Abstract
Maribavir is an orally bioavailable benzimidazole riboside in clinical development for treatment of cytomegalovirus infection in patients who undergo transplantation. Maribavir was evaluated in a thorough QT (TQT) study to determine any effects on cardiac repolarization. The effect of maribavir 100 and 1,200 mg oral doses on the baseline‐adjusted and placebo‐adjusted corrected QT (QTc) interval (delta delta QTc (ddQTc)) and other electrocardiogram (ECG) parameters was assessed in a randomized, phase I, placebo‐controlled, four‐period crossover study in healthy participants (men and women ages 18–50 years). Additionally, maribavir pharmacokinetics, safety, and tolerability were investigated. Moxifloxacin (400 mg) was used as a positive control to demonstrate the study’s ability to detect QT prolongation. Digital 12‐lead Holter ECG monitoring was performed over 22 hours following study drug administration. Individual, Fridericia’s, and Bazett’s QTc intervals were calculated. Of 52 randomized participants (29 ± 8.1 years old; 31 men (60%)), 50 (96%) completed the study. For both 100‐mg and 1200‐mg doses of maribavir, analysis of ddQTc demonstrated that the upper bound of the two‐sided 90% confidence interval was below the 10‐ms threshold at all time points. The concentration–effect analysis demonstrated no relationship between ddQTc and plasma concentrations of maribavir (and its metabolite). There were no clinically meaningful changes in heart rate and systolic blood pressure. The most common adverse event was dysgeusia; no serious adverse events were reported. This TQT study demonstrated that maribavir did not have impact on cardiac repolarization.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The effect of maribavir on the QT/corrected QT (QTc) interval in healthy participants is not known.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Maribavir’s potential effect on the QT/QTc interval was investigated in healthy participants in accordance with International Conference on Harmonization E14 guidelines.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The study provides the evidence of maribavir’s supratherapeutic dose of 1,200 mg in healthy adult participants.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The study provides the evidence of cardiovascular safety of maribavir, an orally bioavailable benzimidazole riboside drug currently in phase III development for cytomegalovirus infection in patients who undergo transplantation.
Cytomegalovirus (CMV) infection or reactivation is a significant complication following both hematopoietic stem cell transplantation (HSCT) and solid organ transplantation, and it is associated with increased morbidity and reduced long‐term survival. 1 , 2 Use of approved anti‐CMV agents may carry risks of treatment‐limiting toxicities or of significant drug interactions leading to contraindication or requiring dose adjustment and monitoring. 3 , 4 , 5 , 6 , 7 Such drawbacks may contribute to failure to prevent CMV infection and disease, or to the development of drug resistance. 8 , 9 , 10
Maribavir (1263W94, GW 1263, GW 1263W94, VP 41263, SHP620, and TAK‐620) is a potent and selective, orally bioavailable, benzimidazole riboside drug with a novel mechanism of action that exerts its effects primarily on viral DNA assembly and on egress of CMV viral capsids from the nuclei of infected cells. 11 , 12 Maribavir is metabolized to its primary metabolite VP 44469 by cytochrome (CYP) P450 isoenzymes CYP3A4 (and, to a minor extent, CYP1A2 and CYP2C19). 13 , 14 Originally developed for CMV prophylaxis in transplant recipients, 15 , 16 maribavir is now in phase III clinical development for the treatment of CMV infection and disease. In January 2018, the US Food and Drug Administration (FDA) granted maribavir a “breakthrough therapy” designation based on data from phase II studies for the treatment of CMV infection and disease (NCT00223925 and NCT01611974).
Maribavir is rapidly absorbed, and peak plasma concentration (Cmax) is generally achieved between 1 and 3 hours after dosing. In single‐dose studies in healthy participants (50–1,600 mg) and patients with HIV (100–1,600 mg), the pharmacokinetics (PKs) of maribavir and VP 44469 were approximately linear. 17 In an ascending, multiple‐dose study in patients with HIV, there was a dose‐proportional increase in plasma maribavir area under the curve to infinity (AUC∞), Cmax, and area under the concentration‐time curve over 24 hours steady‐state (AUC24,ss) over the dose range tested on the first day of maribavir administration (100–200 mg) and following 28 days of drug administration (100, 200, or 400 mg t.i.d., or 600, 900, or 1,200 mg b.i.d.). 18 In phase II studies, maribavir at doses ranging from 400 mg b.i.d. to 1,200 mg b.i.d. was generally well‐tolerated; taste disturbance (dysgeusia), nausea, and diarrhea were notable treatment‐emergent adverse events (TEAEs) that seemed to be associated with maribavir. 19 , 20 , 21 Currently, maribavir at a dose of 400 mg b.i.d. is under investigation in two phase III studies for the treatment of transplant recipients with CMV infection, including resistant or refractory CMV and CMV disease (NCT02927067 and NCT02931539).
The potential of maribavir to prolong ventricular repolarization was previously evaluated in an in vitro study according to the International Conference on Harmonisation (ICH) S7B guidelines. 22 In line with the ICH E14 guidance, 23 this study was conducted in healthy participants to determine the effect of maribavir on the corrected QT (QTc) interval prolongation when compared with placebo as a negative control and moxifloxacin as a positive control. In addition, the PK of maribavir and its metabolite VP 44469, as well as the safety and tolerability of maribavir were evaluated.
METHODS
Study design
The effect of maribavir (100 mg or 1,200 mg) on QTc interval prolongation was evaluated in healthy adult men and women in a phase I randomized, placebo‐controlled, and positive‐controlled (moxifloxacin), four‐sequence crossover study (Figure 1 ). Moxifloxacin (400 mg) was used as a positive control to demonstrate that the study method was sensitive enough to detect a specified change in QTc. PK sample collection and digital 12‐lead Holter electrocardiogram (ECG) monitoring were performed over 22 hours following each study drug administration.
Figure 1.
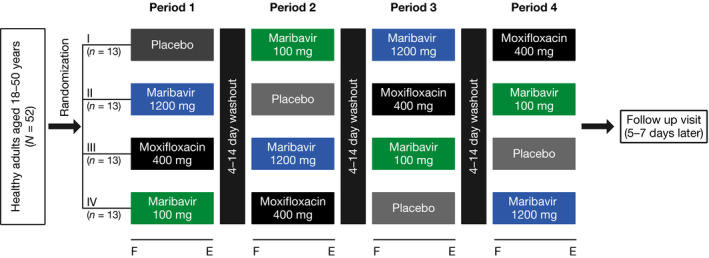
Study schematic. F indicates overnight fast; E indicates pharmacokinetic (PK), echocardiogram (ECG), and safety evaluation (≤ 22 hours). Safety population (N = 52) included all participants who received at least one dose of the study drug (maribavir, moxifloxacin or placebo). PK population (N = 52) included all participants who received at least one dose of the study drug and had sufficient plasma concentration to calculate the primary PK parameters. ECG population (N = 52) included all participants who received at least one dose of the study drug and had at least one baseline (predose) ECG and on‐treatment (postdose) ECG within the same treatment period. PK/pharmacodynamic population included all participants in the ECG population with time‐matched plasma concentrations.
The study protocol was approved by an independent ethics committee and institutional review board. The study was conducted at Covance Clinical Research Unit, Madison, WI, USA, from December 2007 to February 2008 in accordance with the Declaration of Helsinki, the ICH Tripartite Guideline for Good Clinical Practice, and ICH E14 guidelines. 22 , 23 Written informed consent was obtained before any participant initiated study‐related procedures.
Study population
Healthy, nonsmoking men and women between 18 and 50 years of age, with a body mass index of 18.5–32.0 kg/m2 were recruited for the study. Women were of nonchildbearing potential or agreed to use protocol‐specified methods of contraception. Participants were excluded if they had participated in any investigational clinical trial 30 days before the screening visit, or had a known intolerance to moxifloxacin or other quinolones. Participants were also excluded if there was evidence or history of a clinically significant disease/condition, any risk factor for torsades de pointes, ECG abnormalities (including Bazett’s corrected QT (QTcB) interval > 440 ms for men or > 460 ms for women), supine heart rate (HR) > 90 beats per minute (bpm), or supine blood pressure outside 90–140 mm (systolic) or > 90 mm (diastolic). Additional exclusion criteria and agreed behaviors are presented in the Supplementary Material .
Study treatments
A single dose of placebo, maribavir 100 mg (one tablet), maribavir 1,200 mg (six 200‐mg tablets), or Avelox (moxifloxacin) 400 mg (Bayer; Wayne, NJ; positive control) was administered orally on day 1 of each treatment period after an overnight fast based on a randomization schedule. Moxifloxacin was administered in a single‐blind manner, and maribavir and placebo were administered in a double‐blind manner. To maintain blinding, seven tablets were administered for each of the four treatments and participants were blindfolded prior to dosing. Participants continued to fast for 6 hours after the dose administration. The washout period between doses was 4 days (not to exceed 14 days). In the selection of the higher maribavir dose for this study, available safety and PK exposure data by sex were considered. At the time this study was conducted, maribavir had been evaluated in phase III clinical trials for CMV prophylaxis at the dose of 100 mg b.i.d. Therefore, the effect of maribavir on QTc was evaluated at two dose levels: 100 mg (the dose evaluated for preventing CMV disease in two phase III studies) and 1,200 mg (a supratherapeutic dose).
Electrocardiography
ECGs were recorded digitally on days 1 and 2 of each treatment period by a 12‐Lead Holter (H12 + Mortara Instruments, Milwaukee, WI) from 1 hour prior to dosing through 22 hours postdose. Three 10‐second ECG recordings (separated by 1‐minute intervals) were extracted at predose (0 hours) and at 0.5, 1, 1.5, 2, 3, 5, 6, 8, 12, 16, and 24 hours after dosing. Digital 12‐lead Holter ECG analyses were performed at a central laboratory using controlled standard procedures. The ECG data were centrally read by cardiologists using a high‐resolution manual on‐screen caliper method with annotations. All ECG data from each individual was read by a single reader, blinded to treatment. QT (ms) and other ECG intervals (ms) (QTc, PR, and QRS) were calculated using the cardiologists’ annotations on the extracted ECGs. The QT interval results from ventricular repolarization and is measured from the beginning of the QRS complex to the end of the T‐wave. In addition, 12‐lead ECGs for standard assessment of safety were recorded using dual‐snap electrodes. Details of ECG recordings are presented in the Supplementary Material .
Safety and tolerability assessments
Safety was monitored through the recording of adverse events (AEs), changes in physical examinations, vital signs, and standard ECGs. TEAEs included all AEs that started on or after the first dose of the study drug, or increased in severity after the first dose of the study drug. All AEs and TEAEs were coded by system organ class and preferred term using the Medical Dictionary for Regulatory Activities (MedDRA) version 10.0 and were summarized by treatment group.
Pharmacokinetic assessments
Maribavir and VP 44469 plasma concentrations were determined in the samples collected during each treatment period predose and at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, and 22 hours postdose by a validated liquid chromatography/tandem mass spectrometry method from QPS, LLC (Newark, DE). The minimum detectable concentration in plasma was 0.2 μg/mL for both maribavir and VP 44469.
PK parameters were calculated by noncompartmental analysis based on concentration–time profiles and actual postdose times using Phoenix WinNonLin version 5.2 (Pharsight, Mountain View, CA). PK parameters included Cmax; time to reach Cmax (Tmax), AUC from time 0 to time of last quantifiable concentration (AUC0–t), AUC from time 0 to infinity (AUC0–∞), terminal elimination half‐life, and terminal elimination rate constant.
Statistical methods
The analysis of QTc data and concentration–QTc analysis were performed using SAS for Windows (version 8.2 or higher). All Holter ECG data were analyzed by S‐Plus (TIBCO Software, Palo Alto, CA). The relationship between the correction methods and RR interval, and slopes of QT interval corrected individually with placebo data (QTcIb)–RR and QT interval corrected using Fridericia’s formula (QTcF)–RR were assessed based on the coefficient estimates from the linear mixed‐effects model.
Sample size and power calculation
Following a repeated measure design 24 and assuming a one‐sided 0.05 significance level, a within‐participant pretreatment correlation of 0.8, a within‐participant on‐treatment correlation of 0.8, a within‐participant pretreatment vs. on‐treatment correlation of 0.7, and three replicated Holter ECGs at each ECG assessment time point, it was estimated that with a total of 42 participants the study would achieve at least 90% power to detect a prolongation in time‐matched, placebo‐corrected, baseline‐adjusted individualized corrected QT interval (ddQTcIb) of > 5 ms for the largest time‐matched difference between maribavir and placebo among all ECG assessment time points.
A total of 52 participants were randomized to ensure 42 participants were included in the analysis.
QTc calculations
A QTc interval > 500 ms is considered to be prolonged. 25 Because the duration of the QT interval depends on the HR, it is typically standardized by using QTcF or QTcB correction formula. For drugs that increase the HR by > 5–10 bpm, fixed correction may be less accurate than individual QT correction. 26 , 27
QTcIb, QTcF, and QTcB intervals were calculated as follows:
QTcIb: QT rate corrected individually with placebo QT‐RR data (ms).
QTcF: QT corrected for HR by Fridericia’s formula (ms).
QTcB: QT corrected for HR by Bazett’s formula (ms).
The RR interval = 60/HR (seconds) (HR = bpm).
The RR coefficient is the slope obtained from the linear regression of QT on RR derived from the QT–RR pairs recorded on a given participant’s placebo day Holter recording, in a given treatment period. The QT–RR hysteresis (i.e., the change in the preceding RR interval history that influences QT interval) adjustment was accounted for while obtaining the QT–RR coefficients. For any given participant, the participant‐specific QT–RR parameters were calculated. These were fitted into linear regression models to obtain the best fitting curve with least difference from individual data points. The curvature of the individual QT–RR determined the variance in participant‐specific QTcIb. This correction procedure reduces the variance compared with conventional corrections, such as QTcF or QTcB, producing a more reliable and reproducible QT estimate for calculating the primary end point of the study. 28 , 29 The baseline‐corrected QTcIb, where baseline was the average of the three predose values obtained on day 1 of treatment period 1, were calculated for each treatment.
The QTc for a given time point was recorded as the mean/median of the triplicates (three 10‐second ECG recordings separated by 1‐minute intervals). Differences among QTcIb, QTcF, and QTcB at each post‐treatment time point and at baseline (dQTcIb, dQTcF, and dQTcB, respectively) were calculated for every participant by treatment. Individual QTc measurements were summarized with descriptive statistics by treatment and time point.
Statistical analyses of QTc
The primary end point of this study was the mean difference in the treatment‐specific baseline‐adjusted change in postdose QTcIb at each time point between maribavir 1,200 mg and placebo, between maribavir 100 mg and placebo, and between moxifloxacin and placebo (ddQTcIb). The primary ECG analysis was conducted using a repeated‐measures, linear mixed‐effect model with ddQTcIb as the dependent variable, a random effect for “participant” and period, treatment, and time, and treatment‐by‐time interaction as fixed effects. Least squares means and 90% confidence interval (CI) of ddQTcIb were calculated for each postdose time point for maribavir 100 mg, maribavir 1,200 mg, and moxifloxacin. The analysis included the following 10 postdose time points: 0.5, 1, 2, 3, 4, 5, 6, 8, 12, and 22 hours. The same analysis was conducted for QTcF and QTcB as secondary QTc end points. The study’s ability to detect QT prolongation by about 5 ms was validated if moxifloxacin’s lower limit of the two‐sided 90% CI for ddQTcIb (moxifloxacin–placebo) was > 5 ms for at least one of the four postdose time points.
According to the ICH E14 criteria, a thorough QT (TQT) study is negative if an upper one‐sided 95% CI of QTc prolongation effect is < 10 ms. 23 To investigate potential QTc interval prolongation associated with maribavir therapy, the upper bound of the two‐sided 90% CI of the primary end point was compared with 10 ms; if it was < 10 ms in this study, it was concluded that QTc interval prolongation was not clinically meaningful.
Pharmacokinetic analysis
Descriptive statistics (number of participants, arithmetic mean, SD, percentage coefficient of variation, median, minimum, and maximum) were used to summarize PK parameters of interest for maribavir and VP 44469 for both doses at each scheduled time point.
Concentration–QTc analysis
To evaluate the potential of maribavir to prolong the QTc interval, an exploratory post hoc PK–pharmacodynamic analysis based on a linear mixed‐effects model with ddQTcIb or ddQTcF as the dependent variable, maribavir concentration as a fixed effect, and participant as a random effect was performed. As 400 mg b.i.d. maribavir is being evaluated for the treatment of CMV infection in transplant recipients in phase III clinical trials, the effect of 400 mg b.i.d. maribavir dose on QTc at Cmax was predicted using the model developed from the concentration–QTc analysis in this study. The analysis was performed on pooled data from 100‐mg and 1,200‐mg maribavir dose cohorts. The estimated mean slope and intercept from the model were used to calculate predicted ddQTcIb and ddQTcF and 90% CI for the geometric mean Cmax observed at 400 mg b.i.d.. Similar analysis was conducted for the relationship between the metabolite VP 44469 plasma concentration and ddQTc.
RESULTS
Study population
A total of 52 healthy participants (31 men; 60%) were randomly assigned to one of four treatment sequence groups and received at least one dose of the study drug (Figure 1 ). The mean age was 29 ± 8.1 years (18–49 years). The majority of participants were white (44; 85%), with an average weight of 74.1 ± 13.3 kg and body mass index of 24.5 ± 2.9 kg/m2. Demographic and baseline characteristics for participants are shown in Table 1 .
Table 1.
Demographics and baseline characteristics (ITT population, N = 52)
| Characteristic | |
|---|---|
| Age, mean ± SD (range), years | 29 ± 8.1 (18–49) |
| Female, n (%) | 21 (40) |
| Male, n (%) | 31 (60) |
| Race, n (%) | |
| White | 44 (85) |
| Black/African American | 5 (10) |
| Asian | 1 (2) |
| Native Hawaiian or other Pacific Islander | 1 (2) |
| Other | 1 (2) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 6 (12) |
| Not Hispanic/Latino | 46 (89) |
| Height, mean ± SD, cm | 173.3 ± 9.7 |
| Weight, mean ± SD, kg | 74.1 ± 13.3 |
| BMI, mean ± SD (range), kg/m2 | 24.5 ± 2.9 (18–32) |
BMI, body mass index; ITT, intent‐to‐treat.
Fifty participants (96%) completed the study and received all four regimens; two participants discontinued treatment. One participant discontinued the study due to a TEAE of an upper respiratory infection. This participant received maribavir 1,200 mg, placebo, and moxifloxacin 400 mg in the first 3 treatment periods, but did not receive maribavir 100 mg. The second participant received maribavir 100 mg, moxifloxacin 400 mg, and placebo in the first 3 treatment periods, but did not receive maribavir 1,200 mg as he did not return for the fourth treatment period due to a family emergency.
QTc results
The summary of QTcIb and QTcF values (mean ± SD) by treatment and time are shown in Table S1 . The mean QTcIb–RR and QTcF–RR slope, respectively, for placebo was − 0.0014 and − 0.0280 (P = 1.73 × 10−6), for moxifloxacin was 0.0047 and − 0.0243 (P = 2.66 × 10−7), for maribavir 100 mg was 0.0008 and − 0.0263 (P = 7.31 × 10−7), and for maribavir 1,200 mg was 0.0004 and − 0.0260 (P = 8.55 × 10−7). The absolute QTc–RR slope values for QTcIb were smaller than those for QTcF, validating superiority of the individualized correction over Fridericia’s formula. QTc outlier analysis showed no trends in any of the treatment arms. Analysis of the QTc intervals by interval category for QTcIb and QTcF are provided in Table 2 . Four participants had a QTcIb increase of > 30 ms from baseline and two participants had a QTcF increase of > 30 ms from baseline; they had all received moxifloxacin 400 mg.
Table 2.
QTcIb and QTcF interval categorical analysis (N = 52)
| Number of participants (%) | |||||
|---|---|---|---|---|---|
|
Baseline N = 52 |
Placebo N = 52 |
Maribavir 100 mg n = 51 |
Maribavir 1,200 mg n = 51 |
Moxifloxacin 400 mg N = 52 |
|
| QTcIb | |||||
| > 450 ms | 0 | 0 | 0 | 0 | 2 (3.8) |
| > 30 ms increase from baseline | 0 | 0 | 0 | 4 (7.7) | |
| > 30 ms increase from baseline and > 450 ms | 0 | 0 | 0 | 0 | |
| QTcF | |||||
| > 450 ms | 0 | 0 | 0 | 0 | 1 (1.9) |
| > 30 ms increase from baseline | 0 | 0 | 0 | 2 (3.8) | |
| > 30 ms increase from baseline and > 450 ms | 0 | 0 | 0 | 0 | |
QTcF, QT interval corrected using Fridericia’s formula; QTcIb, QT interval corrected individually with placebo QT–RR data.
No participant had QTcIb/QTcF ≥ 480 ms or ≥ 500 ms. In addition, no participant had QTcIb/QTcF increased > 60 ms from baseline. Two participants completed three of the four treatment periods; one missed maribavir 1,200 mg, and the other missed maribavir 100 mg.
The time‐matched analysis demonstrated that for both the 100‐mg and 1,200‐mg maribavir doses, at all time points the upper bound of the 2‐sided 90% CI of the difference in the means of dQTcIb from placebo (ddQTcIb) was below the 10‐ms threshold, confirming no significant effect on cardiac repolarization. Study sensitivity was demonstrated by an increase of ddQTcIb for moxifloxacin with a lower confidence bound exceeding 5 ms at multiple time points during the expected period of peak plasma concentration (Figure 2a ).
Similar results to the primary end point analysis were observed for ddQTcF, the difference between the time‐matched, baseline‐adjusted mean Fridericia‐corrected QT interval for maribavir 1,200 mg (dQTcF), and the dQTcF for placebo (Figure 2b ). In addition, similar results were observed when QTc was corrected using the QTcB formula. Table S2 shows the least squared mean values for the ddQTcIb, ddQTcF, and ddQTcB for maribavir and moxifloxacin over time. In addition, observed ECG abnormalities were judged by the investigator to not be clinically relevant.
Figure 2.
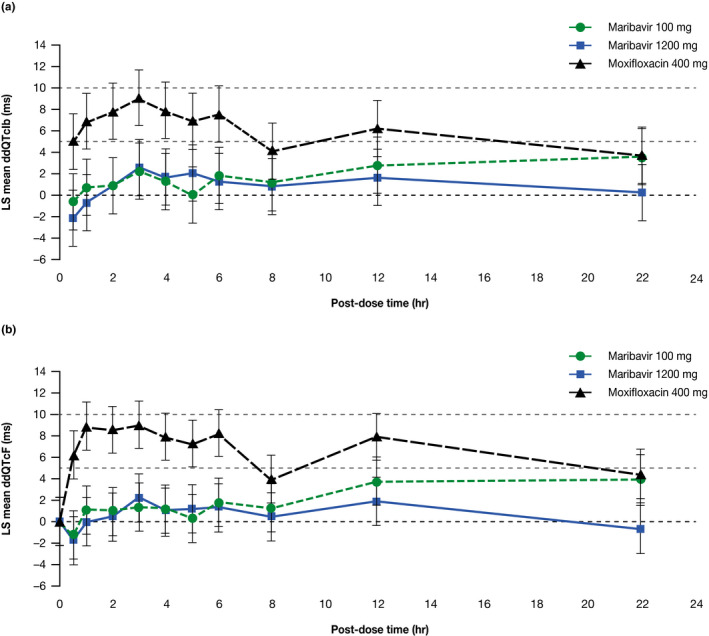
LS mean and 90% CI for time‐matched, placebo‐corrected, baseline‐adjusted corrected QT interval (ddQTc) (ms) vs. time after administration of maribavir (100 mg or 1,200 mg) or moxifloxacin (400 mg) (electrocardiographic PD population); (a) individualized corrected QT interval (ddQTcIb); (b) Fridericia‐corrected QT interval (ddQTcF) (ms). Dashed horizontal lines are reference lines depicting 0, 5, and 10 ms. CI, confidence interval; LS, least squares; PD, pharmacodynamic; QTcF, QT interval corrected using Fridericia’s formula; QTcIb, QT interval corrected individually with placebo QT–RR data.
Pharmacokinetic results
Table 3 shows PK parameters for maribavir and maribavir metabolite VP 44469. Maribavir was rapidly absorbed and readily eliminated after dosing. A slight delay in maribavir mean Tmax and longer mean terminal elimination half‐life values were observed with the increase in dose from 100 mg to 1,200 mg. Mean Cmax values increased 8.8‐fold with a 12‐fold increase in dose level from 100 mg to 1,200 mg maribavir. Mean AUC0–t and AUC0–∞ values increased ~ 18‐fold with a 12‐fold increase in dose level. For VP 44469, Cmax increased 7.3‐fold with a 12‐fold increase in dose level from 100 mg to 1,200 mg maribavir whereas mean AUC0–t and AUC0–∞ values for VP 44469 increased 19‐fold and 10‐fold, respectively, with a 12‐fold increase in maribavir dose level.
Table 3.
Maribavir and VP 44469 PK parameters (PK population; N = 50 a )
| Maribavir | VP 44469 | |||
|---|---|---|---|---|
| Maribavir 100 mg | Maribavir 1,200 mg | Maribavir 100 mg | Maribavir 1,200 mg | |
| Tmax, hour, median (range) | 1.00 (0.500–4.03) | 3.00 (1.00–6.00) | 2.02 (1.00–6.00) | 4.00 (2.00–12.00) |
| Cmax, µg/mL, mean (SD) | 4.18 (1.41) | 36.9 (10.8) | 0.425 (0.125) | 3.12 (0.993) |
| Geometric mean (%CV) | 3.94 (33.8) | 35.4 (29.0) | 0.407 (29.4) | 2.97 (31.9) |
| λz, 1/hour, mean (SD) | 0.265 (0.0811) | 0.135 (0.0388) | 0.190 (0.0273) | 0.0839 (0.0223) |
| t1/2, hour, mean (SD) | 2.95 (1.27) | 5.66 (1.97) | 3.71 (0.501) | 8.83 (2.31) |
| AUC0–t, µg × hour/mL, mean (SD) | 16.8 (7.7) | 307 (105) | 2.12 (0.887) | 40.9 (9.72) |
| Geometric mean (%CV) | 15.3 (45.7) | 290 (33.9) | 1.89 (41.8) | 39.8 (23.8) |
| AUC0–∞, µg × h/mL, mean (SD) | 18.4 (8.5) | 335 (131) | 4.80 (0.776) | 49.8 (11.0) |
| Geometric mean (%CV) | 16.8 (46.0) | 313 (38.8) | 4.76 (16.2) | 48.7 (22.0) |
%CV, percentage coefficient of variation; AUC, area under the concentration–time curve; AUC0–t, AUC from time 0 to the time of the last measurable concentration; AUC0–∞, AUC from time 0 to infinity; Cmax, maximum plasma concentration; PK, pharmacokinetic; Tmax, time to maximum plasma concentration; t1/2, elimination half‐life; λz, terminal elimination rate constant.
Fifty‐two participants were enrolled and treated with the study drug. Fifty (96%) completed the four treatment periods and the study. Two participants completed three of the four treatment periods; one missing maribavir 1,200 mg, and the other missing maribavir 100 mg. Concentrations were not determined for the placebo and moxifloxacin 400 mg treatment groups.
Concentration–QTc relationship analysis
Results from the linear model to evaluate the relationship between ddQTc and plasma maribavir concentration showed that there was no increase in the QTc interval with increasing concentration of maribavir (Figure 3a,b ). At the mean plasma maribavir Cmax of 16.5 µg/mL observed at the maribavir therapeutic dose of 400 mg b.i.d. (Shire Study SHP620‐202, unpublished data), the model‐based estimates of ddQTcIb and ddQTcF were 1.1255 ms (90% CI −0.1612 ms to 2.4122 ms) and 0.9308 ms (90% CI −0.4889 ms to 2.3504 ms), respectively. The CI for both estimates included zero, suggesting that at the maximal concentration at 400 mg b.i.d. maribavir effect on the QTc interval was not significant. As plotted in Figure 3c,d , the relationship between the maribavir metabolite VP 44469 and ddQTc was similar, indicating no increase in the QTc interval with increasing concentration of VP 44469.
Figure 3.
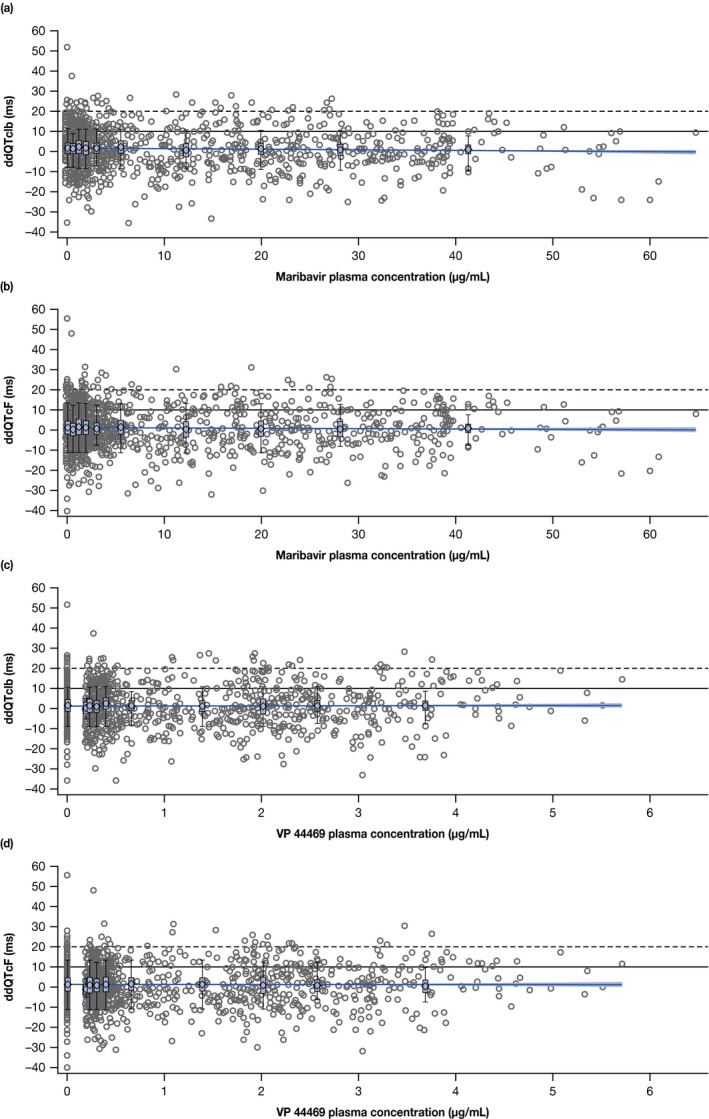
Relationship between maribavir and metabolite plasma concentration and change in time‐matched, placebo‐corrected, baseline‐adjusted QT interval from the linear mixed‐effects PK–PD model for (a) maribavir and ddQTcIb, (b) maribavir and ddQTcF, (c) VP 44469 and ddQTcIb, and (d) VP 44469 and ddQTcF. The solid line with shaded area represents the PK–PD model‐predicted mean change in ddQTcIb or ddQTcF and the 90% confidence interval as a function of maribavir or VP 44469 plasma concentration. Dashed horizontal lines are reference lines depicting 10 ms and 20 ms of ddQTc change. Box plots display the distribution of predicted ddQTcIb or ddQTcF at each concentration decile. Circles represent individual data points from pooled data from 100‐mg and 1,200‐mg maribavir dose cohorts. ddQTcF, time‐matched, placebo‐corrected, baseline‐adjusted Fridericia‐corrected QT interval; ddQTcIb, time‐matched, placebo‐corrected, baseline‐adjusted individualized corrected QT interval; PD, pharmacodynamic; PK, pharmacokinetic.
Furthermore, the geometric mean Cmax value (35.4 µg/mL) observed at the maribavir supratherapeutic dose of 1,200 mg b.i.d. did not show any association with predicted QTc interval prolongation. Mean predicted ddQTcIb and ddQTcF values and their 90% CIs from the linear mixed‐effects model were 0.4876 ms (90% CI −1.0717 ms to 2.047 ms) and 0.5863 ms (90% CI −1.0823 ms to 2.2548 ms), respectively.
Safety and tolerability results
There were no deaths or other serious TEAEs reported during the study. No TEAEs associated with ECG abnormalities were reported. Two participants discontinued the study; the first participant due to a TEAE of upper respiratory infection, which occurred after receiving moxifloxacin, and the second participant discontinued the study due to a family emergency before they would have received maribavir 1,200 mg. There was a total of 133 TEAEs reported (13, 20, 29, and 71, among participants receiving placebo, moxifloxacin 400 mg, maribavir 100 mg, and maribavir 1,200 mg, respectively). All TEAEs were mild in intensity. TEAEs were reported in a higher proportion in participants receiving maribavir 1,200 mg (84%) than those receiving placebo, moxifloxacin 400 mg, or maribavir 100 mg (15%, 21%, and 37%, respectively). Table 4 shows an overall summary of TEAEs and Table S3 shows the number of participants who reported individual TEAEs.
Table 4.
Summary of TEAEs a (ITT‐S population) (N = 52)
| Participants with ≥ 1 TEAE, a n (%) m | Maribavir 100 mg b (n = 51) | Maribavir 1,200 mg c (n = 51) | Moxifloxacin (N = 52) | Placebo (N = 52) |
|---|---|---|---|---|
| All TEAEs | 19 (37) 29 | 43 (84) 71 | 11 (21) 20 | 8 (15) 13 |
| TEAEs related to study drug a , d | 14 (28) 14 | 41 (80) 41 | 2 (4) 2 | 1 (2) 1 |
| Serious TEAEs | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 |
ITT, intent‐to‐treat; ITT‐S, ITT safety; TEAE, treatment‐emergent AE.
TEAEs: All adverse events that started on or after the first dose of the study drug (day 1 of treatment period 1) or increased in severity after the first dose of the study drug. Events that had an onset date during the washout period were counted under the previous treatment period.
One participant was discontinued due to a TEAE of an upper respiratory infection and did not receive maribavir 100 mg.
One participant did not return for the fourth treatment period and did not receive maribavir 1,200 mg.
Related adverse events included events with the relationship to the study drug recorded as possible, probable, or definite, and events with an unknown or unrecorded relationship.
Dysgeusia, the most common TEAE, was reported in 22% (11/51) and 80% (41/51) of participants after receiving maribavir 100 mg and 1,200 mg, respectively. Dysgeusia was also reported by one participant who received moxifloxacin. All TEAEs of dysgeusia were mild in intensity and considered related to the study drug. The next most frequent TEAEs were nausea and headache, which were reported only after treatment with maribavir 100 mg (2% and 6%, respectively) and maribavir 1,200 mg (10% and 2%, respectively). Contact dermatitis was reported in 2–12% of participants across treatments and all cases were attributed to ECG patch application and were not considered to be treatment related.
No clinically meaningful changes in vital signs were observed and no clinically meaningful trends were noted in median change from baseline in any vital sign parameters following any of the four treatments. No participants had standard 12‐lead ECG findings that were considered clinically significant by the investigator.
DISCUSSION
This phase I, randomized, double‐blind, placebo‐controlled, four‐period crossover study in healthy participants evaluated the effect of single‐dose maribavir (100 mg and 1,200 mg) administered orally on ECG parameters with a focus on the QTc interval. No clinically significant repolarization effect and no other significant ECG effect of single‐dose maribavir was detected. Moxifloxacin, which was used as a positive control, demonstrated the study sensitivity.
The results showing the absence of the effect of maribavir on QTc intervals observed in this study are consistent with maribavir in vitro data from human embryonic kidney (HEK293) transfected cells expressing a high level of human ether‐à‐go‐go–related gene channels. Blockade of the human ether‐à‐go‐go–related gene channels, induced by a drug or its metabolites, may cause repolarization abnormalities. Maribavir at concentrations up to 1,500 µg/mL in HEK293 transfected cells did not show an effect on the potassium selective Ik current (ViroPharma study VP1521, unpublished data). In anesthetized beagle dogs, a transient increase in HR without a change in mean arterial pressure was observed following the 30 mg/kg intravenous maribavir dose. 30
The single‐dose, placebo‐controlled and positive‐controlled crossover study design for phase I study in healthy participants used here is a standard design for TQT studies. 25 ICH E14 guidelines also suggest that such studies should evaluate, in healthy participants, the effects of doses that substantially exceed the anticipated therapeutic dose of the drug on ECG parameters (with a focus on QTc interval prolongation).
Because the QT interval duration depends on HR, it is typically standardized to obtain the QTc interval using Fridericia’s or Bazett’s correction formulas, which assume that the relationship between QT and RR is similar in all individuals—although QTcF is often considered more appropriate than QTcB, as the latter may have a greater tendency for overcorrection or undercorrection when there are changes in HR. 25 , 31 For drugs that increase the HR by > 5–10 bpm, it may be more appropriate to use individual‐specific QT correction. 26 , 27 QTc data were reported in this study using QTcIb. The QTc–RR slope values for QTcIb for placebo, moxifloxacin, and maribavir were smaller than those for QTcF, supporting the superiority of the individualized correction over Fridericia’s formula for reducing the HR dependency of QT. Regardless of the correction method used (QTcIb, QTcF, or QTcB), our results consistently showed no significant effect on cardiac repolarization.
Initially, clinical development of maribavir as an anti‐CMV agent was focused on the prevention of CMV disease in patients who underwent transplantation, and, thus, phase II and III CMV prophylaxis studies evaluated maribavir at a dose of 100 mg b.i.d. In phase III studies of CMV prophylaxis, maribavir 100 mg b.i.d. did not show sufficient antiviral activity to prevent CMV disease. 15 , 16 However, maribavir has demonstrated plausible antiviral activity in the treatment of active CMV infections at higher doses (400–1,200 mg b.i.d) in two phase II studies. 19 , 20 Two ongoing phase III studies are being conducted to evaluate the efficacy and safety of maribavir 400 mg b.i.d. for the treatment of CMV infection in treatment‐naive HSCT recipients (NCT02927067) and in solid organ transplantation or HSCT patients with CMV resistant or refractory to (val)ganciclovir or foscarnet (NCT02931539). The concentration–QTc post hoc analysis showed that maribavir at a single dose of 400 mg did not cause QTc prolongation. In addition, the maribavir metabolite VP 44469 did not have an effect on QTc intervals. Maribavir is primarily metabolized through the CYP3A4 pathway, with CYP1A2 being a secondary pathway. Therefore, the administration of concomitant CYP3A4 inhibitors has the potential to increase maribavir exposure significantly. A phase I drug–drug interaction study demonstrated that coadministration with ketoconazole (a strong CYP3A4 inhibitor) increased maribavir AUC by 46% and Cmax by 10%. Other less potent CYP3A4 inhibitors are likely to have a smaller effect on maribavir exposure than ketoconazole. Therefore, the 1,200‐mg dose used in this study is considered a supratherapeutic dose.
In the selection of the higher maribavir dose for this study, available safety and PK exposure data by sex were considered. The highest single dose of maribavir that was administered in early clinical studies was 1,600 mg; however, this dose was evaluated only in male participants. 17 When this TQT study was conducted, the highest maribavir dose that had been administered to female participants was 400 mg b.i.d. Sex difference in maribavir PK has not been observed based on phase I and phase II data (unpublished data, Shire, a Takeda company). Thus, selection of a supratherapeutic dose of 1,200 mg was expected to provide maribavir exposure in male and female participants, which would remain within the upper limit of drug exposure that has been achieved in prior human studies.
The current study used moxifloxacin as a positive control with known QTc prolongation characteristics, to confirm the ability of the study and ECG measurement methods to detect a specified change in QTc interval. From a methodological perspective, the expected effect of moxifloxacin (positive control) on QTc prolongation was observed, supporting the validity of the study results. Several methods for reducing variability in the measurement of QTc interval were used, including recording ECGs in triplicate, QTc hysteresis control, and centralized reading of the ECG data by cardiologists using a high‐resolution manual on‐screen caliper method with annotations, with all ECG data from one participant read by a single reader blinded to treatment. In addition, the study was sufficiently powered to exclude 10‐ms QTc prolongation, using the upper bound of the two‐sided 90% CI of ddQTcIb.
A limitation of this TQT study is that it was performed when maribavir was being developed for prophylaxis of CMV infection. As such, the dose of 100 mg was used as a therapeutic dose, whereas maribavir is now in phase III development at a dose of 400 mg b.i.d. This limitation was overcome by use of the linear model and the estimates of ddQTcIb and ddQTcF at the mean maribavir plasma Cmax seen at the 400‐mg b.i.d. therapeutic dose. 20
There were no unexpected safety findings reported for maribavir during this study. There were no deaths or serious AEs reported, and there were no clinically meaningful abnormalities in vital signs observed at any time point.
In conclusion, in this TQT study, performed in compliance with ICH E14 guidelines, maribavir administered orally at single doses of 100 mg and 1,200 mg in healthy adult participants did not show evidence of a prolonged QT interval or effects on blood pressure or HR. Overall, no significant electrocardiographic effects of maribavir were found, and the PK–pharmacodynamic model indicated no significant effect of maribavir or its metabolite VP 44469 on the QTc interval at the maximal concentration at 400‐mg b.i.d. dosing. These study results are consistent with the cardiac safety findings from previous studies of maribavir 20 , 30 , 32 and provide further insight into maribavir’s safety profile.
Funding
This study was funded by Shire Development LLC, a Takeda company, Lexington, MA, USA. Shire International GmbH, a Takeda company, Zurich, Switzerland, provided funding to Caudex Health, Oxford, UK, for support in writing and editing this manuscript.
Conflicts of Interest
K.I., I.S., and J.W., are employees of Shire, a Takeda company, and own stocks in Takeda. P.M. was an employee of Shire, a Takeda company, at the time of this study.
Author Contributions
K.I. and P.M. wrote the manuscript. K.I., I.S., and J.W. performed the research. K.I., I.S., and J.W. designed the research. K.I., I.S., and J.W. analyzed the data.
Ethical Approval
The protocol was reviewed and approved by an independent ethics committee and institutional review board (Covance Clinical Research Unit Institutional Review Board, Madison, WI).
The study was performed in accordance with the ethical principles stated in the Declaration of Helsinki and the ICH Harmonisation Tripartite Guideline: Guideline for Good Clinical Practice.
The trial was not initiated until written approval of the research plan and the informed consent document were received. Prior to the initiation of any study procedures, the investigator obtained written informed consent from each participant.
Prior Presentation
Data from this manuscript have not been presented previously.
Supporting information
Acknowledgments
The authors acknowledge Steven Villano, MD, who at the time of the research was an employee of Shire, a Takeda company, for contributing to the design of the research; Debra Mandarino, MD, of Covance Clinical Research Unit Inc., for conduct of the study; and Prasant Mohanty, MBBS, MPH, of Shire, a Takeda company, for his statistical input and contributions to the study. Under the direction of the authors, Robert Coover, MPH, of Caudex Health, New York, NY, provided writing assistance for this publication, funded by Shire International GmbH, a Takeda company. Editorial assistance in formatting, proofreading, copyediting, fact checking, coordination, and collation of comments was also provided by Caudex Health, funded by Shire International GmbH, a Takeda company.
Data Availability Statement
The datasets, including redacted study protocol, redacted statistical analysis plan, and individual participant data behind the results reported in this article, will be available 3 months after the submission of a request to researchers who provide a methodologically sound proposal after de‐identification in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
References
- 1. Teira, P. et al Early cytomegalovirus reactivation remains associated with increased transplant‐related mortality in the current era: a CIBMTR analysis. Blood 127, 2427–2438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hakimi, Z. et al Burden of cytomegalovirus disease in solid organ transplant recipients: a national matched cohort study in an inpatient setting. Transpl. Infect. Dis. 19, e12732 (2017). [DOI] [PubMed] [Google Scholar]
- 3. Martin‐Gandul, C. et al Clinical impact of neutropenia related with the preemptive therapy of CMV infection in solid organ transplant recipients. J. Infect. 69, 500–506 (2014). [DOI] [PubMed] [Google Scholar]
- 4. Salzberger, B. , Bowden, R.A. , Hackman, R.C. , Davis, C. & Boeckh, M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood 90, 2502–2508 (1997). [PubMed] [Google Scholar]
- 5. Kotton, C.N. et al The third international consensus guidelines on the management of cytomegalovirus in solid‐organ transplantation. Transplantation 102, 900–931 (2018). [DOI] [PubMed] [Google Scholar]
- 6. Jacobsen, T. & Sifontis, N. Drug interactions and toxicities associated with the antiviral management of cytomegalovirus infection. Am. J. Health Syst. Pharm. 67, 1417–1425 (2010). [DOI] [PubMed] [Google Scholar]
- 7. McCrea, J.B. et al Pharmacokinetic drug‐drug interactions between letermovir and the immunosuppressants cyclosporine, tacrolimus, sirolimus, and mycophenolate mofetil. J. Clin. Pharmacol. 59, 1331–1339 (2019). [DOI] [PubMed] [Google Scholar]
- 8. Hantz, S. et al Drug‐resistant cytomegalovirus in transplant recipients: a French cohort study. J. Antimicrob. Chemother. 65, 2628–2640 (2010). [DOI] [PubMed] [Google Scholar]
- 9. Eid, A.J. , Arthurs, S.K. , Deziel, P.J. , Wilhelm, M.P. & Razonable, R.R. Emergence of drug‐resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin. Transplant. 22, 162–170 (2008). [DOI] [PubMed] [Google Scholar]
- 10. Limaye, A.P. , Raghu, G. , Koelle, D.M. , Ferrenberg, J. , Huang, M.L. & Boeckh, M. High incidence of ganciclovir‐resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J. Infect. Dis. 185, 20–27 (2002). [DOI] [PubMed] [Google Scholar]
- 11. Biron, K.K. et al Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L‐riboside with a unique mode of action. Antimicrob. Agents Chemother. 46, 2365–2372 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krosky, P.M. , Baek, M.C. & Coen, D.M. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77, 905–914 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma, J.D. , Nafziger, A.N. , Villano, S.A. , Gaedigk, A. & Bertino, J.S. Jr. Maribavir pharmacokinetics and the effects of multiple‐dose maribavir on cytochrome P450 (CYP) 1A2, CYP 2C9, CYP 2C19, CYP 2D6, CYP 3A, N‐acetyltransferase‐2, and xanthine oxidase activities in healthy adults. Antimicrob. Agents Chemother. 50, 1130–1135 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song, I. , Sun, K. , Ilic, K. & Martin, P. Summary of maribavir (SHP620) drug–drug interactions based on accumulated clinical and nonclinical data. Biol. Blood Marrow Transplant. 25, S370–S371 (2019); presented at the February 20–24, 2019; Transplantation and Cellular Therapy (TCT) Meeting, Houston, TX. [Google Scholar]
- 15. Marty, F.M. et al Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem‐cell transplants: a phase 3, double‐blind, placebo‐controlled, randomised trial. Lancet Infect. Dis. 11, 284–292 (2011). [DOI] [PubMed] [Google Scholar]
- 16. Winston, D.J. et al Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double‐blind, multicenter controlled trial. Am. J. Transplant. 12, 3021–3030 (2012). [DOI] [PubMed] [Google Scholar]
- 17. Wang, L.H. , Peck, R.W. , Yin, Y. , Allanson, J. , Wiggs, R. & Wire, M.B. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti‐human cytomegalovirus agent, in healthy and human immunodeficiency virus‐infected subjects. Antimicrob. Agents Chemother. 47, 1334–1342 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lalezari, J.P. et al Phase I dose escalation trial evaluating the pharmacokinetics, anti‐human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus‐infected men with asymptomatic HCMV shedding. Antimicrob. Agents Chemother. 46, 2969–2976 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maertens, J. et al Maribavir for preemptive treatment of cytomegalovirus reactivation. N. Engl. J. Med. 381, 1136–1147 (2019). [DOI] [PubMed] [Google Scholar]
- 20. Papanicolaou, G.A. et al Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic‐cell or solid‐organ transplant recipients: a randomized, dose‐ranging, double‐blind, phase 2 study. Clin. Infect. Dis. 68, 1255–1264 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song, I. , Ilic, K. , Sun, K. & Martin, P. Clinical pharmacology of maribavir (SHP620): A comprehensive overview. Biol. Blood Marrow Transplant. 25 (suppl. 3), S342 (2019). [Google Scholar]
- 22. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Harmonised Tripartite Guideline . The non‐clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. S7B 2005 <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/s7b‐nonclinical‐evaluation‐potential‐delayed‐ventricular‐repolarization‐qt‐interval‐prolongation>. Accessed May 12, 2005.
- 23. Department of Health and Human Services (DHHS), Food and Drug Administration & Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) (US) . E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs questions and answers (R3) guidance for industry 2017 <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/e14‐clinical‐evaluation‐qtqtc‐interval‐prolongation‐and‐proarrhythmic‐potential‐non‐antiarrhythmic‐1>.
- 24. Frison, L. & Pocock, S.J. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat. Med. 11, 1685–1704 (1992). [DOI] [PubMed] [Google Scholar]
- 25. Department of Health and Human Services (DHHS), Food and Drug Administration & Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) (US) . Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs 2005. <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073153.pdf>.
- 26. Garnett, C.E. et al Methodologies to characterize the QT/corrected QT interval in the presence of drug‐induced heart rate changes or other autonomic effects. Am. Heart J. 163, 912–930 (2012). [DOI] [PubMed] [Google Scholar]
- 27. Panicker, G.K. , Kadam, P. , Chakraborty, S. , Kothari, S. , Turner, J.R. & Karnad, D.R. Individual‐specific QT interval correction for drugs with substantial heart rate effect using holter ECGs extracted over a wide range of heart rates. J. Clin. Pharmacol. 58, 1013–1019 (2018). [DOI] [PubMed] [Google Scholar]
- 28. Malik, M. , Farbom, P. , Batchvarov, V. , Hnatkova, K. & Camm, A.J. Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart (British Cardiac Society) 87, 220–228 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turner, R. , Panicker, G.K. , Kadam, P. , Chakraborty, S. , Karnad, D.R. & Kothari, S. Individual‐specific corrected QT interval (QTcI) obtained from ECGs recorded at fixed timepoints versus QTcI derived using a wider range of stable heart rates from 24‐hour Holter recordings. Eur. Heart J. 38 (suppl. 1), P2318 (2017). [Google Scholar]
- 30. Koszalka, G.W. et .al Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 46, 2373–2380 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turner, J.R. , Karnad, D.R. , Cabell, C.H. & Kothari, S. Recent developments in the science of proarrhythmic cardiac safety of new drugs. Eur. Heart J. Cardiovasc. Pharmacother. 3, 118–124 (2017). [DOI] [PubMed] [Google Scholar]
- 32. Goldwater, D.R. , Dougherty, C. , Schumacher, M. & Villano, S.A. Effect of ketoconazole on the pharmacokinetics of maribavir in healthy adults. Antimicrob. Agents Chemother. 52, 1794–1798 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets, including redacted study protocol, redacted statistical analysis plan, and individual participant data behind the results reported in this article, will be available 3 months after the submission of a request to researchers who provide a methodologically sound proposal after de‐identification in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.


