FDP, j), fibrinogen (FIB, k), antithrombin III (AT III, l), international normalized ratio (INR, m) and CRP (n) levels in patients with COVID‐19. Data are expressed as mean ± SD (n = 21). C1 vs. H1 or C2 vs. H2, a P < 0.05, aa P < 0.01, aaa P < 0.001; C1 vs. C2 or H1 vs. H2, b P < 0.05, bb P < 0.01, bbb P < 0.001; C3 vs. H3, c P < 0.05, cc P < 0.01, ccc P < 0.001. (C1: control group, indices at admission; C2: control group, indices at discharge; C3: control group, changes in indices during hospitalization; H1: LMWH group, indices before LMWH treatment; H2: LMWH group, indices after LMWH treatment; H3: LMWH group, changes in indices before and after LMWH treatment.)
Effect of LMWH on coagulation parameters
As shown in Figure 4 f–h, there was no significant difference in thrombin time, activated partial thromboplastin time, and prothrombin time levels between the two groups before and after LMWH treatment. As shown in Figure 4 i,j the levels of D‐dimer and fibrinogen degradation products (FDPs) in the LMWH group were significantly higher compared with those in the control group before treatment (D‐dimer: 3.75 ± 4.04, 1.23 ± 1.15, respectively, P = 0.009; FDP: 14.35 ± 14.6, 4.05 ± 3.9, respectively, P = 0.002). The changes in D‐dimer and FDP levels in patients in the LMWH group before and after LMWH treatment were significantly different from those in the control group (D‐dimer: −2.85 ± 3.90, −0.05 ± 0.85, respectively, P = 0.002; FDP: −9.05 ± 13.14, −1.78 ± 3.15, respectively, P = 0.035). However, there was no significant difference in fibrinogen (Figure 4 k), antithrombin III (Figure 4 l), and international normalized ratio (INR; Figure 4 m) levels between the two groups.
Effect of LMWH on CRP levels
As shown in Figure 4 n, LMWH treatment had no significant effect on CRP levels. There is no difference between the two groups of patients before LMWH treatment (31.15 ± 26.62, 29.00 ± 23.79, respectively, P = 0.497), nor after LMWH treatment (8.95 ± 10.44, 8.76 ± 16.66, respectively, P = 0.620). Consequently, there were no significant differences in the changes in CRP levels between the two groups of patients before and after LMWH treatment (−22.62 ± 23.79, −20.23 ± 33.91, respectively, P = 0.660).
DISCUSSION
Cytokine storms are associated with deterioration in several infectious diseases, including SARS and avian influenza, 14 , 15 and IL‐6 is one of the core cytokines that causes cytokine storms. 7 In recent years, studies have revealed that heparin has various non‐anticoagulant properties, for example, LMWH can exert anti‐inflammatory effects by reducing the release of IL‐6. 11 , 12 , 13 , 16
IL‐6 levels in severely ill patients with COVID‐19 are significantly higher than in patients with mild disease. 6 , 17 Transition from mild to severe conditions in patients with COVID‐19 occur when cytokine levels reach and/or exceed a certain threshold, leading to a cytokine release syndrome. 7 Hence, reducing IL‐6 release is expected to attenuate the cytokine storm syndrome caused by the virus, 8 thereby improving the condition of patients with COVID‐19. LMWH was reported to reduce the release of IL‐6 in the body by inhibiting the expression of NF‐κB. 11 , 12 , 13 Measuring the levels of proinflammatory cytokines in patients with COVID‐19, we have found a marked decrease in the levels of IL‐6 in the LMWH‐treated patients compared with the patients without LMWH treatment (P < 0.001), consistent with the proposed protective effect of LMWH. Changes in other inflammatory factors were not statistically significant. In addition, IL‐6 can bind to heparan sulfate (HS) on the cell surface, yielding a sufficiently high local concentration to activate signaling receptors, 18 protect them against proteolysis, and promote paracrine action. 16 , 19 An earlier study has reported that heparin binds IL‐6, with affinity much higher than that of HS, 16 thereby reducing its availability to its receptor complex. It, therefore, appears that LMWH reduces both the release of IL‐6 and its biological activity.
In addition, there are other routes to explain a favorable effect of LMWH on patients with COVID‐19. With the increasing interest in the use of heparin/LMWH for the treatment of COVID‐19, our study signifies an additional activity of heparin apart from anticoagulation. 20 Having demonstrated a marked reduction in IL‐6 levels in the LMWH‐treated patients, we further emphasize the well‐documented anti‐inflammatory/anti‐sepsis effects of non‐anticoagulant species of heparin. 21
HS, a linear polyanionic polysaccharide, is a major constituent of all mammalian cells and tissues. 22 It highly resembles heparin and LMWH in its structural properties and sugar composition. 22 Importantly, HS has been known to serve as the first point of contact between target cells and a large number of human viruses (i.e., dengue virus, hepatitis C virus, HIV, human papillomavirus, and herpes viruses), 23 , 24 including the SARS‐CoV‐2 virus. 25 , 26 A very recent online paper has used surface plasmon resonance and circular dichroism and showed that the SARS‐CoV‐2 Spike S1 protein receptor binding domain interacts with heparin. 26 Heparin, LMWH, and heparin‐like compounds have been shown to efficiently compete with HS and thereby attenuate viral attachment and infection, 27 providing a straightforward explanation for the antiviral effect of LMWH in clinical settings.
Importantly, as clarified below, recent studies expanded the established role of HS from a viral attachment molecule to an essential receptor required for entry. Heparin, LMWH, and non‐anticoagulants species of heparin are known to inhibit the enzymatic activity of heparanase, 28 the sole HS‐degrading endoglycosidase, shown recently to promote viral infection and spread. 29 , 30 , 31 It appears that heparanase behaves as a molecular switch in viral infection, which transforms the cell from a virus‐permissive mode in which viral attachment and entry are favored, to a virus‐deterring mode, which allows for viral detachment and egress from cells. 29 Briefly, it was found that upregulation and activation of heparanase is a strategy common to a broad range of viral species (i.e., porcine reproductive and respiratory syndrome virus (PRRSV) and vaccinia virus) to increase egress, spread, and transmission. 31 Interestingly, it appears that heparanase plays a role also in driving the undesirable cytokine storm discussed above. In individuals with SARS‐CoV‐2 infection, the level of inflammatory cytokines is markedly higher than normal and held responsible for the severity of the disease. Agelidis et al. documented that upon herpes simplex virus‐1 infection, heparanase translocate to the nucleus of the infected cells and promotes inflammatory signaling, mediated primarily via NF‐κB. 31 In fact, transcription of IL‐6 was significantly decreased after treatment with an inhibitor of heparanase enzymatic activity. 31 LMWH, which inhibits heparanase activity, 28 may have a similar effect, possibly providing a mechanistic explanation for the decrease in IL‐6 that we observed in the LMWH‐treated patients. Collectively, the above considerations suggest that heparanase inhibitors (i.e., LMWH) may be an effective strategy in a therapeutic or combination therapy against viral infection, including COVID‐19. Additional studies showed that inhibition of the glycocalyx‐degrading enzymes sialidase, cathepsin L and heparanase, using a combination therapy of zanamivir, cathepsin‐L, and heparanase inhibitors, decreased vascular leakage after exposure to the influenza virus NS1 protein in vitro and in vivo. 32 It will be interesting to see if an analogous therapeutic inhibition of glycocalyx breakdown can provide similar benefit clinically.
Several studies have recommended CRP and LYM% as indices for evaluating the effectiveness of clinical drugs or treatments. 4 , 33 , 34 In the various analyses applied in this study, there was no statistically significant difference in CRP levels between the two groups, indicating that LMWH treatment has no effect on this parameter. Notably, the changes in LYM% in patients of the LMWH group before and after LMWH treatment were significantly different from those in the control group (P < 0.05), consistent with the results of Derhaschnig et al. 35 This result suggests that LMWH can increase LYM% in patients with COVID‐19 and thereby improve their condition. Furthermore, it was reported that proinflammatory cytokines, such as TNF‐α and IL‐6, can induce lymphopenia. 5 Hence, the decrease in IL‐6 (Figure 3 a) may contribute to the increase in LYM% observed in the LMWH‐treated patients.
Tang et al. suggested a correlation among D‐dimer, FDP, and COVID‐19 severity. 36 However, there is currently no conclusive evidence supporting the use of D‐dimer as an evaluation index. 37 , 38 , 39 A broad sample analysis is required to determine whether D‐dimer is associated with COVID‐19 severity. Therefore, the present study does not consider this parameter as an evaluation index for disease progression. The average values of D‐dimer and FDP before treatment was higher in the LMWH group than in the control group (3.75, 1.23, respectively, P < 0.01; 14.35, 4.05, respectively, P < 0.01), therefore, LMWH was applied. Because this study is a retrospective analysis, we did not intervene in the type of treatment given to the patients, inferring that the purpose of medication in the LMWH group was to improve hypercoagulability. Because D‐dimer and FDP are not considered as factors that designate patient’s disease progression, their levels had no effect on subsequent analysis of the results. Notably, it appears (Table 2 ) that patients treated with LMWH had somewhat higher incidence of comorbidities and seemingly more frequent signs and symptoms of COVID‐19. This may be due to the single‐center retrospective study and the limited sample size, which may not fully reflect the overall characteristic status of patients in these aspects. Although there was no significant difference in baseline between the two groups, the lack of adjustment analysis was still a limitation of this study. These apparent effects are being evaluated in a prospective clinical study that evaluates the efficacy and safety of enoxaparin in the treatment of patients with COVID‐19 (see below).
Importantly, LMWH exerts an anti‐inflammatory effect by means of reducing IL‐6 and increasing LYM%. We, therefore, favor the use of LMWH as a potential therapeutic drug for the treatment of COVID‐19. We also suggest that non‐anticoagulant species of LMWH that can be applied at high doses should be considered as a complement to conventional LMWH. To further support this conclusion, we are conducting a prospective clinical study to evaluate the efficacy and safety of one LMWH (enoxaparin) in the treatment of hospitalized adult patients with COVID‐19 (Chinese Clinical Trial Registry, number: chiCTR2000030700), with the objective of providing a more powerful reference for the treatment conditions.
This study still has several limitations. First, due to the retrospective design, we were unable to control the time intervals between examinations of the various indices in patients and the LMWH treatment schedule. Likewise, we could not estimate and manage the effective dose and timing of LMWH. Second, there were no critical cases in the two groups of patients; the treatment outcome of all cases was improvement and discharge, and there were no deaths. Finally, the findings are limited by the sample size and single‐center design of our study.
Funding
This study was funed by the National Key Research and Development Plan of China (2017YFC0909900) National Natural Science Foundation of China (No. 81603037 to S.C.).
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
C.S., C.W., H.X., J.P.L., and I.V. wrote the manuscript. C.S., J.P.L.. and Y.Z. designed the research. C.W. and H.X. performed the research. F.Cai, Y.H.L., T.Z., and B.D. analyzed the data. C.Y., F.Chang, and F.Z. contributed new analytical tools.
References
- 1. Wang, C. , Horby, P.W. , Hayden, F.G. & Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 395, 470–473 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang, C. et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou, W. et al Potential benefits of precise corticosteroids therapy for severe 2019‐nCoV pneumonia. Signal Transduct. Target. Ther. 5, 18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan, L.I. et al Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 5, 33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liao, Y.C. , Liang, W.G. , Chen, F.W. , Hsu, J.H. , Yang, J.J. & Chang, M.S. IL‐19 induces production of IL‐6 and TNF‐alpha and results in cell apoptosis through TNF‐alpha. J. Immunol. 169, 4288–4297 (2002). [DOI] [PubMed] [Google Scholar]
- 6. Wan, S.X. et al Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) [preprint] (2020). https://doi.org/ 10.1101/2020.02.10.20021832. Posted on MedRxiv. February 12, 2020. [DOI]
- 7. Shimabukuro‐Vornhagen, A. et al Cytokine release syndrome. J. ImmunoTherapy Cancer 6, 56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanaka, T. , Narazaki, M. & Kishimoto, T. Immunotherapeutic implications of IL‐6 blockade for cytokine storm. Immunotherapy 8, 959–970 (2016). [DOI] [PubMed] [Google Scholar]
- 9. Hunter, C.A. & Jones, S.A. IL‐6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015). [DOI] [PubMed] [Google Scholar]
- 10. Teachey, D.T. et al Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T‐cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qian, Y. , Xie, H. , Tian, R. , Yu, K. & Wang, R. Efficacy of low molecular weight heparin in patients with acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. COPD 11, 171–176 (2014). [DOI] [PubMed] [Google Scholar]
- 12. Liu, Y. , Mu, S. , Li, X. , Liang, Y. , Wang, L. & Ma, X. Unfractionated heparin alleviates sepsis‐induced acute lung injury by protecting tight junctions. J. Surg. Res. 6, 175–185 (2019). [DOI] [PubMed] [Google Scholar]
- 13. Li, X. , Ma, Y. , Chen, T. , Tang, J. & Ma, X. Unfractionated heparin inhibits lipopolysaccharide‐induced expression of chemokines in human endothelial cells through nuclear factor‐κB signaling pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 28, 117–121 (2016). [DOI] [PubMed] [Google Scholar]
- 14. De Wit, E. , van Doremalen, N. , Falzarano, D. & Munster, V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14, 523–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rao, D. Research progress on cytokine storm induced by pathogen infection. Med. Informat. 27, 480–481 (2014). [Google Scholar]
- 16. Mummery, R.S. & Rider, C.C. Characterization of the heparin‐binding properties of IL‐6. J. Immunol. 165, 5671–5679 (2000). [DOI] [PubMed] [Google Scholar]
- 17. Pedersen, S.F. & Ho, Y.C. SARS‐CoV‐2: a storm is raging. J. Clin. Invest. 130, 2202–2205 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernfield, M. et al Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999). [DOI] [PubMed] [Google Scholar]
- 19. Sarrazin, S. , Lamanna, W.C. & Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 3, a004952 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thachil, J. Clinical differentiation of anticoagulant and non‐anticoagulant properties of heparin. J. Thromb. Haemost. 18, 2424–2425 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hippensteel, J.A. , LaRiviere, W.B. , Colbert, J.F. , Langouët‐Astrié, C.J. & Schmidt, E.P. Heparin as a therapy for COVID‐19: current evidence and future possibilities. Am. J. Physiol. Lung Cell Mol. Physiol. 319, L211–L217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Esko, J.D. & Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Invest. 108, 169–173 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen, Y. et al Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3, 866–871 (1997). [DOI] [PubMed] [Google Scholar]
- 24. Shukla, D. & Spear, P.G. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Invest. 108, 503–510 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milewska, A. et al Entry of human coronavirus NL63 into the cell. J. Virol. 92, e01933–e2017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mycroft‐West, D. S. et al The 2019 coronavirus (SARS‐CoV‐2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding [preprint] (2020). https://doi.org/ 10.1101/2020.02.29.971093. Posted on BioRxiv. March 2, 2020. [DOI]
- 27. Guo, Y. , Wang, Z. , Dong, L. , Wu, J. , Zhai, S. & Liu, D. Ability of low‐molecular‐weight heparin to alleviate proteinuria by inhibiting respiratory syncytial virus infection. Nephrology 13, 545–553 (2008). [DOI] [PubMed] [Google Scholar]
- 28. Vlodavsky, I. , Ilan, N. , Naggi, A. & Casu, B. Heparanase: structure, biological functions, and inhibition by heparin‐derived mimetics of heparan sulfate. Curr. Pharm. Des. 13, 2057–2073 (2007). [DOI] [PubMed] [Google Scholar]
- 29. Hadigal, S.R. et al Heparanase is a host enzyme required for herpes simplex virus‐1 release from cells. Nat Commun. 6, 6985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khanna, M. , Ranasinghe, C. , Browne, A.M. , Li, J.P. , Vlodavsky, I. & Parish, C.R. Is host heparanase required for the rapid spread of heparan sulfate binding viruses? Virology 529, 1–6 (2019). [DOI] [PubMed] [Google Scholar]
- 31. Agelidis, A.M. , Hadigal, S.R. , Jaishankar, D. & Shukla, D. Viral activation of heparanase drives pathogenesis of herpes simplex virus‐1. Cell Rep. 20, 439–450 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glasner, D.R. , Ratnasiri, K. , Puerta‐Guardo, H. , Espinosa, D.A. , Beatty, P.R. & Harris, E. Dengue virus NS1 cytokine‐independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 13, e1006673 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou, F. et al Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li, X. et al Clinical characteristics of 25 death cases with COVID‐19: a retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 94, 128–132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Derhaschnig, U. , Pernerstorfer, T. , Knechtelsdorfer, M. , Hollenstein, U. , Panzer, S. & Jilma, B. Evaluation of antiinflammatory and antiadhesive effects of heparins in human endotoxemia. Crit. Care Med. 31, 1108–1112 (2003). [DOI] [PubMed] [Google Scholar]
- 36. Tang, N. , Li, D. , Wang, X. & Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 18, 844–847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Querol‐Ribelles, J.M. et al Plasma d‐dimer levels correlate with outcomes in patients with community‐acquired pneumonia. Chest 126, 1087–1092 (2004). [DOI] [PubMed] [Google Scholar]
- 38. Snijders, D. , Schoorl, M. , Schoorl, M. , Bartels, P.C. , van der Werf, T.S. & Boersma, W.G. D‐dimer levels in assessing severity and clinical outcome in patients with community‐acquired pneumonia. A secondary analysis of a randomised clinical trial. Eur. J. Intern. Med. 23, 436–441 (2012). [DOI] [PubMed] [Google Scholar]
- 39. Duarte, J.C. , TavareseCastro, A. , Silva, R. , Correia, L. , Simão, A. & Carvalho, A. Prognostic value of plasma D‐dimer level in adults with community‐acquired pneumonia: a prospective study. Rev. Port. Pneumol. (2006) 21, 218–219 (2015). [DOI] [PubMed] [Google Scholar]


