Abstract
The novel coronavirus disease 2019 (COVID‐19) global pandemic has shifted how many patients receive outpatient care. Telehealth and remote monitoring have become more prevalent, and measurements taken in a patient’s home using biometric monitoring technologies (BioMeTs) offer convenient opportunities to collect vital sign data. Healthcare providers may lack prior experience using BioMeTs in remote patient care, and, therefore, may be unfamiliar with the many versions of BioMeTs, novel data collection protocols, and context of the values collected. To make informed patient care decisions based on the biometric data collected remotely, it is important to understand the engineering solutions embedded in the products, data collection protocols, form factors (physical size and shape), data quality considerations, and availability of validation information. This article provides an overview of BioMeTs available for collecting vital signs (temperature, heart rate, blood pressure, oxygen saturation, and respiratory rate) and discusses the strengths and limitations of continuous monitoring. We provide considerations for remote data collection and sources of validation information to guide BioMeT use in the era of COVID‐19 and beyond.
In an effort to mitigate the spread of the novel coronavirus disease 2019 (COVID‐19), the disease caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), healthcare providers are increasingly using telehealth for remote patient visits. At the beginning of the pandemic, amidst fears of being infected and having to visit overcrowded hospitals, individuals were rapidly purchasing technologies, such as pulse oximeters, to use at home to monitor for early signs of infection. 1 Entering early summer, the Centers for Disease Control and Prevention (CDC) reported an increase in cases in several regions of the United States; without a vaccine, experts are concerned for a second wave of the virus. 2 , 3 , 4 , 5 As the healthcare system faces an unprecedented need for remote monitoring due to the COVID‐19 pandemic, Biometric Monitoring Technologies (BioMeTs) offer solutions for collecting disease‐related measurements from patients at home. 6 , 7 , 8
BioMeTs are internet‐connected digital medicine products, such as smart thermometers or heart rate monitors with Bluetooth connectivity, that process data captured by mobile sensors using algorithms to generate measures of behavioral and/or physiological function. 9 These connected technologies are used in a variety of contexts, including but not limited to healthcare delivery, 10 clinical trials, 11 and public health. 12 , 13
BioMeTs offer convenient opportunities to collect frequent and objective data and disease‐related measurements, which facilitates assessing trends 12 and detecting changes in vital signs not traceable by conventional spot check data collection protocols. 14 In response to the COVID‐19 pandemic, BioMeTs can be used for many clinical needs, such as aiding preliminary patient physical assessments, assisting with triage of patients with COVID‐19 symptoms, and monitoring patients post‐hospital discharge for risks of readmission. 8 , 15 , 16 , 17 , 18
For clinical teams implementing remote monitoring for the first time or for those already familiar with these technologies and exploring new options, there is an overwhelming variety of BioMeTs available as the market has seen an exponential growth over the past 2 decades. 11 Navigating engineering solutions, form factors (physical size and shape), corresponding data collection protocols, and knowing how to interpret generated values can be challenging, especially if a healthcare provider is unfamiliar with how a BioMeT compares with conventional clinical instruments. Healthcare providers may question the accuracy of measurements taken by patients at home without supervision and it may be unclear how a BioMeT collects and processes data. Understanding data quality and potential biases in data collection is key to drawing appropriate inferences, especially because some of the data may be used for clinical decision making.
In this paper, we will discuss the following: (i) sources of information one can use to identify high‐quality BioMeTs, (ii) products and engineering solutions for remote vital sign monitoring, including temperature, heart rate, blood pressure (BP), oxygen saturation, and respiratory rate, and (iii) considerations for choosing a product, including form factors, usability and data collection protocols, and interfering factors that can produce altered readings. Although certain vital sign abnormalities have been associated with COVID‐19 and will be highlighted in this review, we believe the foundations of evaluating these BioMeTs can be applied broadly whenever remote vital sign monitoring is needed. Although overviews of wearable sensor applications for COVID‐19 have been published, 8 , 19 this paper provides a critical review of technologies and is intended as an aid to navigate the plethora of remote monitoring sensors.
HOW TO IDENTIFY HIGH‐QUALITY BioMeTs
When implementing new technologies for remote monitoring of vital signs, healthcare providers may ask “how do I know if I can trust this data?” A key characteristic to determine the quality of a BioMeTs’ measurement performance is validity. The term “validation” or “validity” means that a BioMeT was tested according to a defined protocol to determine if its performance characteristics meet predefined criteria. 9 A closely related term is “fit for purpose,” which means that a data collection method is adequate for its purpose and confirmed experimentally. The term “fit for purpose” is broader and may include validation parameters along with many other useful BioMeT characteristics, such as utility and usability, security risks, data protections, and economic feasibility that are beyond the scope of this paper and can be found elsewhere. 20 It is also important to distinguish between “validation” and “evaluation” studies commonly found in the literature. Validation studies are conducted prior to product release to the market or use in a specific study; the validation testing is done according to predefined methods and parameters. In contrast, evaluation studies can be conducted any time, the evaluation criteria may not be defined before the experiment and may include any evaluation parameters of interest, such as accuracy or usability.
Healthcare providers may look to multiple sources, including (i) decision summaries by regulatory agencies as to whether a product can be marketed as a medical device, 21 (ii) professional organizations, and/or (3) product manuals for validation information.
Device regulatory review
The majority of BioMeTs marketed in the United States as medical devices have been cleared under the auspices of the US Food and Drug Administration (FDA) 510(k) program. 22 The guiding principle of this program is a risk‐based classification. Low‐risk, medium‐risk, and high‐risk devices require different levels of evidence for regulatory reviews. Many products used for vital sign monitoring fall into the medium‐risk device category and are cleared by being substantially equivalent to a predicate device using a similar engineering solution. However, the content of regulatory reviews, such as 510(k) summaries, may contain highly abbreviated information not sufficient to reconstitute the details of conducted studies and understand generalizability of results. There is also a great degree of variability of evidence required for clearance of different types of devices. Some device categories, such as pulse oximeters, require robust evidence generated in human subjects, 23 whereas other device categories may not have such requirements. There is also a degree of variability in data quality among 510(k)‐cleared devices in the same category. 24 Moreover, BioMeTs not intended for medical use can provide useful information. 25 , 26 Consequently, a medical device designation does not render a BioMeT fit for purpose by default. 27
Professional organizations
In the clinical realm, groups, such as the American Heart Association and the European Society of Hypertension, have provided overviews of validation protocols. 28 , 29 The British and Irish Hypertension Society provides lists of validated products, particularly for ambulatory BP monitoring. 30 Understandably, a lot of focus has been spent on home BP monitoring given the prevalence of hypertension; however, validation protocols, lists of validated products, and recommendations to ensure accurate measurements for other vital signs are less common or do not exist. The robustness of available validation information varies depending on what is being monitored and may lag behind the creation, development, and deployment of BioMeTs.
Product manuals
Manuals do commonly state that measurements are accurate within a certain range and include an error rate. However, the methods of how the values were determined are often not clear. This lack of transparency can contribute to skepticism on the product’s performance. 31 Additionally, manufacturers may use phrases such as “medical‐grade,” “clinically validated,” “FDA‐approved,” or “research‐grade” in manuals or marketing, but in practice these terms do not infer that a product is better than a product labeled “consumer‐grade.” In independent studies, some commercial products have shown better accuracy compared with “medical‐grade” and “research‐grade” tools. 25
Currently, there is no single source for reporting on BioMeTs’ performance characteristics. Because 510(k) submissions often do not require validation in human subjects and predefined criteria for validation protocols do not exist for all product types, healthcare providers cannot yet rely on availability of validation information as a sole parameter to determine the trustworthiness of a BioMeT’s data. In the absence of uniform requirements for all BioMeTs, the information obtained from a study determining whether a product is fit for purpose for a specific application can be valuable. Moreover, the results of studies conducted independently of BioMeT manufacturers are considered to be most reliable. An understanding of engineering solutions, form factors, body placements, and factors that may impact accuracy are also key elements to consider. In the next section, we discuss these elements for BioMeTs that can remotely collect vital signs.
BIOMETRIC MONITORING TECHNOLOGIES (BioMeTs) FOR REMOTE MONITORING IN THE ERA OF COVID‐19
There are many versions of BioMeTs available to remotely collect vital signs, including body temperature, heart rate, BP, blood oxygen saturation (SpO2), and respiratory rate that may be important for COVID‐19 and patient care generally, as shown in Figure 1 . We chose not to include diagnostic early warning signs for each measurement in Figure 1 as symptoms of COVID‐19 are highly variable and clinical recommendations are changing rapidly. 7 , 32 Instead, we will focus on how BioMeTs can be used for at‐home monitoring. 33 , 34 , 35
Figure 1.
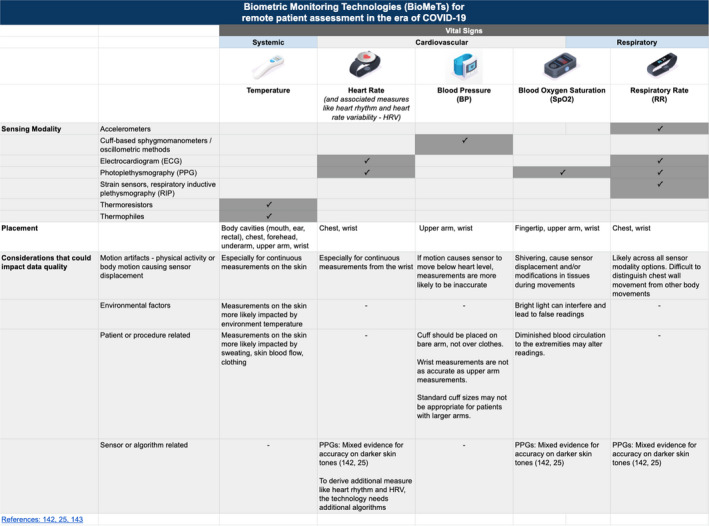
Biometric Monitoring Technologies (BioMeTs) can collect vital signs for remote patient assessment in the era of coronavirus disease 2019 (COVID‐19). Healthcare providers should be familiar with the sensor modality, body placement and other considerations that could impact data quality to facilitate informed care decisions. PPG, photoplethysmogram.
VITAL SIGNS ‐ BODY TEMPERATURE
In COVID‐19, fever is a common but variable symptom present in 50–87% of patients on hospital admission. 7 , 32 , 36 Continuous real‐time temperature monitoring offers expanded opportunities to capture changes in asymptomatic patients or those with mild symptoms being monitored at home. 8
Body temperature can be divided into measurements of core and skin temperature. As indicated in Figure 1 , two common thermometer modalities—thermoresistors and thermopiles—can measure either core or skin temperature, depending on if and where the sensor is worn. 37 , 38 Thermoresistors function on the principle of resistance, the ease at which electricity flows through metal, and require direct contact with the body for measurement. 39 As a metal gets hot, resistance increases, and an algorithm computes changes in resistance to changes in temperature. Thermophiles measure the intensity of thermal radiation from a surface. BioMeTs with thermophile sensors, often called infrared thermometers, do not require direct contact with the body.
Due to the variety of form factors available, BioMeTs can measure temperature from different body locations. The reference ranges at each body location can be inconsistent. 40 Variations in body location may be more important to account for than differences in product accuracy ranges. For example, readings from the armpit and forehead are often 0.5°F (0.3°C) to 1°F (0.6°C) lower than oral temperature. 41 Comparatively, product manuals from nine BioMeTs spanning multiple form factors and wear locations, claim an accuracy range of ±0.2–0.4°F (±0.1–0.2°C). 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 When interpreting measurements from BioMeTs, comparisons of measurements taken on the skin to measurements of core temperature may vary as skin temperature is more likely impacted by external factors. 37 For example, measurements taken on the skin using a patch worn on the chest have shown no correlation with measurements taken from the mouth. 51
Different form factors are designed to perform measurements as intermittent spot checks or continuously over multiple days. Intermittent measurements can be collected from a body cavity (e.g., oral, ear, or rectal) with portable monitors, such as Kinsa’s Quick Care or Smart Ear models using a metal tip with a thermoresistor. 52 Alternatively, intermittent measurements can be collected from the forehead using infrared thermometers, such as Withings Thermo. 53 In contrast, continuous measurements can involve underarm patches with embedded thermoresistors, such as Fever Scout from VivaNKL or TempTraq, and straps worn on the wrist, arm, or chest. 54 , 55 , 56 , 57 , 58 Compared with intermittent spot checks, continuous measurements are more prone to variability due to external factors, such as clothing, ambient temperature, and body movements. Furthermore, adhesive patches are convenient and well accepted in children but may be more difficult for elderly people with frail skin or those susceptible to contact dermatitis. 59 , 60
Some key considerations are as follows:
Form factor options include patch, portable monitor, and strap/band.
Continuous measurements from the skin are more likely to be impacted by external factors.
When interpreting measurements, knowing the body location is just as important as knowing the accuracy ranges of the tools’ themselves.
VITAL SIGNS ‐ CARDIOVASCULAR
Heart rate
There are numerous causes of an elevated basal heart rate or changes in heart rhythm, and these abnormalities can be seen in patients presenting with infection. 12 Research is ongoing to determine how changes in heart rate and heart rate variability can predict or monitor COVID‐19 progression. 15 , 61 , 62
As indicated in Figure 1 , electrocardiograms (ECGs) and photoplethysmograms (PPGs) are the two sensor modalities commonly found in BioMeTs to measure heart rate and associated heart rhythm abnormalities. Many BP monitors also have capabilities to measure heart rate and will be discussed in the corresponding section below. Monitors often include an ECG that uses electrodes to measure the heart’s electrical activity. Many consumer fitness trackers use PPGs, also known as optical heart rate sensors. 63 A PPG uses light to measure blood volume pulse, which is the change in blood volume determined by the amount of light passing through the skin. The light sensor determines heart rate by calculating the amount of time between the changes in blood volume. 63 Although ECG is a conventional standard for comprehensive cardiovascular assessments, including the detection and classification of cardiovascular events, PPG is a convenient method for capturing heart rate, heart rate variability, and some heart rhythm abnormalities.
Both sensor modalities can detect heart rhythm abnormalities with appropriate algorithms. Historically, Holter monitors have commonly been used for ambulatory ECG data collection. However, Holter monitors can be inconvenient for patients and the use is usually limited to 24–48‐hour monitoring. Additionally, Holter monitors do not allow for real‐time monitoring, as the data can only be analyzed retrospectively. The new generation of cardiac monitoring BioMeTs often come in the form of more convenient chest‐worn single‐lead ECG patches.
Certain models also provide an opportunity to monitor and review data in real‐time. For example, some ECG patches, such as iRhythm ZioXT, have demonstrated improved ability to detect hidden arrhythmias compared with Holter monitors. 64 , 65 A large‐scale Apple Watch study investigating the ability of PPGs to identify atrial fibrillation found that, among participants who received notification of an irregular pulse, 34% had atrial fibrillation on subsequent ECG patch readings and 84% of notifications were consistent with atrial fibrillation. 66
Since the method of data collection is different (e.g., electrical vs. light), ECG and PPG waveforms look different, which can impact analysis and the capacity to detect nuanced cardiac conditions. For example, some patients take medications that can cause QT prolongation, a measure of delayed ventricular repolarization that can place patients at risk for dangerous tachycardias. To monitor such patients, a single‐lead ECG has been proven inaccurate, so a multiple‐lead ECG is needed. 67 A new generation of BioMeTs, such as AliveCor KardiaMobile 6L or Preventice BodyGuardian Mini models, incorporate multi‐lead designs and may be of interest to monitor nuanced cardiac conditions. 68 , 69 They provide more convenient and user‐friendly methods compared with Holter monitors, but do not provide the same level of details.
For at‐home monitoring, BioMeTs with either sensor come in different form factors. For example, BioMeTs with ECGs are often chest straps, portable monitors, or patches. Chest straps and patches often require specific placement and may not be comfortable for all populations to wear continuously. 70 PPGs can take many forms, including wristwatches, armbands, clips worn on the chest, or unobstructive rings on the hand. When selecting a form factor, it is important to determine the minimum amount of data required for making informed care decisions, as certain BioMeTs are designed to collect data in intermittent or continuous modes. Continuous data collection, while useful in certain clinical contexts, can be more cumbersome because ambulatory normal ranges are not well‐established and the data are prone to motion artifacts, as described below.
The quality of data collection in both ECGs and PPGs is impacted by motion artifacts, measurements coupled with high accelerometer sensor values often occuring during physical activity causing sensor displacement. Motion artifacts can manifest as missing data or false beat detection. 25 Thus, measurements at rest and during low‐intensity activity are more likely to be accurate. 25 , 71 Most consumer products have algorithms to automatically identify and delete PPG measurements that are coupled with large amounts of motion data. One should be aware that poor data‐filtering algorithms can be a source of a measurement error. For example, one study reported a number of artifacts in a chest patch leading to falsely elevated readings above 180 beats per minute, which requires a manual review and can be time‐consuming. 51 The ability to access electrical or blood volume pulse preprocessed data directly from the sensor will vary by manufacturer. Especially for PPG products marketed to consumers, access to preprocessed data to evaluate artifacts may not be possible.
A limitation of data quality unique to PPGs is concerns of inaccurate measurements on darker skin tones because measurements are based on light absorption. However, multiple studies have found no statistical difference in measurements for individuals with darker skin. 25 , 72
In general, the information to determine quality for heart rate detecting BioMeT is scattered and often limited to evaluation studies pursuing variable goals: establishing accuracy of heart rate detection, assessing the ability to detect a certain cardiovascular condition, etc. There are no uniform standards for validation, largely because of high variability in engineering solutions associated with different form factors and wide range of product abilities to collect certain variables ranging from simple processed variables, such as heart rate and heart rate variability, to comprehensive detection of certain cardiovascular conditions.
Some key considerations are as follows:
Form factor options include chest strap, patch, portable monitor, watch, armband, clip, and ring.
Measurements of interest (e.g., resting heart rate or diagnosis of certain cardiovascular conditions) should be considered when selecting sensor modality.
Duration of data collection and data volume ranges from spot checks to continuous monitoring.
Blood pressure
Hypertension has been identified as a frequent comorbidity in hospitalized patients with COVID‐19. A higher incidence of hypertension was found in patients who died of COVID‐19 compared with survivors. 73
Unlike others vital signs, remote BP monitoring has well‐established practices for product validation and widely accepted recommendations for specific patient populations and settings, including in clinic, ambulatory, or at home. These robust practices are driven by the high prevalence of hypertension in adults, 74 requiring frequent assessments of patient conditions. Remote BP monitoring is an integral part of clinical care delivery 75 addressing concerns about “white‐coat hypertension” 76 and masked hypertension. 77 BioMeTs enable data collection over extended periods of time to understand patterns in BP changes (e.g., sustained, white‐coat, masked, and nocturnal hypertension) that cannot be detected during clinical office visits. Professional organizations have developed standardized product validation protocols, 29 , 78 , 79 , 80 and lists of products validated by independent parties are available in the peer review literature or on validation websites. 81 , 82 Moreover, there are guidelines for product choice in specific circumstances such as for at‐home monitoring, measurements in a pediatric populations or measurements in patients with obesity. 75
A BP monitor consists of an inflatable cuff releasing the brachial artery under the cuff in a controlled manner. Systolic BP and diastolic BP are the most commonly reported BP variables in care delivery and human research because the relationship with cardiovascular outcomes are well established. 83 , 84 Additionally, many BP monitors can also report heart rate, which makes these products convenient tools for obtaining several parameters at once.
Oscillometric products were originally developed to measure BP in home settings to eliminate human error associated with the auscultatory method, 85 but they have become widely accepted for clinical BP measurements as well. The readings are based on the amplitude of oscillations recorded in the lateral walls of the upper arm. The systolic number is determined when the cuff is deflated and goes from having no blood flow through the artery to when there is vibration felt in the arterial wall by blood pushing the arterial wall open in order to flow through. 86 The diastolic number is recorded when this vibration stops as the cuff continues to decrease, indicating the point at which the blood is flowing smoothly without any arterial compression from the cuff. 86 Oscillometric products use proprietary algorithms; there are no requirements to report changes when the algorithm is modified, making it difficult to compare product‐to‐product measures even from the same manufacturer. 85 There is a recognition in the field that only products independently validated according to an established protocol should be used. 75 , 85 , 87
Wrist monitors have become popular because of their ease of use, and especially for patients with very large upper arms. A number of products have been developed conforming to the validation standards used to evaluate the accuracy of BP products. 81 However, these products have two important limitations. First, BP can be measured accurately only if the sensor is at the heart level. Second, the product has to be placed directly over the radial artery. Despite the convenience and ease of use, the recommendation is to use wrist worn monitors only in cases when measurements in the upper arm are not feasible. 88 Other BioMeTs, like finger cuffs and smartphone‐based BP measurements, also do not provide the same quality of measurements as conventional upper arm cuffs and/or lack validation studies. 75 They are not yet recommended for hypertension diagnosis and patient monitoring.
Common sources of BP measurement errors include patient‐related (e.g., recent food consumption and movement), product‐related (e.g., using a noncalibrated or nonvalidated monitor), and procedure‐related (e.g., talking during the measurement or improper cuff placement) factors. 75 There is also a pronounced diurnal rhythm of BP, with a decrease of 10–20 mm Hg during sleep and a “morning surge” after waking and rising in the morning when the prevalence of many cardiovascular morbid events tend to be highest. 89
Some key considerations are as follows:
BP monitors provide the best example of validated BioMeTs. There are well‐established validation protocols, measurement techniques, and recommendations for specific models based on independent studies.
Upper arm cuffs using the oscillometric measurement technique are the most accurate for remote data collection.
Other options, such as wrist worn cuffs, should be used with caution as strict adherence to the data collection method is required to obtain accurate values.
VITAL SIGNS ‐ RESPIRATORY
Blood oxygen saturation (SpO2)
A patient’s SpO2, in conjunction with their respiratory rate, are frequently evaluated when deciding whether a patient may need mechanical ventilation. 90 A variety of respiratory and cardiac pathology can result in low SpO2, including COVID‐19. In COVID‐19, low SpO2 has been found to be related to poor outcomes. 36 , 91 Silent hypoxemia 92 , 93 has been described in certain patients with COVID‐19 who do not experience respiratory distress while having dangerously low oxygen levels. Using pulse oximeters at home may be beneficial for remote triaging of patients and may help identify patients who should seek in‐person medical advice and treatment sooner.
SpO2 measures oxygen saturation using different light absorption properties of oxygen‐saturated hemoglobin and deoxygenated hemoglobin. The accuracy of a pulse oximeter is established by comparing SpO2, which is an estimate of oxygen saturation with the reference standard, SaO2, the direct measurement of oxygen in arterial blood samples. As indicated in Figure 1 , pulse oximeters measure SpO2 with PPG sensors, which use a light source and a photodetector. 63 For a full description of this modality, see the heart rate section.
In pulse oximeters, the light absorption by oxygen‐saturated hemoglobin and hemoglobin cannot be measured directly; it must be determined experimentally by calibration using empirical calibration curves developed from studies of healthy volunteers to calculate SpO2 by the pulse oximeter and SaO2 measured by co‐oximetry in extracted blood, the gold standard, under normal oxygen saturation conditions and during hypoxia. 94 , 95 Most manufacturers claim an accuracy of 2%. 96 If manufacturers choose to pursue a 510k clearance, the FDA requires accuracy of root mean square error of < 3% at SaO2 between 70% and 100%. 23 Measurements should be interpreted with caution. An independent study of six low‐cost fingertip pulse oximeters with no FDA regulatory clearance, 23 found only two out of six products met the standard. 94 The biases were attributed mostly to the measurements done under hypoxic conditions, raising concerns about these oximeters’ ability to accurately measure blood oxygenation in disease conditions.
When performing SpO2 measurements at home, there are important usability considerations related to form factor and wear location. Traditionally, measurements are taken at discrete time points and on areas of the body that have good blood perfusion, such as a fingertip, earlobe, and forehead. For example, the iHealth Air is a BioMeT that is placed on the fingertip. 97 , 98 Continuous monitoring has been made possible by newer wearables, such as the WristOx2 Pulse Oximeter Model 3150 by Nonin: a recent observational study performed in patients with chronic obstructive pulmonary disease demonstrated that continuous SpO2 monitoring is feasible with the highest rates of valid data collected at night. 99 Another pulse oximeter, Oxitone‐1000, is a wrist‐worn model with improved usability characteristics. This device was tested side‐by‐side with another 510(k) cleared pulse oximeter in normal healthy volunteers and patients with lung conditions, demonstrating comparable results. 100 However, the value ranges received during this side‐by‐side comparisons were different for the two oximeters examined (Oxitone‐1000 range was 83.8–99.0 and for the reference product it was 91.3–100.0), indicating that measurements may differ for oximeters with different wear locations and may need further investigation. A number of fitness trackers, such as certain Fitbit and Garmin models, also collect SpO2 data from the wrist. Importantly, Fitbit has stated measurements from their products are “not intended to track the kind of changes in blood oxygen levels that might occur with acute or chronic respiratory problems” but rather to track breathing patterns during sleep. 101
It is important to understand that a number of factors can produce altered readings. First, evidence is mixed on the impact of dark color nail polish. Prior studies showed dark nail polish affected SpO2 values 102 but later studies indicated differences of < 2%. 103 , 104 Second, some physiological conditions can impact SpO2 measurements, such as limited blood circulation, cold hands, or hypotension. Carbon monoxide poisoning can also falsely overestimate SpO2 by binding hemoglobin and absorbing light in the wavelengths. Third, the motion artifacts (e.g., shivering), can impact measurements due to sensor displacement and/or modifications in tissues during movements. 63 Fourth, environmental factors, such as bright light, can interfere with measurements and lead to false readings. 95 The measurement protocol recommended by a manufacturer, especially in the home setting, has to be followed diligently. In the case of abnormal readings, the procedure may need to be repeated or a follow‐up telephone call may be required to ensure adherence to the data collection protocol.
Some key considerations are as follows:
Form factor options include clip and strap/band.
Both medical use and nonmedical use pulse oximeters can be accurate under normal conditions. The main gap is accuracy under hypoxic conditions. 94 , 105
Devices receiving 510(k) clearance are a better choice because of the requirement to establish accuracy under hypoxic conditions.
Results from independent validation studies 105 , 106 can be a reliable source for information on product accuracy.
Respiratory rate
Respiratory rate (RR) is an important measurement for monitoring respiratory dysfunction. In COVID‐19, elevated RR may be present on arrival to the hospital and may be a prognostic factor associated with poor outcomes. 36 , 73 , 91
In traditional clinical settings, respiratory rate is often measured by a manual count of the chest rising and falling, which is prone to error and not suitable for continuous remote monitoring. 9 , 107 , 108 BioMeTs that measure respiratory rate often do so by measuring chest wall movements or by modulation of cardiac activity. 109 For both methods, as indicated in Figure 1 , there are multiple sensor modalities and a variety of form factors to consider.
Accelerometers and strain sensors have been implemented to measure chest wall movements. 110 , 111 , 112 , 113 With every breath, air enters and leaves the lungs causing the chest wall diameter to expand up to 7 cm. 114 Accelerometers function by measuring acceleration forces caused by gravity or motion. Thus, an accelerometer can detect the motion of the chest wall as a person inhales and exhales. 109 Meanwhile, strain sensors include respiratory inductive plethysmography (RIP), which uses two belts around the abdomen 115 , 116 to capture breathing patterns. 109
In addition to chest wall movements, RR can be derived from cardiac activity measured with ECGs or PPGs. 117 , 118 For a full description of these modalities, see the heart rate section. As seen in Table 1 , algorithms estimate RR from one or more of the following signals: baseline wander, amplitude modulation, and frequency modulation. 119 Baseline wander and amplitude modulation are caused by different mechanisms in an ECG and PPG and frequency modulation is caused by the same manifestation. 120 , 121 , 122
Table 1.
Respiratory modulations comparison in ECGs vs. PPGs
| ECGs | PPGs | |
|---|---|---|
| Baseline wander | Changes in the orientation of the heart’s electrical axis relative to the electrodes and changes in thoracic impedance | Changes in tissue blood volume |
| Amplitude modulation | Changes in the orientation of the heart’s electrical axis relative to the electrodes and changes in thoracic impedance | Changes in intrathoracic pressure that reduce pulse amplitude and stroke volume during inhalation |
| Frequency modulation | Manifestation of respiratory sinus arrhythmia, the spontaneous increase in heart rate during inspiration, and decreases during exhalation | Manifestation of respiratory sinus arrhythmia, the spontaneous increase in heart rate during inspiration, and decreases during exhalation |
ECG, electrocardiogram; PPG, photoplethysmogram.
Similar to other measurements, form factor is an important consideration that allows for continuous monitoring over multiple days. For example, accelerometers may be clipped onto clothes or embedded in a patch worn on the chest. 109 RIP bands can be manufactured directly into clothing, like Hexoskin’s smart shirts that have RIP belts around the abdomen. 115 , 116 Smart clothes have unique considerations, such as ease of taking the garment on and off, comfort of wiring, and cultural aesthetic. 123 As previously described, ECGs and PPGS also come in chest straps, portable monitors or patches, and wristbands.
Regardless of sensor modality, RR measurements are complicated by motion artifacts and are best taken at rest. 124 Biovotion strongly recommends as such in the user manual for its Everion product. 125 Especially for measurements taken from accelerometers, inaccuracies arise due to difficulties distinguishing breathing from unrelated body movements. 109 , 126 There are also concerns of data volume and cleaning algorithms for ECG and PPG readings similar to heart rate measurements.
There is a lack of uniformity for manufacturer’s reporting acceptable error rates. One study reports two breaths per minute (bpm) as an accepted discrepancy because that would not be significant enough to lead to a change in medical treatment. 71 In review of BioMeTs on the market, we found that accuracy is woefully inconsistent: we found one that does not mention accuracy ranges in their product manual at all, 127 one states measurement ranges without an error rate, 128 three claim an error rate of < 3 bpm in 510(k) summary or product manuals, 46 , 129 , 130 and one has a white paper claiming an mean absolute error of 1.8 bpm. 131 The user manual for Hexoskin does not report accuracy ranges, yet it has an independent validation study in an older adult population where measurement differences between the smart shirt and a metabolic cart were found to be almost 0. 115 , 132
Some key considerations are as follows:
Form factor options include clip, strap/band, patch, and smart clothing.
Sensor based monitoring provides a measure of RR that is not subject to the possible human error involved in manually counting breaths.
Knowing the sensor modality will indicate the most likely sources of measurement error.
DISCUSSION
The COVID‐19 pandemic has brought an unprecedented crisis to the modern healthcare system. Resource constraints during the initial peak of this pandemic catalyzed the shift to remote monitoring as a primary means of healthcare provision for millions of Americans. 133 This method of healthcare delivery has reduced exposure to SARS‐CoV‐2 for patients and healthcare providers alike. Remote monitoring systems using BioMeTs can monitor patients with mild COVID‐19 symptoms at home and patients discharged from the hospital after initial improvement for an acute worsening later in their course. 8 Research projects using wearables, such as WHOOP, Oura Ring, and smartwatches, are ongoing to explore how measurements from BioMeTs can be used for early detection and prediction of disease severity. 15 , 134 , 135 But perhaps even more notably, remote monitoring can be used to deliver routine healthcare for patients who were unable to come in for their scheduled clinic visits for chronic conditions, diseases, and illnesses that consumed our system before the pandemic. The five vital signs form the cornerstone of many initial physical examinations, helping to determine “sick” vs. “not sick.” To guide healthcare providers in navigating the plethora of options for remote monitoring, this review highlights BioMeT engineering solutions, form factors, body placements, and factors that may impact accuracy when measuring vital signs at home.
Because many BioMeT types do not have well‐established validation protocols, it is not possible to gather validation information for every BioMeT described in this paper. As highlighted in a recent systematic review of evidence for validation, feasibility, clinical outcomes, and costs of wearables for early detection of patient deterioration, guidelines for the acceptable mean differences, and limits of agreement for continuous monitoring of vital signs are unfortunately lacking. 136 BP is the only measure with internationally accepted validation protocols and a conduct of independent validation studies as a routine practice. In contrast, there are no widely agreed upon protocols for validating respiratory rate from ECGs or PPGs. To address the lack of validation practices, some colleagues have proposed a framework describing a three‐part process called “V3” that BioMeTs need verification (accuracy of sample level sensor data compared with a bench standard), analytical validation (ability of a sensor plus an algorithm to capture the physiological concept accurately), and clinical validation (whether the measurement is meaningful to answer a specific clinical question in a specific population). 9 Information about verification, analytical validation, and clinical validation is highly variable based on BioMeT type; and some of the information, like sensor verification, is often not available in the public domain. In the absence of validation data, other important resources include evaluation studies that describe experiments testing accuracy and usability. However, the studies included in the recent systematic review referred above were predominantly rated low or moderate quality due to a lack of reporting on clinical outcomes, lack of information about cost effectiveness, small sample size, and the risk of biases when performed or funded by the product manufacturer itself. The authors argue the need for large well‐controlled studies to guide implementation of vital sign monitoring devices on a large scale in clinical practice or in‐home monitoring. 136
New generations of BioMeT have form factors and body placements that allow for continuous real‐time monitoring of vital signs. For example, a patch worn for multiple days can collect temperature, heart rate, or RR. Unobtrusive wristbands could be used to measure temperature, heart rate, BP, or oxygen saturation. With continuous monitoring, BioMeTs have the potential to provide rich data sets, allowing physicians to know a patient’s baseline over time.
While monitoring for COVID‐19 or any change in health status, these technologies have a potential to help doctors identify clinically meaningful changes in vital signs due to diseases that differ from normal variations due to biological variability, time of day, food and drink, a person’s age, exercise, or underlying physiological conditions. 14 , 99 , 137
More broadly, this knowledge allows for a more personalized approach to medicine. Continuous at‐home monitoring may enable the diagnosis of conditions that are difficult to detect in the clinic or hospital, such as masked hypertension 77 or atrial fibrillation. 66 Such findings can guide future diagnostic tests and treatment.
Continuous monitoring introduces new challenges when collecting and interpreting vital sign measurements taken at home: motion artifacts and usability for patients.
Motion artifacts
Physical activity causing sensor displacement can cause altered readings for any vital sign, especially for wrist‐worn products. Taking measurements at rest and assessing measurements within the context of an individual’s daily activities can reduce the impact of making a care decision on inaccurate data.
Usability for patients
Patients may be unlikely to wear a product that is uncomfortable, despite how accurate it may be. For instance, sticky adhesives or bulky smart clothing may not be acceptable in all patient populations. Other practical features impacting wear adherence include the tool’s battery life, waterproof level, and the tech savvy of the patient population. Considering those at the highest risk for COVID‐19 and most likely to receive care remotely are older patients, assistance or training for using the technology may be required. Healthcare providers could consider form factors that have had positive results in usability evaluations conducted in target patient populations. In a survey earlier this year, one in five Americans reported regularly wearing a smartwatch or wearable fitness tracker. 138 To maximize adherence, healthcare providers could consider using BioMeTs that their patient population is already comfortable with.
It is important to note limitations in BioMeT capabilities. First, connected sensor technologies will not be applicable or appropriate to measure all potential signs and symptoms. For example, vital sign abnormalities in COVID‐19 can be highly variable, and diagnosis and treatment for the disease are not standardized. 7 , 32 There is a wide range of possible symptoms, including shortness of breath, fever, chills, muscle pain, sore throat, nausea, diarrhea, and even loss of smell and taste 139 , 140 that a sensor cannot reliably measure. For instance, shortness of breath is a subjective measurement collected via patient self‐report. Additionally, even for commonly seen symptoms of COVID‐19, such as cough, there is no robustly tested technology for at‐home monitoring. 7 Furthermore, BioMeTs will not be appropriate for all elements of a complete clinical assessment, such as laboratory tests or imaging. Finally, BioMeTs are not intended to be a sole diagnostic tool, they collect measurements that require interpretation by a healthcare professional.
There are limitations of this review. This is not a systematic review or meta‐analysis of individual studies evaluating product measurement performance. Rather, this is a compilation of information available at the time of manuscript submission (June 2020). Information about COVID‐19 and digital medicine in general is rapidly accumulating. This review is not intended to describe all the possible versions of products. There are also other important components when evaluating BioMeTs, such as data rights and security that are beyond the scope of this paper. 20
As we enter the summer months with stay‐at‐home restrictions lifting, the CDC is reporting an increase in COVID‐19 cases across the country, raising concerns that a second wave is upon us. 5 , 141 Consequently, remote monitoring is likely to become a permanent aspect of clinical care and research. To support this growth, more information in the public domain is needed to inform a product choice based on the quality of measurements and usability. Moreover, the field would benefit from unified sources of information on certain types of BioMeTs and results from independent evaluation or validation studies.
Funding
There are no funding sources to disclose. We include specific product manufacturers and models as illustrative examples, not as recommendations or endorsements.
Conflicts of Interest
A.R.C. is an employee of Elektra Labs. D.M. is an employee of Elektra Labs. C.M. is an employee of Elektra Labs. E.S.I. is an employee of Koneksa Health and may own company stock. All other authors declared no competing interests for this work.
Acknowledgments
The authors would like to thank Eric Perakslis and Adam Conner‐Simons for review and feedback on the manuscript.
References
- 1. Daily, L. What is a pulse oximeter, and does the coronavirus pandemic mean you need one? <https://www.washingtonpost.com/lifestyle/wellness/pulse‐oximeter‐covid‐19‐coronavirus/2020/05/18/5b6f8a98‐96df‐11ea‐9f5e‐56d8239bf9ad_story.html> (2020). Accessed July 1, 2020.
- 2.Centers for Disease Control and Prevention (CDC) . Coronavirus Disease 2019 (COVID‐19) <https://www.cdc.gov/coronavirus/2019‐ncov/covid‐data/covidview/index.html> (2020). Accessed July 1, 2020.
- 3. Xu, S. & Li, Y. Beware of the second wave of COVID‐19. Lancet. 395, 1321–1322 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le, T.T. et al The COVID‐19 vaccine development landscape. Nat. Rev. Drug Discov. 19, 305–306 (2020). [DOI] [PubMed] [Google Scholar]
- 5. New York Times . States pause plans to reopen as cases soar <https://www.nytimes.com/2020/06/29/world/coronavirus‐updates.html> (2020). Accessed July 1, 2020.
- 6. Coravos, A. et al Digital medicine: a primer on measurement. Digit. Biomark. 3, 31–71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenhalgh, T. , Koh, G.C.H. & Car, J. Covid‐19: a remote assessment in primary care. BMJ 368, m1182 (2020). [DOI] [PubMed] [Google Scholar]
- 8. Ding, X.R. et al Wearable sensing and telehealth technology with potential applications in the coronavirus pandemic. IEEE Rev. Biomed. Eng. https://doi.org/10.1109/RBME.2020.2992838. [DOI] [PubMed] [Google Scholar]
- 9. Goldsack, J.C. et al Verification, analytical validation, and clinical validation (V3): the foundation of determining fit‐for‐purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit. Med. 3, 55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunn, J. , Runge R., Snyder M. Wearables and the medical revolution. Personalized Medicine 15, 429–448 (2018). [DOI] [PubMed] [Google Scholar]
- 11. Izmailova, E.S. , Wagner, J.A. & Perakslis, E.D. Wearable devices in clinical trials: hype and hypothesis. Clin. Pharmacol. Ther. 104, 42–52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radin, J.M. , Wineinger, N.E. , Topol, E.J. & Steinhubl, S.R. Harnessing wearable device data to improve state‐level real‐time surveillance of influenza‐like illness in the USA: a population‐based study. Lancet Digit. Health 2, e85–e93 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu, G. et al Learning from large‐scale wearable device data for predicting epidemics trend of COVID‐19. Hindawi Discrete Dyn. Nat. Soc. 2020, 1–8 (2020). [Google Scholar]
- 14. Izmailova, E.S. et al Continuous monitoring using a wearable device detects activity‐induced heart rate changes after administration of amphetamine. Clin. Transl. Sci. 12, 677–686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouro Ring (OURA) . UCSF TemPredict Study <https://ouraring.com/ucsf‐tempredict‐study> (2020). Accessed July 1, 2020.
- 16. Lovell, T. COVID‐19 has accelerated adoption of non‐contact patient monitoring technology, says Frost & Sullivan analysis<https://www.healthcareitnews.com/news/europe/covid‐19‐has‐accelerated‐adoption‐non‐contact‐patient‐monitoring‐technology‐says‐frost> (2020). Accessed July 1, 2020.
- 17. Kapoor, A. , Guha, S. , Das, M.K. , Goswami, K.C. & Yadav, R. Digital healthcare: the only solution for better healthcare during COVID‐19 pandemic? Indian Heart J. 72, 61–64 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alwashmi, M.F. The use of digital health in the detection and management of COVID‐19. Int. J. Environ. Res. Public Health 17, 2906 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seshadri, D.R. et al Wearable sensors for COVID‐19: a call to action to harness our digital infrastructure for remote patient monitoring and virtual assessments. Front. Digit. Health 2, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coravos, A. et al Modernizing and designing evaluation frameworks for connected sensor technologies in medicine. NPJ Digit. Med. 3, 37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration (FDA). 510(k) Premarket notification search database<https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm> (2020). Accessed July 1, 2020.
- 22.US Food and Drug Administration (FDA) . Premarket Notification 510(k) <https://www.fda.gov/medical‐devices/premarket‐submissions/premarket‐notification‐510k> (2020). Accessed July 1, 2020.
- 23.US Food and Drug Administration (FDA) . Pulse Oximeters ‐ Premarket Notification Submissions [510(k)s]: Guidance for Industry and Food and Drug Administration Staff <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/pulse‐oximeters‐premarket‐notification‐submissions‐510ks‐guidance‐industry‐and‐food‐and‐drug> (2013). Accessed July 1, 2020.
- 24. Batchelder, P.B. Fingertip pulse oximeter performance in dyspnea and low perfusion during hypoxic events <https://www.nonin.com/resource/clinimark‐white‐paper/>. Accessed July 1, 2020.
- 25. Bent, B. et al Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 3, 18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kodjak, A. A fitbit saved his life? Well, maybe <https://www.npr.org/sections/health‐shots/2016/04/11/473393761/a‐fitbit‐saved‐his‐life‐well‐maybe> (2016). Accessed July 1, 2020.
- 27. Izmailova, E. et al Remote cardiac monitoring for clinical trials <https://fnih.org/sites/default/files/final/pdf/CS2_Cardiac%20Monitoringv2.pdf> (2020). Accessed July 1, 2020.
- 28. Shimbo, D. et al Self‐measured blood pressure monitoring at home: a joint policy statement from the American Heart Association and American Medical Association. Circulation 142, e42–e63 (2020). [DOI] [PubMed] [Google Scholar]
- 29. O'Brien, E. et al European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 15, 23–38 (2010). Published correction appears in Blood Press Monit. 15, 171–172. [DOI] [PubMed] [Google Scholar]
- 30.British and Irish Hypertension Society (BIHS) . BP monitors <https://bihsoc.org/bp‐monitors/>. Accessed July 1, 2020.
- 31. Sieverink, F. , Kelders, S. , Poel, M. & van Gemert‐Pijnen, L. Opening the black box of electronic health: collecting, analyzing, and interpreting log data. JMIR Res. Protoc. 6, e156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goyal, P. et al Clinical characteristics of Covid‐19 in New York City. N. Engl. J. Med. 382, 2372–2374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dunn, J. , Runge, R. & Snyder, M. Wearables and the medical revolution. Per. Med. 15, 429–448 (2018) [DOI] [PubMed] [Google Scholar]
- 34. Yetisen, A.K. , Martinez‐Hurtado, J.L. , Ünal, B. , Khademhosseini, A. & Butt, H. Wearables in medicine. Adv. Mater. 30, 1–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Junger, D. , Madrid, N.M. , Member, I.E.E.E. , Malek, N.P. & Thies, C. Open wearables mobile platform to support personalized medicine. Telemed. Telemon. AAL Home Environ. 7 (2019). [Google Scholar]
- 36. Wang, Z. , Yang, B. , Li, Q. , Wen, L. & Zhang, R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 71, 769–777 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamura, T. , Huang, M. & Togawa, T. Current developments in wearable thermometers. Adv. Biomed. Eng. 7, 88–99 (2018). [Google Scholar]
- 38. Dias, D. & Paulo Silva Cunha, J. Wearable health devices‐vital sign monitoring, systems and technologies. Sensors (Basel) 18, 2414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan, Y. , Ostfeld, A.E. , Lochner, C.M. , Pierre, A. & Arias, A.C. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater. 28, 4373–4395 (2016). [DOI] [PubMed] [Google Scholar]
- 40. Sund‐Levander, M. , Forsberg, C. & Wahren, L.K. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand. J. Caring Sci. 16, 122–128 (2002). [DOI] [PubMed] [Google Scholar]
- 41. Medline Plus . Temperature measurement <https://medlineplus.gov/ency/article/003400.htm>. Accessed July 1, 2020.
- 42. Tyto Care . Tyto Thermometer User Guide <https://static‐cloud.tytocare.com/4_0/manuals/en‐us/760‐00262_A01_TytoHome‐Tyto_Thermometer.pdf> (2019). Accessed July 1, 2020.
- 43. iHealth PT3 . User’s Manual <https://ca0cm30uzh03yhb8b41bu5n1‐wpengine.netdna‐ssl.com/wp‐content/uploads/iHealth‐thermometer‐V1.0‐20180402.pdf>. Accessed July 1, 2020.
- 44. Withings Thermo . Smart Temporal Thermometer Installation and Operating Instructions (iOS users) <https://images‐na.ssl‐images‐amazon.com/images/I/91LDFQ6W7jS.pdf> (2016). Accessed July 1, 2020.
- 45. Empatica . E4 wristband user’s manual <https://empatica.app.box.com/v/E4‐User‐Manual> (2018). Accessed July 1, 2020.
- 46. VitalPatch® Biosensor . VitalConnect® Platform. Instructions for Use. <https://vitalconnect.com/docs/ifu006/revS/IFU‐06%20Rev%20S%20VitalPatch%202.0%20Instructions%20for%20Use.pdf> (2019). Accessed July 1, 2020.
- 47. TempTraq® User Manual Model TT‐100 <https://www.temptraq.com/Instructions/Files/2016‐03‐09_TempTraq‐IFU>. Accessed July 1, 2020.
- 48. Fever™ Scout Instructions for Use <https://images‐na.ssl‐images‐amazon.com/images/I/B1R7hbXkGyS.pdf>. Accessed July 1, 2020.
- 49. Kinsa QC . Kinsa QuickCare Thermometer User Manual KelvinIFU Dec7 <https://usermanual.wiki/Kinsa/QC1/html>. Accessed July 1, 2020.
- 50. Kinsa Smart Ear Thermometer™ Instructions for Use <https://images‐na.ssl‐images‐amazon.com/images/I/A1Q2Jl%2B7G9L.pdf>. Accessed July 1, 2020.
- 51. Izmailova, E.S. et al Evaluation of wearable digital devices in a phase I clinical trial. Clin. Transl. Sci. 12, 247–256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kinsa Product <https://www.kinsahealth.co/products/#Kinsa>. Accessed July 1, 2020.
- 53.US Food and Drug Administration (FDA) . 510(k) Premarket Notification Withings Thermo (Model SCT01) <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K160544>. Accessed July 1, 2020.
- 54.US Food and Drug Administration (FDA) . 510(k) Premarket Notification Fever Scout <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K181013>. Accessed July 1, 2020.
- 55.US Food and Drug Administration (FDA) . 510(k) Premarket Notification TempTraq TT‐100 <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K143267>. Accessed July 1, 2020.
- 56. Kamišalić, A. , Fister, I. Jr , Turkanović, M. & Karakatič, S. Sensors and functionalities of non‐invasive wrist‐wearable devices. A review. Sensors (Basel, Switzerland) 18, 1714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Biovotion . Everion® ‐ Revealing medical grade data you can act on <https://www.biovotion.com/everion/>. Accessed July 1, 2020.
- 58. BioIntelliSense . A New Era of Continuous Health Monitoring and Clinical Intelligence <https://biointellisense.com/>. Accessed July 1, 2020.
- 59. Sampson, M. , Hickey, V. , Huber, J. , Alonso, P.B. , Davies, S.M. & Dandoy, C.E. Feasibility of continuous temperature monitoring in pediatric immunocompromised patients: a pilot study. Pediatr. Blood Cancer 66, e27723 (2019). [DOI] [PubMed] [Google Scholar]
- 60. Khatsenko, K. , Khin, Y. & Maibach, H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis 31, 283–286 (2020). [DOI] [PubMed] [Google Scholar]
- 61. WHOOP . WHOOP Investigating Respiratory Rate Pattern and Relationship with COVID‐19 Symptoms <https://www.prnewswire.com/news‐releases/whoop‐investigating‐respiratory‐rate‐pattern‐and‐relationship‐with‐covid‐19‐symptoms‐301033339.html> (2020). Accessed July 1, 2020.
- 62. Stanford Medicine . Stanford Medicine scientists hope to use data from wearable devices to predict illness, including COVID‐19 <https://med.stanford.edu/news/all‐news/2020/04/wearable‐devices‐for‐predicting‐illness‐.html> (2020). Accessed July 1, 2020.
- 63. Castaneda, D. , Esparza, A. , Ghamari, M. , Soltanpur, C. & Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectr. 4, 195–202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steinhubl, S. et al Effect of a home‐based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 10, 146–155 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fung, E. et al Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front. Physiol. 6, 149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Perez, M.V. et al Large‐scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 381, 1909–1917 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bekker, C.L. , Noordergraaf, F. , Teerenstra, S. , Pop, G. & van den Bemt, B.J.F. Diagnostic accuracy of a single‐lead portable ECG device for measuring QTc prolongation. Ann. Noninvasive. Electrocardiol. 25, e12683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. AliveCor . This is your heart × 6. Introducing KardiaMobile 6L. The only FDA‐cleared personal 6‐lead EKG <https://www.alivecor.com/kardiamobile6l/>. Accessed July 1, 2020.
- 69. Preventice Solutions, Inc. , BodyGuardian® MINI <https://www.preventicesolutions.com/hcp/body‐guardian‐mini>. Accessed July 1, 2020.
- 70. Jeffs, E. , Vollam, S. , Young, J.D. , Horsington, L. , Lynch, B. & Watkinson, P.J. Wearable monitors for patients following discharge from an intensive care unit: practical lessons learnt from an observational study. J. Adv. Nurs. 72, 1851–1862 (2016). [DOI] [PubMed] [Google Scholar]
- 71. Weenk, M. et al Continuous monitoring of vital signs using wearable devices on the general ward: pilot study. JMIR Mhealth Uhealth 5, e91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hailu, R. Fitbits and other wearables may not accurately track heart rates in people of color <https://www.statnews.com/2019/07/24/fitbit‐accuracy‐dark‐skin/> (2019). Accessed July 1, 2020.
- 73. Zhou, F. et al Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Whelton, P.K. et al ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e426–e483 (2018). [DOI] [PubMed] [Google Scholar]
- 75. Muntner, P. et al Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension 73, e35–e66 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pickering, T.G. , James, G.D. , Boddie, C. , Harshfield, G.A. , Blank, S. & Laragh, J.H. How common is white coat hypertension? JAMA 259, 225–228 (1988). [PubMed] [Google Scholar]
- 77. Anstey, D.E. et al An update on masked hypertension. Curr. Hypertens. Rep. 19, 94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. AAMI . Objectives and uses of AAMI standards and recommended practices <https://my.aami.org/aamiresources/previewfiles/8106002_1306_preview.pdf>. Accessed July 1, 2020.
- 79. O'Brien, E. et al Short report: an outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J. Hypertens. 11, 677–679 (1993). [DOI] [PubMed] [Google Scholar]
- 80. Tholl, U. , Anlauf, M. , Lichtblau, U. , Dammer, R. & Roggenbuck, U. [The Stamp of Quality (Prüfsiegel) of the German Hypertension League for the clinical validation of blood pressure measuring devices. Results from the testing of 51 devices]. Dtsch. Med. Wochenschr. 131(46 Spec No), H31–H36 (2006). [DOI] [PubMed] [Google Scholar]
- 81. dabl® Educational Trust <http://www.dableducational.org/sphygmomanometers/recommended_cat.html>. Accessed July 1, 2020.
- 82. US Blood Pressure Validated Device Listing <https://www.validatebp.org/>. Accessed July 1, 2020.
- 83. Lewington, S. , Clarke, R. , Qizilbash, N. , Peto, R. & Collins, R. Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 22, 1060 (2003). [DOI] [PubMed] [Google Scholar]
- 84. Rapsomaniki, E. et al Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1•25 million people. Lancet 383, 1899–1911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cohen, J.B. et al History and justification of a national blood pressure measurement validated device listing. Hypertension 73, 258–264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Berger, A. Oscillatory blood pressure monitoring devices. BMJ. 323, 919 (2001). [Google Scholar]
- 87. Bello, N.A. et al Accuracy of blood pressure measurement devices in pregnancy: a systematic review of validation studies. Hypertension 71, 326–335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Casiglia, E. , Tikhonoff, V. , Albertini, F. & Palatini, P. Poor reliability of wrist blood pressure self‐measurement at home: a population‐based study. Hypertension 68, 896–903 (2016). [DOI] [PubMed] [Google Scholar]
- 89. Ogedegbe, G. & Pickering, T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 28, 571–586 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Del Sorbo, L. et al Mechanical ventilation in adults with acute respiratory distress syndrome. Summary of the experimental evidence for the clinical practice guideline. Ann. Am. Thorac. Soc. 14, S261–S270 (2017). [DOI] [PubMed] [Google Scholar]
- 91. Caputo, N.D. , Strayer, R.J. & Levitan, R. Early self‐proning in awake, non‐intubated patients in the emergency department: a single ED's Experience during the COVID‐19 pandemic. Acad. Emerg. Med. 27, 375–378 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ottestad, W. , Seim, M. & Mæhlen, J.O. COVID‐19 with silent hypoxemia. Tidsskr. Nor. Laegeforen. https://doi.org/10.4045/tidsskr.20.0299. [DOI] [PubMed] [Google Scholar]
- 93. Pappas, S. Silent hypoxia' may be killing COVID‐19 patients. But there's hope <https://www.livescience.com/silent‐hypoxia‐killing‐covid‐19‐coronavirus‐patients.html> (2020). Accessed July 1, 2020.
- 94. Lipnick, M.S. , Feiner, J.R. , Au, P. , Bernstein, M. & Bickler, P.E. The accuracy of 6 inexpensive pulse oximeters not cleared by the food and drug administration: the possible global public health implications. Anesth. Analg. 123, 338–345 (2016). [DOI] [PubMed] [Google Scholar]
- 95. DeMeulenaere, S. Pulse oximetry: uses and limitations author links open overlay. J. Nurse Pract. 3, 312–317 (2007). [Google Scholar]
- 96. Nitzan, M. , Romem, A. & Koppel, R. Pulse oximetry: fundamentals and technology update. Med. Devices (Auckl). 7, 231–239 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. iHealth Air Wireless Pulse Oximeter Product <https://ihealthlabs.com/fitness‐devices/wireless‐pulse‐oximeter/>. Accessed July 1, 2020.
- 98.US Food and Drug Administration (FDA) . 510(k) Premarket Notification iHealth Finger Tip Pulse Oximeter <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K131111>. Accessed July 1, 2020.
- 99. Buekers, J. et al Wearable finger pulse oximetry for continuous oxygen saturation measurements during daily home routines of patients with chronic obstructive pulmonary disease (COPD) over one week: observational study. JMIR Mhealth Uhealth 7, e12866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Guber, A. , Epstein Shochet, G. , Kohn, S. & Shitrit, D. Wrist‐sensor pulse oximeter enables prolonged patient monitoring in chronic lung diseases. J. Med. Syst. 43, 230 (2019). [DOI] [PubMed] [Google Scholar]
- 101. Rabkin Peachman, R. People concerned about COVID‐19 are using pulse oximeters to measure oxygen levels. These are the pros and cons<https://www.consumerreports.org/medical‐symptoms/covid‐19‐pulse‐oximeters‐oxygen‐levels‐faq/> (2020). Accessed July 1, 2020.
- 102. Coté, C.J. , Goldstein, E.A. , Fuchsman, W.H. & Hoaglin, D.C. The effect of nail polish on pulse oximetry. Anesth. Analg. 67, 683–686 (1988). [PubMed] [Google Scholar]
- 103. Science Daily . Can the oxygen in the blood be measured if the nails have been painted? <https://www.sciencedaily.com/releases/2015/11/151130130215.htm> (2015). Accessed July 1, 2020.
- 104. Yamamoto, L.G. , Yamamoto, J.A. , Yamamoto, J.B. , Yamamoto, B.E. & Yamamoto, P.P. Nail polish does not significantly affect pulse oximetry measurements in mildly hypoxic subjects. Respir. Care 53, 1470–1474 (2008). [PubMed] [Google Scholar]
- 105. Hudson, A.J. et al Clinical interpretation of peripheral pulse oximeters labeled "not for medical use". Ann. Fam. Med. 16, 552–554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Areia, C. et al Protocol for a prospective, controlled, cross‐sectional, diagnostic accuracy study to evaluate the specificity and sensitivity of ambulatory monitoring systems in the prompt detection of hypoxia and during movement. BMJ Open 10, e034404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Marjanovic, N. , Mimoz, O. & Guenezan, J. An easy and accurate respiratory rate monitor is necessary. J. Clin. Monit. Comput. 34, 221–222 (2020). [DOI] [PubMed] [Google Scholar]
- 108. Kellett, J. , Li, M. , Rasool, S. , Green, G.C. & Seely, A. Comparison of the heart and breathing rate of acutely ill medical patients recorded by nursing staff with those measured over 5 min by a piezoelectric belt and ECG monitor at the time of admission to hospital. Resuscitation. 82, 1381–1386 (2011). [DOI] [PubMed] [Google Scholar]
- 109. Massaroni, C. , Nicolò, A. , Lo Presti, D. , Sacchetti, M. , Silvestri, S. & Schena, E. Contact‐based methods for measuring respiratory rate. Sensors (Basel) 19, 908 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chu, M. et al Respiration rate and volume measurements using wearable strain sensors. NPJ Digit. Med. https://doi.org/10.1038/s41746‐019‐0083‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cherif, N.H. , Mezghani, N. , Gaudreault, N. , Ouakrim, Y. , Mouzoune, I. & Boulay, P. Physiological data validation of the hexoskin smart textile. 150–156 (2018).
- 112. Lapi, S. et al Respiratory rate assessments using a dual‐accelerometer device. Respir. Physiol. Neurobiol. 191, 60–66 (2014). [DOI] [PubMed] [Google Scholar]
- 113. Aliverti, A. Wearable technology: role in respiratory health and disease. Breathe (Sheff). 13, e27–e36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Moll, J.M. & Wright, V. An objective clinical study of chest expansion. Ann. Rheum. Dis. 31, 1–8 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Banerjee, T. , Peterson, M. , Oliver, Q. , Froehle, A. & Lawhorne, L. Validating a commercial device for continuous activity measurement in the older adult population for dementia management. Smart Health (Amst). 5, 51–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Abdallah, S. et al Late Breaking abstract ‐ validation of hexoskin biometric technology to monitor ventilatory responses at rest and during exercise in COPD. Eur. Respir. J. 50 (suppl. 61) PA1359 (2017). [Google Scholar]
- 117. Chan, A.M. , Ferdosi, N. & Narasimhan, R. Ambulatory respiratory rate detection using ECG and a triaxial accelerometer. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 4058–4061 (2013). [DOI] [PubMed] [Google Scholar]
- 118. Pimentel, M.A.F. , Charlton, P.H. & Clifton, D.A. Probabilistic Estimation of Respiratory Rate from Wearable Sensors Wearable Electronics Sensors. Smart Sensors, Measurement and Instrumentation, Vol. 15, 241–262 (Springer International Publishing Switzerland, Cham, 2015). https://link.springer.com/chapter/10.1007/978‐3‐319‐18191‐2_10#citeas [Google Scholar]
- 119. Charlton, P. et al Breathing rate estimation from the electrocardiogram and photoplethysmogram: a review. IEEE Rev. Biomed. Eng. 11, 2–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nitzan, M. , Faib, I. & Friedman, H. Respiration‐induced changes in tissue blood volume distal to occluded artery, measured by photoplethysmography. J. Biomed. Opt. 11, 040506 (2006). [DOI] [PubMed] [Google Scholar]
- 121. Meredith, D.J. , Clifton, D. , Charlton, P. , Brooks, J. , Pugh, C.W. & Tarassenko, L. Photoplethysmographic derivation of respiratory rate: a review of relevant physiology. J. Med. Eng. Technol. 36, 1–7 (2012). [DOI] [PubMed] [Google Scholar]
- 122. Berntson, G.G. , Cacioppo, J.T. & Quigley, K.S. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 30, 183–196 (1993). [DOI] [PubMed] [Google Scholar]
- 123. Li, J. , Ma, Q. , Chan, A.H. & Man, S.S. Health monitoring through wearable technologies for older adults: smart wearables acceptance model. Appl. Ergon. 75, 162–169 (2019). [DOI] [PubMed] [Google Scholar]
- 124. Shen, C.L. et al Respiratory rate estimation by using ECG, impedance, and motion sensing in smart clothing. J. Med. Biol. Eng. 37, 826–842 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Biovotion Instructions for Use for Handbook <https://biovotion.zendesk.com/hc/en‐us/articles/211452869‐Instructions‐for‐Use‐for‐Handbook‐>. Accessed July 2, 2020.
- 126. Bates, A. , Ling, M.J. , Mann, J. & Arvind, D.K. Respiratory rate and flow waveform estimation from tri‐axial accelerometer data. 2010 International Conference on Body Sensor Networks, Singapore, pp.144–150 (2010). 10.1109/BSN.2010.50 [DOI]
- 127. BioStamp nPoint User Manual <https://cdn2.hubspot.net/hubfs/2433253/2019/Resources/PL‐0145%20Rev%20B%20‐%20BioStamp%20nPoint%20Instructions%20for%20Use%20(1).pdf>. Accessed July 2, 2020.
- 128. Caretaker4 User Manual <https://www.biopac.com/wp‐content/uploads/NIBP‐A‐MRI‐CareTaker4‐Guide.pdf>. Accessed July 2, 2020.
- 129.US Food and Drug Administration (FDA) . 510(k) premarket notification Loop System <https://www.accessdata.fda.gov/cdrh_docs/pdf18/K181352.pdf> (2019). Access July 2, 2020.
- 130. Bio Sticker . Wear Instructions and User Guide <https://fccid.io/2ASE7‐BIOST0308/User‐Manual/user‐manual‐4477967>. Accessed July 2, 2020.
- 131. Holt, M. , Yule, B. , Jackson, D. , Zhu, M. & Moraveji, N. Ambulatory monitoring of respiratory effort using a clothing‐adhered biosensor. IEEE <https://cdn.shopify.com/s/files/1/1549/3297/files/PID5364855.pdf?12662736693242338563> (2018).
- 132. Hexoskin . Hexoskin Shirts Technical Specifications <https://cdn.shopify.com/s/files/1/0284/7802/files/Hexoskin.pdf>. Accessed July 2, 2020.
- 133. Hollander, J.E. & Carr, B.G. Virtually perfect? Telemedicine for Covid‐19. N. Engl. J. Med. 382, 1679–1681 (2020). [DOI] [PubMed] [Google Scholar]
- 134. Capodilupo, E. Leveraging WHOOP Technology to Predict COVID‐19 Risk <https://www.whoop.com/thelocker/predict‐covid‐19‐risk/> (2020). Accessed July 2, 2020.
- 135. Duke Covidentify <https://covidentify.covid19.duke.edu/>. Accessed July 2, 2020.
- 136. Leenen, J.P. et al Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: systematic review. J. Med. Internet Res. 22, e18636 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Li, X. et al Digital health: tracking physiomes and activity using wearable biosensors reveals useful health‐related information. PLoS Biol. 15, e2001402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vogels, E.A. About one‐in‐five Americans use a smart watch or fitness tracker <https://www.pewresearch.org/fact‐tank/2020/01/09/about‐one‐in‐five‐americans‐use‐a‐smart‐watch‐or‐fitness‐tracker/> (2020). Accessed July 2, 2020.
- 139.Centers for Disease Control and Prevention (CDC) . Symptoms of Coronavirus <https://www.cdc.gov/coronavirus/2019‐ncov/symptoms‐testing/symptoms.html> (2020). Accessed July 2, 2020.
- 140. Yan, C.H. , Faraji, F. , Prajapati, D.P. , Boone, C.E. & DeConde, A.S. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int. Forum Allerg. Rhinol. 10, 806–813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. CDC . Coronavirus Disease 2019 (COVID‐19) <https://www.cdc.gov/coronavirus/2019‐ncov/covid‐data/covidview/index.html> (2019). Accessed July 2, 2020.
- 142. Shcherbina, A. et al Accuracy in wrist‐worn, sensor‐based measurements of heart rate and energy expenditure in a diverse cohort. J. Pers. Med. 7, 3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Elektra Labs . Measure COVID‐19 related symptoms at home <https://www.elektralabs.com/covid‐19>. Accessed July 2, 2020.


