Abstract
In December 2019, the severe acute respiratory syndrome virus‐2 pandemic began, causing the coronavirus disease 2019. A vast variety of drugs is being used off‐label as potential therapies. Many of the repurposed drugs have clinical pharmacogenetic guidelines available with therapeutic recommendations when prescribed as indicated on the drug label. The aim of this review is to provide a comprehensive summary of pharmacogenetic biomarkers available for these drugs, which may help to prescribe them more safely.
In December 2019, the pandemic of the severe acute respiratory syndrome virus‐2 (SARS‐CoV‐2) started in Wuhan, China. This virus causes the novel coronavirus disease 2019 (COVID‐19). By the end of May 2020, more than 5 million cases and 330,000 deaths were declared. 1 The management of the disease includes preventive and therapeutic strategies and the treatment of the acute respiratory distress syndrome and the cytokine storm. 2 A vast variety of drugs are being used as off‐label therapies. 3 , 4 Until the effectiveness of any of these repurposed drugs against the SARS‐CoV‐2 virus is demonstrated in randomized clinical trials, their off‐label use will likely continue. The first outbreak of the pandemic ended in June 2020 in Europe, as the pandemic slowed down in the summer. However, in the American continent, the pandemic is currently in an accelerated phase. In addition, the risk for outbreaks and a second wave in autumn exists, both in Europe and worldwide.
Due to the novelty of the disease, physicians may not be aware of the usefulness of pharmacogenetic biomarkers. This applies especially to those clinicians whose medical specialties are not related to infectious or respiratory diseases but had to treat patients with COVID‐19 due to the health crisis. Many of the investigational repurposed drugs have clinical pharmacogenetic guidelines available with therapeutic recommendations when prescribed as indicated on the drug label. In addition to pharmacogenetic biomarkers, it is of great importance to assess drug interactions. Researchers in the University of Liverpool developed an online tool, which includes many (but not all) of the repurposed drugs used in clinical practice for the detection of these interactions. 5 As an example of the usefulness of pharmacogenetic information, two of the prescribed agents are chloroquine and hydroxychloroquine. They are originally indicated for malaria and autoimmune diseases 6 and were related to severe hemolysis crisis in a patient with deficient glucose‐6‐phosphate dehydrogenase (G6PD). 7 Although other authors published opposing results, 8 for primaquine, which belongs to the same family of antimalarial drugs, this association is well‐demonstrated. 9 As an alternative to enzymatic assays, this deficit can be determined with pharmacogenetic biomarkers, as it will be further explained below. Hence, the purpose of this review is not to confirm or deny the effectiveness of drugs used for COVID‐19. Alternatively, we aimed to provide a summary of pharmacogenetic biomarkers available for these drugs, which may help prescribe them more safely. In this review, two criteria were used to include drugs: (1) drugs frequently used to treat COVID‐19 and for which any useful pharmacogenetic information is available 10 or (2) drugs barely used to treat COVID‐19 but with relevant pharmacogenetic information available.
Once the review was finished, we classified the biomarkers into four evidence levels: (1) level 1 evidence involves pharmacogenetic biomarkers with clinical pharmacogenetic guidelines (i.e., those drugs where a particular genotype is related to the efficacy or safety of the drug and where therapy must be adjusted according to the genotype. These biomarkers were extensively studied for years and are already validated in clinical practice; a pharmacogenetic test prior to drug prescription is warranted. (2) Level 2 evidence applies to drugs associated with an adverse drug reaction and a specific pharmacogenetic biomarker, which do not yet have a specific guideline. The evidence for this association is very high and was validated with other drugs. For instance, hydroxychloroquine/chloroquine. G6PD deficiency was reported to be related to the risk of acute hemolytic anemia in patients receiving rasburicase. There is a pharmacogenetic guideline for rasburicase, but not yet for hydroxychloroquine/chloroquine, even though the risk of hemolysis in patients with G6PD deficiency receiving these drugs was reported. A pharmacogenetic test prior to drug prescription is warranted. (3) Level 3 evidence involves drugs without sufficient pharmacogenetic information but related to pharmacogenes (i.e., genes related to metabolism or mechanism of action). In addition, the Pharmacogenomics Knowledgebase includes clinical annotations for these associations, however, no pharmacogenetic recommendations are indicated. These were considered “candidate pharmacogenetic biomarkers” as they need to be validated in clinical practice. Currently, a pharmacogenetic test prior to drug prescription cannot be warranted. (4) Level 4 evidence groups drugs with no or almost no pharmacogenetic information available (i.e., without any Pharmacogenomics Knowledgebase clinical annotation). However, they are related to pharmacogenes, so they were considered “speculative biomarkers.” In summary, levels 1 and 2 group together drugs in which a pharmacogenetic test is warranted and levels 3 and 4 are drugs where, to date, there is insufficient evidence to recommend a pharmacogenetic test (Figure 1 ).
Figure 1.
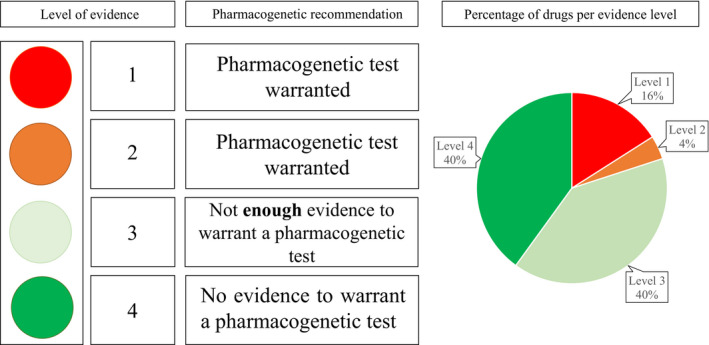
Levels of evidence for the pharmacogenetic testing and application of clinical recommendations for drugs used in the treatment of coronavirus disease 2019 and percentage of drugs per evidence level.
Table 1 depicts a summary of the drugs included in this review, along with the respective pharmacogenetic biomarker and the level of evidence of each association.
Table 1.
Summary of drugs used during COVID‐19 pandemic with corresponding pharmacogenetic information
| Drug | Biomarker | Level of evidence |
|---|---|---|
| Chloroquine and hydroxychloroquine | G6PD | 2 |
| CYP2C8, CYP3A4, and CYP2D6 | 4 | |
| Remdesivir | CYP2C8, CYP2D6, and CYP3A4 | 4 |
| Losartan | CYP2C9 | 3 |
| Captopril, enalapril, and lisinopril | ACE rs1799752 | 3 |
| Spironolactone | ADD1 rs4961 | 3 |
| Ribavirin and peg‐interferon alfa 2a/2b | IFNL3 (IL28B) rs12979860 | 1 |
| Lopinavir/ritonavir | CYP3A4 | 4 |
| Atazanavir/ritonavir | UGT1A1 | 1 |
| Corticosteroids | ABCB1 | 3 |
| Progesterone | CYP2C19 | 3 |
| Nonsteroidal anti‐inflammatory drugs | CYP2C9 | 1 |
| Tocilizumab | IL‐6R rs4329505, rs12083537, and rs11265618 | 3 |
| CYP3A4 | 4 | |
| Sarilumab | CYP3A4 | 4 |
| IL‐6R rs4329505, rs12083537, and rs11265618 | 4 | |
| Siltuximab | CYP3A4 | 4 |
| IL‐6R rs4329505, rs12083537, and rs11265618 | 4 | |
| Sirolimus | CYP3A5 | 3 |
| Nicotine | CYP1A1 and CYP1A2 | 3 |
| Fluvoxamine | CYP2D6 | 1 |
| Ruxolitinib | CYP3A4 and CYP2C9 | 4 |
| Baricitinib | CYP3A4 and ABCB1 | 4 |
| Anakinra | IL‐1 rs17651 | 3 |
| Colchicine | CYP2D6 | 3 |
Levels of evidence. 1: Biomarkers with clinical pharmacogenetic guidelines; 2: Biomarkers with clinical pharmacogenetic guidelines applied to other drugs; 3: Candidate pharmacogenetic biomarkers as published in peer‐review journals without clinical pharmacogenetic guidelines; 4: Speculative biomarkers.
ABCB1, ATP binding cassette subfamily B member 1; ACE, angiotensin‐converting enzyme; ADD1, adducin 1 (alpha); COVID‐19, coronavirus disease 2019; CYP, cytochrome P450; G6PD, glucose‐6‐phosphate dehydrogenase; IFNL3, interferon lambda 3; IL‐1, interleukin‐1; IL‐6R, interleukin‐6 receptor; UGT1A1, UDP glucuronosyltransferase family 1 member A1.
CHLOROQUINE AND HYDROXYCHLOROQUINE
Chloroquine and hydroxychloroquine were among the most utilized repurposed therapeutic agents during the first stages of the pandemic. These drugs demonstrated in vitro activity against SARS‐CoV‐2 virus for what they were vastly used off‐label for the management of COVID‐19. The Centers for Disease Control and Prevention (CDC) guideline “Information for Clinicians on Therapeutic Options for COVID‐19 Patients” included chloroquine as an optional treatment, 11 however, its clinical effectiveness was questioned. 12 , 13 In addition, a recent study reported an increased death rate in patients treated with these drugs. 14 On June 15, 2020, the US Food and Drug Administration (FDA) revoked the Emergency Use Authorization for chloroquine and hydroxychloroquine, due to serious cardiac adverse events reported. The FDA considered that the potential benefits of the use of chloroquine and hydroxychloroquine no longer outweigh the actual potential risks. 15 However, the prophylactic use of chloroquine and hydroxychloroquine in clinical trials continues. 16 The decision against using these drugs for the treatment of COVID‐19 is not applicable to their pre‐exposure and post‐exposure use. 17
Because chloroquine and hydroxychloroquine may still be used, we depict here all the available pharmacogenetic information. To date, their mechanism of action is not perfectly known, and different theories still emerge. Both compounds are weak bases with a half‐life of around 50 days. They inhibit cytokine synthesis by interacting with transcriptional activity; they modulate the release of stimulant molecules, altering lysosomal activity, signaling pathways and autophagy. 18
Although no pharmacogenetic testing is recommended before starting treatment with chloroquine and hydroxychloroquine, the FDA and Swiss drug labels state that they may cause hemolysis in patients who are G6PD deficient. This could be especially important when chloroquine and hydroxychloroquine are co‐administered with other hemolysis‐inducing drugs, such as penicillin, methyldopa, and some cephalosporins. 19 , 20 , 21 , 22 CDC guidelines recommend a reduced dose of 300 mg (half of the standard dose of 600 mg) of chloroquine to those with G6PD deficiency. This dose adjustment can be considered as an indirect recommendation for genetic testing. 23 Although, to date, no specific G6PD deficiency testing is recommended, 11 such deficiency can easily be identified by genotyping the gene. The most common deficiency haplotype is often referred to as G6PD A‐ and composed of rs1050828 (c.202C > T) and rs1050829 (c.376T > C) variants. These two polymorphisms have an average minor allele frequency (MAF) of 15% and 39%, respectively, in African populations. The cause of their high prevalence in Africa is due to its protective effect against malaria infection. The most common white race variant is rs1050829 with 0.4% MAF, and with 0.6% MAF in the Mediterranean population. 24 A higher risk of chloroquine and hydroxychloroquine toxicity may be linked to these genotypes/haplotypes; therefore, physicians should avoid prescribing these drugs to carriers of mutant alleles. In addition, cytochrome P450 (CYP) 2C8, CYP3A4, and CYP2D6 are the main CYP isoforms metabolizing chloroquine. 25 A recently published study reported an association between CYP2C8*2 and *3 and gametocytemia and parasitemia low clearance rates in chloroquine/primaquine‐treated patients. Consequently, these alleles are related to increased toxicity caused by a reduced elimination rate. 26 The role of polymorphisms in these genes should be further investigated concerning chloroquine metabolism. A higher toxicity in individuals with polymorphisms encoding inactive enzymes would be expected.
REMDESIVIR
Remdesivir, an antiviral drug, was developed to treat RNA‐based viruses that maintained global pandemic potential, such as Ebola virus and the Coronaviridae family viruses Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome virus‐1 (SARS‐CoV‐1). Antiviral activity was confirmed against SARS‐CoV‐1 and MERS zoonotic coronaviruses, as well as the circulating human coronaviruses HCoV‐OC43 and HCoV‐229E, which cause the common cold. 27 In vitro and preclinical in vivo animal models supported the effectiveness of remdesivir against SARS‐CoV‐2. 28 , 29 Several clinical trials are being performed to confirm its effectiveness and the clinical improvement of patients with COVID‐19. Based on these initial findings, the FDA issued an Emergency Use Authorization for remdesivir in hospitalized patients. This drug was the first authorized investigational therapeutic option for COVID‐19. 27 Preliminary clinical trials demonstrated remdesivir effectiveness for the treatment of COVID‐19 30 and its use was authorized for the treatment of the disease. 31
Remdesivir is a nucleoside analog and acts as an RNA‐dependent RNA polymerase inhibitor, which is a protein complex used by the virus to replicate its genome. After the host metabolizes remdesivir into active nucleotide triphosphate, the metabolite competes with adenosine triphosphate for incorporation into the RNA strand of the virus causing premature termination of its synthesis. 32
To date, few data are available about the metabolism of remdesivir. The drug appears to be an in vitro substrate of CYP2C8, CYP2D6, and CYP3A4. Nevertheless, its in vivo metabolism seems to be dominated by hydrolase activity. Therefore, no possible drug‐drug interactions are likely to occur with any CYP2C8, CYP2D6, and CYP3A4 inhibitors and inducers. 33 In addition, poor metabolizers (PMs) for any of these enzymes are unlikely to be associated with a different clinical outcome. Congruently, according to current knowledge, no guideline recommends pharmacogenetic testing before remdesivir administration.
THE RENIN‐ANGIOTENSIN‐ALDOSTERONE SYSTEM
Concerns about the possibility that inhibitors of the renin–angiotensin–aldosterone system (RAAS) could predispose individuals to severe COVID‐19 were raised. 34 SARS‐CoV‐2 enters pulmonary cells through the angiotensin converting enzyme (ACE)2, which is expressed in the membrane of pulmonary cells. ACE2 and ACE counterbalance each other and provide a homeostatic regulation of angiotensin (Ang)II. 35 Renin mediates the transformation of angiotensinogen into Ang I, ACE converts Ang I to Ang II, and ACE2 converts Ang II to Ang 1–7. Ang II produces vasoconstriction and hypertension by interacting with angiotensin receptors 1R and 2R (AT1R, AT2R) and Ang 1–7 produces vasodilation by interacting with the Mas receptor. Finally, Ang II stimulates the release of aldosterone on the adrenal cortex, which regulates blood volume, blood pressure, and levels of Na+, K+, and H+. 36 , 37 , 38 Three drug families block the RAAS system at different levels: inhibitors of the ACE, namely enalapril or lisinopril, antagonists of the AT1R receptor, namely losartan or irbesartan, and antagonists of aldosterone, namely spironolactone. The use of any of these families controls blood pressure through the explained mechanisms and upregulates the expression of ACE2. 39 As this enzyme is the entrance for the SARS‐CoV‐2 into the cell, the upregulation of ACE2 was related to a worse COVID‐19 prognosis. 40 The use of RAAS inhibitors in patients with COVID‐19 seems not to have any clinically relevant effect. 34 There is no evidence for a dose‐dependent ACE2 upregulation. Therefore, it is unlikely that the use of higher doses of RAAS inhibitors will produce a clinical effect. Therefore, RAAS inhibitors ought not to be discontinued. Another reason that justifies the use of these drugs in patients with COVID‐19 and hypertension is that hypertension was related to a worse COVID‐19 prognosis. 41
There are some pharmacogenetic predictors of disposition and response of RAAS inhibitors. Their consideration for the management of COVID‐19 would control the upregulation of ACE2. For instance, losartan is metabolized by CYP2C9, 42 therefore, genotyping some of its polymorphisms may condition the clinical benefits of losartan. The most common reduced‐function variants are CYP2C9*2 (Arg144Cys), and CYP2C9*3 (Ile359Leu). Approximately two‐third of the white population express the wild‐type genotype (CYP2C9*1/*1), one‐third express either the *1/*2 or *1/*3 genotype, and < 2.5% of individuals express the *2/*2, *2/*3, and *3/*3 genotypes. 43 In patients with secondary kidney diseases, increased blood pressure was registered more frequently in *2 and *3 variant carriers compared with wild‐type individuals. 42 Although, to date, no pharmacogenetic guideline was published with therapeutic recommendations on losartan, CYP2C9 genotyping could be beneficial for dose adjustment. An impaired metabolism would cause toxicity problems due to the accumulation of the drug.
Other pharmacogenetic biomarkers, namely ATP Binding Cassette Subfamily B Member 1 (ABCB1) gene variants, could be useful predictors of losartan response. 44 These gene encodes the P‐glycoprotein (P‐gp), an efflux transporter that mediates the movements of xenobiotics between different body compartments. 45 Consequently, it can modulate drug pharmacokinetics. The most relevant polymorphisms in ABCB1 are C3435T (rs1045642), G2677T/A (rs2032582) and C1236T (rs1128503). 46 Regarding ACE inhibitors, ACE rs1799752 was related to variability in captopril, 47 enalapril, 48 and lisinopril 49 effectiveness. This polymorphism consists of a 50‐nucleotide deletion (del). Patients with the del/del diplotype were related to a worse clinical outcome. Concerning aldosterone antagonists, the best pharmacogenetic predictor for spironolactone effectiveness is rs4961, located in the alpha‐adducin gene, which predicts drug response in combination with furosemide. 50 Carriers of the G allele exhibited a better response to therapy compared with T allele carriers. Furthermore, polymorphism of the ACE2 gene and the viral ACE2 receptor gene was related to variability in COVID‐19 prognosis. 51 To date, there is no clear evidence on the contribution of this polymorphism to the susceptibility to the disease. However, polymorphisms in the ACE2 and ACE genes were associated with hypertension (ACE2 rs4240157, rs4646155, rs4830542, rs2074192 rs233575, rs2158083, and rs21068809, ACE G8790A, and I/D). 51 Due to the apparent relationship between hypertension and COVID‐19 prognosis, it may be of interest to genotype patients for ACE2 polymorphisms. In the context of the use of RAAS inhibitors, the detection of mutations in one of the system mediators (ACE2) may be of particular relevance.
RIBAVIRIN
Ribavirin is an antiviral medication used to treat respiratory syncytial virus and hepatitis C virus (HCV) infections and some viral hemorrhagic fevers. 52 Its mechanism of action is well‐described: first, it incorporates into replicating RNA, which stops chain elongation; second, it inhibits the de novo synthesis of GTP, required for viral RNA synthesis; and third, it enhances the response of interferon‐stimulated genes, making cells more susceptible against exogenous interferon and increasing the endogenous production. Because it demonstrated in vitro activity against SARS‐CoV‐2 virus, it was used in a variety of clinical settings for the management of COVID‐19. 53
The rs12979860 polymorphism in the interferon lambda 3 (IFNL3 and IL28B) gene is the strongest baseline predictor of response to ribavirin in patients with HCV genotype 1 treated with interferons and ribavirin, according to the Clinical Pharmacogenetics Implementation Consortium (CPIC). Patients with the favorable response genotype (rs12979860 CC) have better response (higher sustained virologic response (SVR)) compared with those with unfavorable response genotype (rs12979860 CT or TT). Precisely, individuals with CC genotype have ~ 70% chance for SVR after 48 weeks of treatment. Contrastively, only 30% of individuals with unfavorable response genotype have SVR after 48 weeks of treatment. 54 Additionally, the Dutch Pharmacogenetics Working Group (DPWG) found some evidence for lower treatment response in HLA‐B*44‐negative patients. However, there are no dosing recommendations currently as ~ 90% of the population is HLA‐B*44‐negative. 55 In summary, IFNL3 genotyping may be a predictor of ribavirin/peginterferon response in patients with COVID‐19, but further research on this biomarker is warranted.
INTERFERONS
Interferons (IFNs) are cytokines, which are produced and released by host cells as an antiviral response to trigger the immune system, therefore, to stimulate protective defenses. IFNs have several other functions: they activate natural killer cells and macrophages, and increase the expression of major histocompatibility antigens. 56 Consequently, they are used to treat viral infections, such as chronic HCV, hepatitis B, human papillomavirus infections, and Kaposi’s sarcoma caused by AIDS. Moreover, they are also used in hairy‐cell leukemia, chronic myelogenous leukemia, metastatic renal‐cell carcinomas, cutaneous melanoma, hemangiomas, and multiple sclerosis. 57 IFNs are classified into three types, type I, II (alfa and beta interferons), and type III (gamma interferon), based on the structure of their receptors on the cell membrane surface. Pegylated interferon, usually called peginterferon, is a recombinant form of the standard interferon. PEG stands for polyethylene glycol. 58 Currently, only IFN beta‐1b is being investigated as a possible treatment for COVID‐19. Nevertheless, its efficacy was determined only in combination with lopinavir, ritonavir, and ribavirin. 59
No pharmacogenetic recommendation is available for IFN beta‐1b. However, the CPIC guideline recommends genotyping the rs12979860 polymorphism in the IFNL3 gene during peginterferon alfa‐2a and peginterferon alfa‐2b therapy of HCV in combination with ribavirin. 54 Several other polymorphisms, such as rs8099917 and rs1188122, are being investigated in IFNL3 and IFNL4 genes associated to IFNs. However, no clinical recommendation is available for any other than rs12979860.
In addition, a recent study showed that homozygosity for the C allele of rs12252 in the IFITM3 gene is associated with more severe COVID‐19 prognosis in an age‐dependent manner. 60 IFITM3 is a potent antiviral protein that enhances cellular resistance to a variety of pathogens, including influenza virus. 61 Consequently, homozygous for the CC genotype of rs12252 may not have this protective effect against the serious symptoms of COVID‐19.
LOPINAVIR AND RITONAVIR
Lopinavir and ritonavir are usually co‐administered antiretroviral drugs of the protease inhibitor class and used against HIV infections. The co‐administration of lopinavir and ritonavir produces its antiviral effect by inhibiting the formation of infectious virions, thus preventing subsequent waves of cellular infection. 62 Based on a recent study, it appears that these drugs are inhibitors of SARS‐CoV‐2 3CLpro protein, which is responsible for the cleavage of polyproteins into an RNA‐dependent RNA polymerase and a helicase 1 during the replication process. 63 CYP3A4 is involved in their metabolism. Lopinavir and ritonavir are contraindicated with drugs that are highly metabolized by CYP3A enzymes or are potent CYP3A inducers, according to the FDA‐approved label. 64 The most studied no function CYP3A4 alleles are CYP3A4*6, CYP3A4*20, and CYP3A4*26. The CYP3A4*20 allele has a frequency of about 1.2% in white patients; with a frequency that reaches up to 3.8% in specific regions. 65 CYP3A4*22, with the MAF of 5% in white patients, is a reduced function allele. 66 Additionally, CYP3A4*2 and *3 were identified as missense polymorphisms 67 decreasing the function of the enzyme with a frequency of 1.1 and 2.1% in white patients, respectively. 68 , 69 Nevertheless, the exact role of these polymorphisms are currently unknown. These polymorphisms are unlikely to have a relevant effect, as ritonavir completely blocks CYP3A4, which is, in fact, prescribed with this intention in order to increase lopinavir exposure.
ATAZANAVIR AND RITONAVIR
Atazanavir is an inhibitor of the HIV protease, one of the viral molecules targeted in the clinical management of AIDS. It is typically co‐administered with ritonavir and other antiviral drugs in highly active antiretroviral therapy. 70 In vitro and ex silico investigations reported atazanavir to mediate SARS‐CoV‐2 major protease inhibition. 71 , 72 The CPIC dosing guideline on atazanavir and UDP glucuronosyltransferase family 1 member A1 (UGT1A1) gene classifies individuals according to UGT1A1 alleles into normal metabolizers (NMs), intermediate metabolizers (IMs) and PMs. The PMs (i.e., carriers of two decreased function alleles) are more likely to develop jaundice, which may cause nonadherence, and alternative drugs should be considered. The risk of discontinuation is low or very low for individuals carrying one or zero decreased‐function alleles (IMs and NMs). 73 Table 2 shows some examples of genotype‐phenotype inference. In conclusion, UGT1A1 genotyping in atazanavir‐treated patients with COVID‐19 could improve therapy tolerability.
Table 2.
UGT1A1 phenotype inference based on genotype information
| UGT1A1 | |
|---|---|
| Normal metabolizers | |
| *1/*1, *1/*36, *36/*36 | 2 reference function (*1/*1) and/or increased function allele (*36) |
| rs887829 C/C | rs887829 C/C homozygosity |
| Intermediate metabolizers | |
| *1/*28, *1/*37, *36/*28, *36/*37 | 1 reference function (*1) or increased function allele (*36) and 1 decreased function allele (*6, *28, *37) |
| rs887829 C/T (*1/*80) | rs887829 C/T heterozygosity |
| Poor metabolizers | |
| *28/*28, *28/*37, *37/*37, *6/*6 | 2 decreased function alleles (*6, *28, *37). |
| rs887829 T/T (*80/*80) | rs887829 T/T homozygosity |
Information obtained from Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline on atazanavir and UGT1A1. 73
UGT1A1, UDP glucuronosyltransferase family 1 member A1.
STEROIDS
Previous epidemics of coronaviruses, such as SARS‐CoV‐1 and MERS, demonstrated that the unregulated inflammatory response could eventually lead to an aggression on the respiratory system. 74 However, patients with severe COVID‐19 undergo inflammatory‐induced lung injuries, for what corticosteroids were indicated, namely hydrocortisone, prednisone, methylprednisolone, prednisolone, or dexamethasone. 75 To date, no pharmacogenetic guideline was published on any corticosteroid and no pharmacogenetic recommendations can be addressed. However, ABCB1 polymorphism was related to variability in dexamethasone effectiveness, and to variability in methylprednisolone, prednisone, and prednisolone tolerability, probably due to the variability on drug exposure. 76 , 77 , 78 , 79 The ABCB1 gene encodes the P‐gp, an efflux transporter responsible for the transport of xenobiotics across different body compartments; its polymorphism can cause loss of function on the transporter and relevant pharmacokinetic alterations. 45 , 46 Apart from traditional corticosteroids, the sexual hormone progesterone was repurposed for the treatment of COVID‐19 (ClinicalTrials.gov: NCT04365127). It is metabolized by CYP3A4, CYP2C19, and CYP2C9 enzymes. However, only CYP2C19 phenotype seems relevant to significantly alter drug bioavailability. 80 In patients with COVID‐19, it could be of interest to closely observe CYP2C19 PMs (*2/*2 or *2/*3) for the risk of toxicity and to titrate dose to reach clinical efficacy for CYP2C19 ultrarapid metabolizers (*17/*17).
NONSTEROIDAL ANTI‐INFLAMMATORY DRUGS
Nonsteroidal anti‐inflammatory drugs (NSAIDs) inhibit cyclooxygenase enzymes 1 or 2 (COX1 and 2) impeding the production of prostaglandins (involved in the process of inflammation) and thromboxane (modulating blood clotting). NSAIDs may be prescribed for the management of pain and fever. Controversy arose on the use of NSAIDs due to the possibility of a worse COVID‐19 prognosis. 81 However, the European Medicines Agency (EMA) declared that there is no evidence in this respect and that NSAIDs may be used for COVID‐19 management. 82
In March 2020, the CPIC published a pharmacogenetic guideline on NSAIDs. 83 Specific therapeutic recommendations are acknowledged for celecoxib, flurbiprofen, ibuprofen, lornoxicam, meloxicam, piroxicam, and tenoxicam based on CYP2C9 phenotype. This phenotype is inferred from an activity score, which is obtained by the sum of two individual allele scores (Table 3 ). A functional allele is assigned a score of 1, whereas reduced‐function (e.g., *2) and no‐function (e.g., *3) alleles receive the score of 0.5 and 0, respectively. IMs or PMs may be overexposed to these drugs, that justifies dose reductions or changes in the prescription. For celecoxib, flurbiprofen, ibuprofen, and lornoxicam, CYP2C9 NMs and IMs with an activity score of 1.5 are recommended to initiate therapy with the approved starting dose. For IMs with an activity score of 1.0, therapy should start with the lowest recommended dose and titrate to clinical effect. Finally, the clinical recommendation for PMs involves sharp dose reductions (initiating therapy with 25–50% of the lowest recommended starting dose) or using alternative drugs (e.g., acetylsalicylic acid, metamizole, or naproxen). For meloxicam, piroxicam, and tenoxicam, the recommendations for CYP2C9 NMs and IMs with an activity score of 1.5 are similar to celecoxib, flurbiprofen, ibuprofen, or lornoxicam. For IMs with activity score of 1.0, meloxicam should be prescribed with 50% of the lowest starting dose or an alternative drug may be used; for PMs, an alternative drug should be used. For piroxicam and tenoxicam, IMs with an activity score of 1.0 and PMs should receive an alternative drug.
Table 3.
CYP2C9 phenotype inference based on genotype information
| CYP2C9 | ||
|---|---|---|
| Normal metabolizers | ||
| AS = 2.0 | *1/*1 | 2 normal‐function alleles |
| Intermediate metabolizers | ||
| AS = 1.5 | *1/*2, *1/*5, 1*/*8, *1/*11 | 1 normal‐function allele + 1 decreased‐function allele |
| AS = 1.0 | *1/*3, *2/*2, *2/*5, *2/*8, *2/*11, *5/*5, *5/*8, *5/*11, *8/*8, *8/11, *11/11 | 1 normal‐function allele + 1 decreased‐function allele or 2 decreased‐function alleles |
| Poor metabolizers | ||
| AS = 0.5 | *2/*3, *3/*5, 3*/*8, *3/*11 | 1 decreased‐function allele + 1 no‐function allele |
| AS = 0 | *3/*3 | 2 no‐function alleles |
Information obtained from Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline on non‐steroidal anti‐inflammatory drugs and CYP2C9. 83
AS, activity score; CYP2C9, cytochrome P450 2C9.
TOCILIZUMAB, SARILUMAB, AND SILTUXIMAB
A large number of T‐lymphocytes and mononuclear macrophages are activated in patients suffering from COVID‐19. Consequently, they induce cytokines, such as IL‐6, which binds to the IL‐6 receptor on the target cells, causing the cytokine storm and severe inflammatory responses, particularly in the lungs. Tocilizumab, sarilumab, and siltuximab are monoclonal antibodies that target IL‐6 receptor (tocilizumab and sarilumab) or IL‐6 (siltuximab). Consequently, they inhibit IL‐6 mediated signaling, mitigating the inflammatory response. 84 Tocilizumab and sarilumab are used for the treatment of rheumatoid arthritis, 85 , 86 whereas siltuximab is used for the treatment of Multicentric Castleman’s disease, a lymphoproliferative disorder often associated to human herpes virus 8, which is more frequently seen in the presence of an HIV infection. 87
To date, no pharmacogenetic testing is recommended for tocilizumab. Nevertheless, several studies were performed to investigate the association between genetic polymorphisms in IL6R gene and tocilizumab response. Patients with IL6R rs4329505 CC and CT genotypes may have decreased response to tocilizumab as compared with patients with TT genotype. 88 Moreover, rs12083537 AA genotype was associated with decreased response to tocilizumab and higher risk for asthma as compared with AG genotype. 88 Finally, rs11265618 CC genotype was associated to increased response to tocilizumab as compared with CT and TT genotypes. 89 Polymorphisms in CD69, FCGR3A, and GALNT18 genes were also associated with tocilizumab response. Patients with the CD69 rs11052877 AA genotype may have an increased response to tocilizumab compared to patients with the AG and GG genotypes. 90 In addition, patients with the FCGR3A rs396991 AA genotype may have an increased response to tocilizumab as compared with patients with the AC or CC genotypes. 91 Finally, GALNT18 rs4910008 CC individuals may have increased response to tocilizumab compared with patients with the CT and TT genotypes. 90 However, these studies state that other genetic and clinical factors may affect the response to tocilizumab.
Concerning sarilumab, very little pharmacogenetic information is available. Similar to tocilizumab, it is metabolized by catabolic pathways and not by CYP450 enzymes. Nevertheless, elevated levels of IL‐6 decrease CYP3A4 activity. Consequently, the use of these monoclonal antibodies enhance CYP3A4 activity and reduce CYP3A4 substrate exposure, such as simvastatin. 86 The same mechanism may occur with CYP2C9 and CYP2C19. 92 Consequently, CYP3A4 metabolizer status should be evaluated in patients receiving sarilumab or tocilizumab with a CYP3A4 substrate. Furthermore, due to their same mechanism of action, the associations established for tocilizumab could also affect sarilumab.
To the best of our knowledge, to date, only an Italian study—SISCO (siltuximab in serious COVID‐19)—administered siltuximab for COVID‐19 treatment. Twenty‐one patients who developed acute respiratory distress syndrome received intravenous siltuximab. An improvement was experienced in the clinical condition of 33% of patients, 43% stabilized and the condition of 24% of patients worsened; among this group, 1 patient died, and 1 patient experienced a cerebrovascular event. 93 Siltuximab is presumably degraded into small peptides and amino acids via catabolism. 87 Nevertheless, similarly to sarilumab, it may interfere with CYP450 activity. The FDA drug label states that siltuximab treatment can lead to the increased metabolism of CYP450 substrates, especially CYP3A4. This could be due to the fact that CYP450 enzymes are suppressed by cytokines, such as IL‐6 in case of infection and inflammation. 94 Consequently, drug levels should be checked and the dose of siltuximab should be adjusted if necessary.
SIROLIMUS
Sirolimus, also known as rapamycin, is a macrocyclic triene antibiotic with potent antifungal, antitumoral, and immunosuppressant activity. Its immunosuppressant activity is the consequence of very complex intracellular interactions; it interacts with immunophilins and blocks the transcriptional activation of several cytokine genes, impeding cytokine production, and inhibiting the activation of T and B cells by reducing their sensitivity to IL‐2. 95 , 96 It is mainly prescribed to prevent organ transplant rejection, especially kidney, and the graft‐vs.‐host disease. Sirolimus, in combination with dactinomycin, was repurposed for the treatment of COVID‐19 as synergistically targeted human coronavirus protein subnetwork. 97 This drug is currently being investigated in phase I/II clinical trials (ClinicalTrials.gov: NCT04371640 and NCT04341675). It is mainly metabolized by CYP3A4/5. 98 Most people are nonexpressers of CYP3A5 (i.e., carriers of the *3/*3 diplotype). However, some of them preserve the *1 allele, therefore, express CYP3A5 and metabolize sirolimus to a greater extent than nonmetabolizers and show reduced sirolimus bioavailability. Consequently, CYP3A5 expressers (*1/*1 and *1/*3) were related to higher dose requirements compared with CYP3A5 nonexpressers (*3/*3). 99 Although there is no pharmacogenetic guideline on sirolimus and CYP3A5, for the structurally related drug tacrolimus, a pharmacogenetic guideline was published in 2015. 100 For CYP3A5 expressors, an initial dose of 1.5 to 2 times the standard one is recommended to reach therapeutic concentrations. Nevertheless, there is not enough evidence currently to apply these dose adjustments to everolimus.
NICOTINE
Smoking, along with its related conditions (e.g., cardiovascular diseases and chronic obstructive pulmonary disease), are known to increase the risk for respiratory infection susceptibility and severity. Nevertheless, nicotine seems to have protective effects by enhancing the cholinergic anti‐inflammatory pathway. 101 Additionally, it is suspected that COVID‐19 may be a disease of the nicotinic cholinergic system. 101
Nicotine induces CYP1A1 and CYP1A2 enzymes in the lungs, which can activate carcinogens. The most common variants in CYP1A1 are *2A (rs4646903), and *2C (rs1048943), whose combination defines *2B. All three have uncertain functions. Moreover, CYP1A1*3 seems to have enhanced enzyme activity, although it is very rare in white people. Finally, CYP1A1*4, with 3% MAF in white people, was also related to greater enzyme catalytic efficiency. 102 Several studies reported increased risk for lung cancer in the presence of some of these variants, however, no clear conclusion was established to date. 103 , 104 The three most common CYP1A2 variants are *1B (probably decreased function), *1C (decreased function), and *1F (confers higher inducibility). 105 Decreased enzyme activity slows down elimination of xenobiotics and can result in increased drug concentrations and enhanced drug effects. 106 Therefore, CYP1A1 and CYP1A2 selected polymorphisms could be genotyped in patients with COVID‐19 when receiving nicotine therapy to evaluate their possible effects.
FLUVOXAMINE
Fluvoxamine is an antidepressant, which belongs to the selective serotonin reuptake inhibitor class. The drug is used primarily for the treatment of obsessive–compulsive disorder, however, it is also used for depression and anxiety disorders, such as panic disorder, social anxiety disorder, and post‐traumatic stress disorder. 107 Although there is no recommendation of fluvoxamine for the treatment of COVID‐19, a phase I clinical trial is being conducted currently in outpatients to test if serious complications, such as shortness of breath, can be prevented. 108 It was shown previously that fluvoxamine reduced the inflammatory response—a cytokine storm—during sepsis. 109 The second phase of COVID‐19 can involve a serious inflammatory reaction, 110 which may be prevented if fluvoxamine is used for treatment.
The CPIC and DPWG Consensus Dosing Guideline recommends a 25–50% dose reduction for the starting dose of fluvoxamine, or the use of an alternative drug, which is not metabolized by CYP2D6 for CYP2D6 PMs (*3/*4, *4/*4, *5/*5, and *5/*6). The guideline does not recommend any dose adjustment for ultrarapid metabolizers, NMs, or IMs. Table 4 shows the inference of CYP2D6 phenotype based on genotypes. 111
Table 4.
CYP2D6 phenotype inference based on genotype information
| CYP2D6 | ||
|---|---|---|
| Ultrarapid metabolizers | ||
| AS > 2.25 |
*1/*1xN, *1/*2xN, *2/*2xN *1x2/*9 |
Duplicated normal function alleles in combination with (1) a decreased function allele other than CYP2D6*10 or (2) another normal function allele |
| Normal metabolizers | ||
| AS = 2.25 | *1x2/*10, *2x2/*10 | Duplicated normal function alleles in combination with CYP2D6*10 |
| AS = 2.0 | *1/*1, *1/*2 | 2 normal function alleles |
| AS = 1.5 | *1/*41, *1/*9 | A normal function allele plus a decreased function allele other than CYP2D6*10 |
| AS = 1.25 | *1/*10 | CYP2D6*10 plus a normal function allele |
| Intermediate metabolizers | ||
| AS = 1.0 | *41/*41, *1/*5 | 2 decreased function alleles other than CYP2D6*10 |
| AS = 0.75 | *9/*10, *10/*41 | CYP2D6*10 plus another decreased function allele |
| AS = 0.5 | *4/*41, *10/*10 | CYP2D6*10/*10 |
| AS = 0.25 | *4/*10 | CYP2D6*10 plus a no function allele |
| Poor metabolizers | ||
| AS = 0 | *3/*4, *4/*4, *5/*5, *5/*6 | 2 no function alleles |
Information obtained from Clinical Pharmacogenetics Implementation Consortium (CPIC) and Dutch Pharmacogenetics Working Group standardizing consensus on CYP2D6 genotype to phenotype translation. 111
AS, activity score; CYP2D6, cytochrome P450 2D6.
Moreover, the FDA‐approved drug label states that patients with low CYP2D6 activity and those receiving other medications known to inhibit CYP2D6 should be monitored. 112 The Swiss drug label concurs with this fact; it states that the pharmacological properties and relative proportions of metabolites of fluvoxamine are altered in CYP2D6 PM. 113 In addition, the DPWG evaluated the therapeutic dose recommendation for fluvoxamine based on CYP2C19 genotypes. They concluded that no dose adjustment is needed. 114
RUXOLITINIB AND BARICITINIB
Ruxolitinib is used for the treatment of high‐risk myelofibrosis and polycythemia vera along with many other possible options, such as psoriasis and steroid‐refractory acute graft‐vs.‐host disease. It is a Janus‐associated kinase (JAK 1/2) inhibitor. JAK1 and JAK2 play key roles in cellular responses during inflammation, growth, regulation of metabolism, and gene transcription. Consequently, ruxolitinib might be effective against the consequences of elevated cytokine levels in patients with COVID‐19. A recent study showed that adding ruxolitinib to standard‐of‐care (usually including remdesivir) treatment was not associated with clinical improvement in patients with COVID‐19. Nevertheless, ruxolitinib recipients had faster clinical improvement compared with the control group who did not receive any treatment. 115
The FDA ruxolitinib drug label does not contain pharmacogenetic information. Nevertheless, it states that ruxolitinib is metabolized by CYP3A4 and CYP2C9, and suggests dosing adjustments and more frequent monitoring of hematology parameters if co‐administered with strong CYP3A4 inhibitors (e.g., ketoconazole) or inducers (e.g., rifampin), or dual inhibitors of CYP3A4 and CYP2C9 (e.g., fluconazole). However, the drug label also states that ruxolitinib and its M18 metabolite do not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4, do not induce CYP1A2, CYP2B6, or CYP3A4, and do not inhibit the P‐gp transport system at clinically relevant concentrations in vitro. 116 More studies are needed in order to access accurate pharmacogenetic information for ruxolitinib. However, the CYP3A4 and CYP2C9 PMs should be closely followed for the risk of drug accumulation and toxicity.
Baricitinib, similarly to tofacitinib, is a JAK 1/2 inhibitor. It is used for the treatment of rheumatoid arthritis in patients whose disease was not well controlled with tumor necrosis factor antagonists. Furthermore, it appears to have antiviral activity by its affinity for AP2‐associated protein AAK1, reducing SARS‐CoV‐2 endocytosis. 117 In a recent clinical trial, in which patients with COVID‐19 showing fever, cough, myalgia, fatigue, or pneumonia were included, baricitinib significantly improved the clinical and laboratory parameters. None of the patients required intensive care unit support and most of them were discharged. 118
The drug label states that baricitinib is a CYP3A4 substrate in vitro. Nevertheless, in clinical pharmacology studies, ketoconazole, a CYP3A4 inhibitor, did not affect the pharmacokinetics of baricitinib. Moreover, fluconazole, a CYP3A, CYP2C9, and CYP2C19 inhibitor, and rifampicin, a CYP3A inducer, did not alter baricitinib pharmacokinetics. In vitro studies suggest that baricitinib is a P‐gp substrate, however, clinical studies concluded that P‐gp inhibitors and substrates, such as cyclosporine and methotrexate, respectively, did not affect its pharmacokinetics. 119 Based on these data, more studies are needed to conclude useful pharmacogenetic information.
ANAKINRA
Anakinra is a recombinant form of human interleukin‐1 receptor antagonist (IL‐1RA), an endogenous key mediator that inhibits the IL‐1 signaling pathway. IL‐1 causes inflammation and hyperproliferative cutaneous disorders. Consequently, anakinra is prescribed for the management of inflammatory 120 or cutaneous 121 diseases. Anakinra does not suffer CYP450 metabolism and undergoes extensive kidney elimination. It was repurposed for COVID‐19 treatment as IL‐1 is one of the cytokines involved in the development of the cytokine storm. 122 Polymorphisms in genes related with pharmacokinetics seem irrelevant. However, the IL‐1α G4845T (rs17651) T allele was significantly related to responsiveness to anakinra; the variant was reported to alter IL‐1α production. 123
COLCHICINE
Colchicine is an alkaloid indicated for several disorders, including, but not limited to: familial Mediterranean fever, Bechet’s disease, pericarditis, coronary artery disease, and inflammatory and fibrotic diseases. The mechanism of action is not fully understood. Colchicine inhibits neutrophil chemotaxis, adhesion, and mobilization; inhibits NACHT‐LRRPYD‐containing protein 3 inflammasomes; inhibits IL‐1β processing and release. 124 It was repurposed for COVID‐19 treatment to direct its anti‐inflammatory properties against the cytokine storm (ClinicalTrial.gov: NCT04326790, NCT04322682, and NCT04322565). 125 Although CYP3A4 is involved in colchicine metabolism and it is a substrate of the P‐gp, polymorphisms in the ABCB1 gene had no effect on the response to colchicine. 126 Contrastively, the no‐function and decreased‐function CYP2D6 alleles *4 and *10 were significantly related to a worse response to colchicine. 127
CONCLUSIONS
More than half a year after the outbreak of the SARS‐CoV‐2 virus worldwide, only remdesivir was proven to be effective against COVID‐19. Despite immense efforts by the scientific, pharmaceutical, and medical communities, more drugs effective in the management of the disease are required. Therefore, until herd immunity is achieved, a vaccine is marketed, or the virus is eradicated, the risk of new outbreaks is high. In the absence of effective drugs, the off‐label use of drugs will continue. In this review, we provide a range of drugs for which therapeutic actions are available based on pharmacogenetics. As long as these drugs continue to be used, they must be used as safely as possible. In the coming months, other drugs may be used to treat the disease with available pharmacogenetic guidelines. It is the responsibility of the specialists in pharmacogenetics to draft and promote this type of review, providing easily accessible information to help improve the clinical management of patients with COVID‐19. Nevertheless, the level of evidence of many of the biomarkers described here is low. Consequently, it is necessary to perform more in‐depth research on very important pharmacogenes to elaborate pharmacogenetic clinical guidelines. The implementation of pharmacogenetics in clinical settings is increasing as it leads to more efficient and cost‐effective treatments. Currently, in times of a global healthcare crisis, these therapeutic improvements become crucial.
Conflicts of Interest
F.A.‐S. has been consultant or investigator in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Chemo, Cinfa, FAES, Farmalíder, Ferrer, GlaxoSmithKline, Galenicum, Gilead, Janssen‐Cilag, Kern, Normon, Novartis, Servier, Silverpharma, Teva, and Zambon. All other authors declared no competing interests for this work.
Funding
M.N.‐G. is co‐financed by the European Social Fund and the Youth European Initiative; grant number PEJ‐2018‐TL/BMD‐11080.
References
- 1. World Health Organization . Coronavirus disease (COVID‐19) Situation Report – 124. <https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200523‐covid‐19‐sitrep‐124.pdf?sfvrsn=9626d639_2>.
- 2. Pooladanda, V. , Thatikonda, S. & Godugu, C. The current understanding and potential therapeutic options to combat COVID‐19. Life Sci. 254, 117765 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders, J.M. , Monogue, M.L. , Jodlowski, T.Z. & Cutrell, J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Off‐label use of medicines for COVID‐19. <https://www.who.int/news‐room/commentaries/detail/off‐label‐use‐of‐medicines‐for‐covid‐19>.
- 5. Back, D. et al COVID‐19 treatment in patients with comorbidities: Awareness of drug‐drug interactions. Br. J. Clin. Pharmacol. 10.1111/bcp.14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh, A.K. , Singh, A. , Shaikh, A. , Singh, R. & Misra, A. Chloroquine and hydroxychloroquine in the treatment of COVID‐19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 14, 241–246 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beauverd, Y. , Adam, Y. , Assouline, B. & Samii, K. COVID‐19 infection and treatment with hydroxychloroquine cause severe haemolysis crisis in a patient with glucose‐6‐phosphate dehydrogenase deficiency. Eur. J. Haematol. 10.1111/ejh.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohammad, S. , Clowse, M.E.B. , Eudy, A.M. & Criscione‐Schreiber, L.G. Examination of hydroxychloroquine use and hemolytic anemia in G6PDH‐deficient patients. Arthritis Care Res. 70, 481–485 (2018). [DOI] [PubMed] [Google Scholar]
- 9. Braga, C.B.E. et al Side effects of chloroquine and primaquine and symptom reduction in malaria endemic area (Mâncio Lima, Acre, Brazil). Interdiscip. Perspect. Infect. Dis. 2015, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agencia Española del Medicamento y Productos Sanitarios (AEMPS) . Tratamientos disponibles sujetos a condiciones especiales de acceso para el manejo de la infección respiratoria por SARS‐CoV‐2. <https://www.aemps.gob.es/la‐aemps/ultima‐informacion‐de‐la‐aemps‐acerca‐del‐covid%E2%80%9119/tratamientos‐disponibles‐para‐el‐manejo‐de‐la‐infeccion‐respiratoria‐por‐sars‐cov‐2/>.
- 11. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health <https://www.covid19treatmentguidelines.nih.gov/>. [PubMed]
- 12. Hernandez, A.V. , Roman, Y.M. , Pasupuleti, V. , Barboza, J.J. & White, C.M. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID‐19: a living systematic review. Ann. Intern. Med. 10.7326/M20-2496. [DOI] [PubMed] [Google Scholar]
- 13. Pastick, K.A. et al Review: hydroxychloroquine and chloroquine for treatment of SARS‐CoV‐2 (COVID‐19). Open Forum Infect. Dis. 7, ofaa130 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehra, M.R. , Desai, S.S. , Ruschitzka, F. & Patel, A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: a multinational registry analysis. Lancet. 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. US Food and Drug Administration (FDA) News Release . Coronavirus (COVID‐19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. June 15, 2020. <https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐fda‐revokes‐emergency‐use‐authorization‐chloroquine‐and>. Last accessed July 9, 2020.
- 16. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health. <https://www.covid19treatmentguidelines.nih.gov/>. Last accessed July 9, 2020. [PubMed]
- 17. World Health Organization . Q&A: Hydroxychloroquine and COVID‐19. <https://www.who.int/news‐room/q‐a‐detail/q‐a‐hydroxychloroquine‐and‐covid‐19>.
- 18. Schrezenmeier, E. & Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 16, 155–166 (2020). [DOI] [PubMed] [Google Scholar]
- 19. Plaquenil® hydroxychloroquine drug label. <https://amiko.oddb.org/de/fi?gtin=53831>. German (2019).
- 20. ARALEN® Chloroquine phosphate drug label. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/006002s044lbl.pdf> (2017).
- 21. Nivaquine® drug label for chloroquine. <https://amiko.oddb.org/de/fi?gtin=18889&highlight>. German. (2019).
- 22. Branch, D.R. Drug‐induced immune haemolytic anaemias. ISBT Sci. Ser. 14, 49–52 (2019). [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). Treatment of Malaria: Guidelines for Clinicians (United States). <https://www.cdc.gov/malaria/diagnosis_treatment/clinicians1.html>.
- 24. Clarke, G.M. et al Characterisation of the opposing effects of G6PD deficiency on cerebral malaria and severe malarial anaemia. eLife 6, e15085 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Projean, D. et al In vitro metabolism of chloroquine: identification of CYP2C8, CYP3A4, and CYP2D6 as the main isoforms catalyzing N‐desethylchloroquine formation. Drug Metab. Dispos. Biol. Fate Chem. 31, 748–754 (2003). [DOI] [PubMed] [Google Scholar]
- 26. Sortica, V.A. et al The effect of SNPs in CYP450 in chloroquine/primaquine Plasmodium vivax malaria treatment. Pharmacogenomics 17, 1903–1911 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eastman, R.T. et al Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID‐19. ACS Cent. Sci. 6, 672–683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang, M. et al Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 30, 269–271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheahan, T.P. et al Broad‐spectrum antiviral GS‐5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 9, eaal3653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grein, J. et al Compassionate use of remdesivir for patients with severe Covid‐19. N. Engl. J. Med. 382, 2327–2336 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. European Medicines Agency . Veklury®, remdesivir. <https://www.ema.europa.eu/en/medicines/human/EPAR/veklury#overview‐section>.
- 32. Amirian, E.S. & Levy, J.K. Current knowledge about the antivirals remdesivir (GS‐5734) and GS‐441524 as therapeutic options for coronaviruses. One Health 9, 100128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCreary, E.K. & Pogue, J.M. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum. Infect. Dis. 7, ofaa105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Abajo, F.J. et al Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet 395, 1705–1714 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turner, A.J. ACE2 cell biology, regulation, and physiological functions In Prot. Arm Renin Angiotensin Syst. RAS 185–189 (Elsevier, New York, NY, 2015). [Google Scholar]
- 36. Patel, S. , Rauf, A. , Khan, H. & Abu‐Izneid, T. Renin‐angiotensin‐aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 94, 317–325 (2017). [DOI] [PubMed] [Google Scholar]
- 37. Acelajado, M.C. , Hughes, Z.H. , Oparil, S. & Calhoun, D.A. Treatment of resistant and refractory hypertension. Circ. Res. 124, 1061–1070 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ocaranza, M.P. et al Enalapril attenuates downregulation of angiotensin‐converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension 48, 572–578 (2006). [DOI] [PubMed] [Google Scholar]
- 39. Kessler, T. & Schunkert, H. Inhibitors of the renin–angiotensin system and SARS‐CoV‐2 infection. Herz 45, 323–324 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Calò, L.A. , Davis, P.A. , Rigato, M. & Sgarabotto, L. ACE2 and prognosis of COVID‐19. Insights from Bartter’s and Gitelman’s syndromes patients. J. Med. Virol. 10.1002/jmv.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Esler, M. & Esler, D. Can angiotensin receptor‐blocking drugs perhaps be harmful in the COVID‐19 pandemic? J. Hypertens. 38, 781–782 (2020). [DOI] [PubMed] [Google Scholar]
- 42. Joy, M.S. et al CYP2C9 genotype and pharmacodynamic responses to losartan in patients with primary and secondary kidney diseases. Eur. J. Clin. Pharmacol. 65, 947–953 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee, C.R. , Goldstein, J.A. & Pieper, J.A. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in‐vitro and human data. Pharmacogenetics 12, 251–263 (2002). [DOI] [PubMed] [Google Scholar]
- 44. Göktaş, M.T. et al Relationship between genetic polymorphisms of drug efflux transporter MDR1 (ABCB1) and response to losartan in hypertension patients. Eur. Rev. Med. Pharmacol. Sci. 20, 2460–2467 (2016). [PubMed] [Google Scholar]
- 45. Zubiaur, P. et al How to make P‐glycoprotein (ABCB1, MDR1) harbor mutations and measure its expression and activity in cell cultures? Pharmacogenomics. 10.2217/pgs-2018-0101. [DOI] [PubMed] [Google Scholar]
- 46. Saiz‐Rodríguez, M. et al Effect of ABCB1 C3435T polymorphism on pharmacokinetics of antipsychotics and antidepressants. Basic Clin. Pharmacol. Toxicol. 123, 474–485 (2018). [DOI] [PubMed] [Google Scholar]
- 47. Parving, H.‐H. et al Effect of deletion polymorphism of angiotensin converting enzyme gene on progression of diabetic nephropathy during inhibition of angiotensin converting enzyme: observational follow up study. BMJ 313, 591–594 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haas, M. et al Angiotensin‐converting enzyme gene polymorphism determines the antiproteinuric and systemic hemodynamic effect of enalapril in patients with proteinuric renal disease. Kidney Blood Press. Res. 21, 66–69 (1998). [DOI] [PubMed] [Google Scholar]
- 49. O’Toole, L. , Stewart, M. , Padfield, P. & Channer, K. Effect of the insertion/deletion polymorphism of the angiotensin‐converting enzyme gene on response to angiotensin‐converting enzyme inhibitors in patients with heart failure. J. Cardiovasc. Pharmacol. 32, 988–994 (1998). [DOI] [PubMed] [Google Scholar]
- 50. Yang, Y.‐Y. et al Identification of diuretic non‐responders with poor long‐term clinical outcomes: a 1‐year follow‐up of 176 non‐azotaemic cirrhotic patients with moderate ascites. Clin. Sci. 121, 509–521 (2011). [DOI] [PubMed] [Google Scholar]
- 51. Devaux, C.A. , Rolain, J.‐M. & Raoult, D. ACE2 receptor polymorphism: Susceptibility to SARS‐CoV‐2, hypertension, multi‐organ failure, and COVID‐19 disease outcome. J. Microbiol. Immunol. Infect. 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Loustaud‐Ratti, V. Ribavirin: Past, present and future. World J. Hepatol. 8, 123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khalili, J.S. , Zhu, H. , Mak, N.S.A. , Yan, Y. & Zhu, Y. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID‐19. J. Med. Virol. 92, 740–746 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Muir, A.J. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for IFNL3 (IL28B) genotype and PEG interferon‐α‐based regimens. Clin. Pharmacol. Ther. 95, 141–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Swen, J.J. et al Pharmacogenetics: from bench to byte— an update of guidelines. Clin. Pharmacol. Ther. 89, 662–673 (2011). [DOI] [PubMed] [Google Scholar]
- 56. Parkin, J. & Cohen, B. An overview of the immune system. Lancet 357, 1777–1789 (2001). [DOI] [PubMed] [Google Scholar]
- 57. Friedman, R.M. Clinical uses of interferons. Br. J. Clin. Pharmacol. 65, 158–162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lin, F. & Young, H.A. Interferons: success in anti‐viral immunotherapy. Cytokine Growth Factor Rev. 25, 369–376 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shalhoub, S. Interferon beta‐1b for COVID‐19. Lancet 395, 1670–1671 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang, Y. et al Interferon‐induced transmembrane protein 3 genetic variant rs12252‐C associated with disease severity in coronavirus disease 2019. J. Infect. Dis. 222, 34–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bedford, J.G. , O’Keeffe, M. , Reading, P.C. & Wakim, L.M. Rapid interferon independent expression of IFITM3 following T cell activation protects cells from influenza virus infection. PLoS One 14, e0210132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shuter, J. Lopinavir/ritonavir in the treatment of HIV‐1 infection: a review. Ther. Clin. Risk Manag. 4, 1023–1033 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu, X. & Wang, X.‐J. Potential inhibitors against 2019‐nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genomics 47, 119–121 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. KALETRA® lopinavir and ritonavir drug label. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021251s052_021906s046lbl.pdf> (2000).
- 65. Apellániz‐Ruiz, M. et al High frequency and founder effect of the CYP3A4*20 loss‐of‐function allele in the Spanish population classifies CYP3A4 as a polymorphic enzyme. Pharmacogenomics J. 15, 288–292 (2015). [DOI] [PubMed] [Google Scholar]
- 66. Tang, J.T. et al Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin. Drug Metab. Toxicol. 12, 555–565 (2016). [DOI] [PubMed] [Google Scholar]
- 67. Sata, F. et al CYP3A4 allelic variants with amino acid substitutions in exons 7 and 12: evidence for an allelic variant with altered catalytic activity. Clin. Pharmacol. Ther. 67, 48–56 (2000). [DOI] [PubMed] [Google Scholar]
- 68. van Schaik, R.H. et al The CYP3A4*3 allele: is it really rare? Clin. Chem. 47, 1104–1106 (2001). [PubMed] [Google Scholar]
- 69. Zhou, Y. , Ingelman‐Sundberg, M. & Lauschke, V.M. Worldwide distribution of cytochrome P450 alleles: a meta‐analysis of population‐scale sequencing projects. Clin. Pharmacol. Ther. 102, 688–700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Achenbach, C.J. , Darin, K.M. , Murphy, R.L. & Katlama, C. Atazanavir/ritonavir‐based combination antiretroviral therapy for treatment of HIV‐1 infection in adults. Future Virol. 6, 157–177 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fintelman‐Rodrigues, N. et al Atazanavir inhibits SARS‐CoV‐2 replication and pro‐inflammatory cytokine production. Microbiology. 10.1101/2020.04.04.020925. [DOI] [Google Scholar]
- 72. Beck, B.R. , Shin, B. , Choi, Y. , Park, S. & Kang, K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS‐CoV‐2) through a drug‐target interaction deep learning model. Comput. Struct. Biotechnol. J. 18, 784–790 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gammal, R. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for UGT1A1 and atazanavir prescribing. Clin. Pharmacol. Ther. 99, 363–369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Channappanavar, R. & Perlman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 39, 529–539 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang, C. , Wang, Y. , Lv, H. , Guan, Z. & Gu, J. Caution against corticosteroid‐based COVID‐19 treatment. Lancet 395, 1759–1760 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maggini, V. et al MDR1 diplotypes as prognostic markers in multiple myeloma. Pharmacogenet. Genom. 18, 383–389 (2008). [DOI] [PubMed] [Google Scholar]
- 77. Jakobsen Falk, I. et al Pharmacogenetic study of the impact of ABCB1 single‐nucleotide polymorphisms on lenalidomide treatment outcomes in patients with multiple myeloma: results from a phase IV observational study and subsequent phase II clinical trial. Cancer Chemother. Pharmacol. 81, 183–193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jordheim, L.P. et al Single nucleotide polymorphisms in ABCB1 and CBR1 can predict toxicity to R‐CHOP type regimens in patients with diffuse non‐Hodgkin lymphoma. Haematologica 100, e204–e206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Asano, T. et al ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid‐induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics 13, 675–682 (2003). [DOI] [PubMed] [Google Scholar]
- 80. Zubiaur, P. et al Effect of polymorphisms in CYP2C9 and CYP2C19 on the disposition, safety and metabolism of progesterone administrated orally or vaginally. Adv. Ther. 36, 2744–2755 (2019). [DOI] [PubMed] [Google Scholar]
- 81. World Health Organization (WHO). Updated: WHO Now Doesn’t Recommend Avoiding Ibuprofen For COVID‐19 Symptoms. <https://www.sciencealert.com/who‐recommends‐to‐avoid‐taking‐ibuprofen‐for‐covid‐19‐symptoms?__twitter_impression=true>.
- 82. EMA advice on the use of NSAIDs for Covid‐19. Drug Ther. Bull. 58, 69 (2020). [DOI] [PubMed] [Google Scholar]
- 83. Theken, K.N. et al Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and nonsteroidal anti‐inflammatory drugs. Clin. Pharmacol. Ther. 10.1002/cpt.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xu, X. et al Effective treatment of severe COVID‐19 patients with tocilizumab. Proc. Natl. Acad. Sci. 117, 10970–10975 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Oldfield, V. , Dhillon, S. & Plosker, G.L. Tocilizumab: a review of its use in the management of rheumatoid arthritis. Drugs 69, 609–632 (2009). [DOI] [PubMed] [Google Scholar]
- 86. McCarty, D. & Robinson, A. Efficacy and safety of sarilumab in patients with active rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 10, 61–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sarosiek, S. , Shah, R. & Munshi, N.C. Review of siltuximab in the treatment of multicentric Castleman’s disease. Ther. Adv. Hematol. 7, 360–366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Enevold, C. et al Interleukin‐6‐receptor polymorphisms rs12083537, rs2228145, and rs4329505 as predictors of response to tocilizumab in rheumatoid arthritis. Pharmacogenet. Genomics. 10.1097/FPC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 89. Maldonado‐Montoro, M. , Cañadas‐Garre, M. , González‐Utrilla, A. & Ángel Calleja‐Hernández, M. Influence of IL6R gene polymorphisms in the effectiveness to treatment with tocilizumab in rheumatoid arthritis. Pharmacogenomics J. 18, 167–172 (2018). [DOI] [PubMed] [Google Scholar]
- 90. Maldonado‐Montoro, M. , Cañadas‐Garre, M. , González‐Utrilla, A. , Plaza‐Plaza, J.C. & Calleja‐Hernández, M.Ÿ. Genetic and clinical biomarkers of tocilizumab response in patients with rheumatoid arthritis. Pharmacol. Res. 111, 264–271 (2016). [DOI] [PubMed] [Google Scholar]
- 91. Jiménez Morales, A. et al FCGR2A/FCGR3A gene polymorphisms and clinical variables as predictors of response to tocilizumab and rituximab in patients with rheumatoid arthritis. J. Clin. Pharmacol. 59, 517–531 (2019). [DOI] [PubMed] [Google Scholar]
- 92. Lee, E.B. et al Disease‐drug interaction of sarilumab and simvastatin in patients with rheumatoid arthritis. Clin. Pharmacokinet. 56, 607–615 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gritti, G. et al Use of siltuximab in patients with COVID‐19 pneumonia requiring ventilatory support. (Respiratory Medicine, 2020). 10.1101/2020.04.01.20048561. [DOI] [Google Scholar]
- 94. SYLVANT®, siltuximab drug label. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125496s000lbl.pdf>.
- 95. Sehgal, S. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant. Proc. 35, S7–S14 (2003). [DOI] [PubMed] [Google Scholar]
- 96. Sehgal, S.N. Rapamune (Sirolimus, Rapamycin): an overview and mechanism of action. Ther. Drug Monit. 17, 660–665 (1995). [DOI] [PubMed] [Google Scholar]
- 97. Zhou, Y. et al Network‐based drug repurposing for novel coronavirus 2019‐nCoV/SARS‐CoV‐2. Cell Discov. 6, 14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Picard, N. , Djebli, N. , Sauvage, F.‐L. & Marquet, P. Metabolism of sirolimus in the presence or absence of cyclosporine by genotyped human liver microsomes and recombinant cytochromes P450 3A4 and 3A5. Drug Metab. Dispos. 35, 350–355 (2007). [DOI] [PubMed] [Google Scholar]
- 99. Anglicheau, D. et al Consequences of genetic polymorphisms for sirolimus requirements after renal transplant in patients on primary sirolimus therapy. Am. J. Transplant. 5, 595–603 (2005). [DOI] [PubMed] [Google Scholar]
- 100. Birdwell, K. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin. Pharmacol. Ther. 98, 19–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Farsalinos, K. et al Editorial: Nicotine and SARS‐CoV‐2: COVID‐19 may be a disease of the nicotinic cholinergic system. Toxicol. Rep. 7, 658–663 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ezzeldin, N. et al Genetic polymorphisms of human cytochrome P450 CYP1A1 in an Egyptian population and tobacco‐induced lung cancer. Genes Environ. 39, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee, K.‐M. et al CYP1A1, GSTM1, and GSTT1 polymorphisms, smoking, and lung cancer risk in a pooled analysis among Asian populations. Cancer Epidemiol. Biomarkers Prev. 17, 1120–1126 (2008). [DOI] [PubMed] [Google Scholar]
- 104. Wynder, E.L. & Hoffmann, D. Smoking and lung cancer: scientific challenges and opportunities. Cancer Res. 54, 5284–5295 (1994). [PubMed] [Google Scholar]
- 105. Sachse, C. et al Polymorphisms in the cytochrome P450 CYP1A2 gene (CYP1A2) in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. Br. J. Clin. Pharmacol. 55, 68–76 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Faber, M.S. , Jetter, A. & Fuhr, U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin. Pharmacol. Toxicol. 97, 125–134 (2005). [DOI] [PubMed] [Google Scholar]
- 107. Figgitt, D.P. & McClellan, K.J. Fluvoxamine: an updated review of its use in the management of adults with anxiety disorders. Drugs 60, 925–954 (2000). [DOI] [PubMed] [Google Scholar]
- 108.A double‐blind, placebo‐controlled clinical trial of fluvoxamine for symptomatic individuals with COVID‐19 infection (STOP COVID). <https://clinicaltrials.gov/ct2/show/NCT04342663> (2020).
- 109. Rosen, D.A. et al Modulation of the sigma‐1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 11, eaau5266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tay, M.Z. , Poh, C.M. , Rénia, L. , MacAry, P.A. & Ng, L.F.P. The trinity of COVID‐19: immunity, inflammation and intervention. Nat. Rev. Immunol. 20, 363–374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Caudle, K.E. et al Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the clinical pharmacogenetics implementation consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 13, 116–124 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Luvox® fluvoxamine maleate drug label. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022235lbl.pdf> (1994).
- 113. Floxyfral® label for fluvoxamine. <https://amiko.oddb.org/de/fi?gtin=44603>.
- 114. Dutch Pharmacogenetics Working Group Guidelines. November 2018. <https://www.knmp.nl/downloads/pharmacogenetic‐recommendations‐november‐2018.pdf> (2018).
- 115. Cao, Y. et al Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID‐19): a multicenter, single‐blind, randomized controlled trial. J. Allergy Clin. Immunol. 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jakavi ruxolitinib drug label. <https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi> (2011).
- 117. Richardson, P. et al Baricitinib as potential treatment for 2019‐nCoV acute respiratory disease. Lancet 395, e30–e31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cantini, F. et al Baricitinib therapy in COVID‐19: a pilot study on safety and clinical impact. J. Infect. 81, 318–356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. OLUMIANT® baricitinib drug label. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf> (2018).
- 120. European Medicines Agency Kineret®, anakinra drug label. <https://www.ema.europa.eu/en/documents/product‐information/kineret‐epar‐product‐information_es.pdf>.
- 121. Pazyar, N. , Feily, A. & Yaghoobi, R. An overview of interleukin‐1 receptor antagonist, anakinra, in the treatment of cutaneous diseases. Curr. Clin. Pharmacol. 7, 271–275 (2012). [DOI] [PubMed] [Google Scholar]
- 122. Muñoz‐Jiménez, A. , Rubio‐Romero, E. & Marenco de la Fuente, J.L. Propuesta de uso de anakinra en el distrés respiratorio agudo secundario a COVID‐19. Reumatol. Clínica. 10.1016/j.reuma.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Camp, N.J. et al Evidence of a pharmacogenomic response to interleukin‐l receptor antagonist in rheumatoid arthritis. Genes Immun. 6, 467–471 (2005). [DOI] [PubMed] [Google Scholar]
- 124. Leung, Y.Y. , Yao Hui, L.L. & Kraus, V.B. Colchicine—update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 45, 341–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wu, R. et al An update on current therapeutic drugs treating COVID‐19. Curr. Pharmacol. Rep. 6, 56–70 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dogruer, D. , Tug, E. , Bes, C. & Soy, M. Lack of an effect of CYP3A4 and MDR1 gene polymorphisms on colchicine pharmacogenetics in the treatment of Familial Mediterranean fever. Genet. Mol. Res. 12, 3521–3528 (2013). [DOI] [PubMed] [Google Scholar]
- 127. Yalcıntepe, S. et al The CYP4502D6 *4 and *6 alleles are the molecular genetic markers for drug response: implications in colchicine non‐responder FMF patients. Eur. J. Drug Metab. Pharmacokinet. 41, 281–286 (2016). [DOI] [PubMed] [Google Scholar]


