Abstract
Introduction
Many type 2 diabetes patients show insufficient levels of physical activity (PA) and are often unmotivated to change PA behaviors. This study investigated whether a newly developed smartphone game delivering individualized exercise and PA promotion through an elaborate storyline can generate sustained improvements in daily PA (steps/d).
Study design
Thirty-six participants were enrolled in this 24-week RCT between August 2016 and April 2018. After baseline assessment, participants were randomized in equal numbers to the intervention or control condition. Data analysis was performed in May/June 2018.
Setting/participants
Inactive, overweight type 2 diabetes patients, aged 45–70 years, were recruited through advertising and from hospitals and diabetes care centers in the Basel, Switzerland metropolitan area.
Intervention
Participants were instructed to play the innovative smartphone game (intervention group) or to implement the recommendations from the baseline lifestyle counseling (control group) autonomously during the 24-week intervention period.
Main outcome measures
Primary outcomes were changes in daily PA (steps/d), changes in aerobic capacity, measured as oxygen uptake at the first ventilatory threshold, and changes in glycemic control, measured as HbA1c.
Results
Daily PA increased by an average of 3,998 (SD 1,293) steps/d in the intervention group and by an average of 939 (SD 1,156) steps/d in the control group. The adjusted difference between the two groups was 3,128 steps/d (95% CI 2,313, 3,943; p<0.001). The increase in daily PA was accompanied by an improved aerobic capacity (adjusted difference of oxygen uptake at the first ventilatory threshold of 1.9 ml/(kg·min), 95% CI 0.9, 2.9; p<0.001). Glycemic control (HbA1c) did not change over the course of the intervention.
Conclusions
A novel, self-developed smartphone game, delivering multidimensional home-based exercise and PA-promotion, significantly increases daily PA (steps/d) and aerobic capacity in inactive type 2 diabetes patients after 24 weeks. The ability of the game to elicit a sustained PA motivation may be relevant for other inactive target groups with chronic diseases.
Trial registration
This study is registered at www.clinicaltrials.gov NCT02657018
Introduction
In recent years, type 2 diabetes has developed into a global health burden that presents one of the great healthcare challenges of our time.1 In 2014, over 400 million adults worldwide had diabetes mellitus, predominantly of type 2,2 representing a key determinant of morbidity and mortality in both developed and developing countries.1
Regular physical activity (PA) with its well-documented health benefits is a cornerstone of a successful diabetes management.3 Despite the apparent benefits of regular PA, only 25% of adults with type 2 diabetes meet PA recommendations and 40% are physically inactive, engaging in less than ten minutes of moderate or vigorous activity per week during work, leisure time, or transportation.4 In contrast, physical inactivity not only increases the risk of type 2 diabetes by over 100% but also significantly contributes to the development of several comorbidities4 and a 1.5-fold increased risk of all-cause mortality.5 Lack of motivation and lack of PA enjoyment, as well as aversion to exercise facilities, missing social support, and health concerns such as hypoglycemia or fear of injury, are the main reasons that keep patients with type 2 diabetes from becoming more physically active or cause them to discontinue participation in a PA promoting program.6 Motivating and enjoyable low-threshold forms of PA are therefore desperately needed to encourage this target group to sustainably become more physically active.
In recent years, PA-promoting games (exergames) have increasingly been used and examined to encourage regular PA in those who cannot be motivated through conventional PA-promoting interventions.7 By making PA an enjoyable and meaningful experience, this “gamified” approach aims to attenuate the negative perception of PA, as often the case in inactive target groups, and thereby lowers the subjectively perceived cost of being physically active.7 Console-based exergaming has been shown to improve glycemic control, quality of life, and subjectively (questionnaire) assessed PA in type 2 diabetes;8 however, evidence is very limited and longer-term results of any gamified approach regarding objectively (accelerometer) measured increases in PA are missing.9 In addition, current exergames seldom offer exercise modes that concur in intensity and duration with established exercise guidelines and fail to provide a sufficient extent of individualization that is required for an effective and safe training in patients with chronic diseases who often have a significantly lower fitness level than healthy target groups.9 Further, for exergames, and more generally, for serious games with a behavioral intervention objective to be enjoyably effective, they need to engage their intended audience in terms of narrative premise, gameplay, and storytelling.7 To address these design challenges, as well as the shortcomings of existing exergames, a mobile smartphone-based game application was developed that is specifically designed for type 2 diabetes patients with the goal to encourage a healthier, more active lifestyle through gamified daily PA.10
The aim of this randomized controlled trial (RCT) was to assess the effect of the game on daily PA (steps/d) in physically inactive individuals with type 2 diabetes. It was hypothesized that the use of the game application would lead to higher increases in steps/d after 24 weeks than a control intervention consisting of a one-time lifestyle counseling. Secondary aims were to assess the effect of the game on glycemic control, circulating cardiovascular risk factors, and aerobic capacity.
Research Design and Methods
Study Design
This 24-week RCT (2-arm) was conducted in accordance with the Declaration of Helsinki11 between August 2016 and April 2018 at the Department of Sport, Exercise and Health of the University of Basel, Switzerland. The study was registered at ClinicalTrials.gov (NCT02657018) and approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz, Reg.-No. EKNZ 2015–424). Permuted block randomization with randomly varying block sizes was used to allocate participants at random and in equal numbers to one of the two groups. All outcome assessors were blinded with respect to group allocation. The detailed study protocol can be found in a previous publication.10
Participants
Participants were recruited in cooperation with several hospitals, doctor’s offices, and diabetes care centers in the Basel metropolitan area and through online and newspaper advertising. Participants were eligible to participate if they met the following inclusion criteria: (I) physician-diagnosed and medically treated non-insulin-dependent diabetes mellitus, (II) body mass index ≥25 kg/m2, (III) 45–70 years of age, (IV) <150 min of moderate-intensity PA per week, and (V) regular smartphone use during the year before the study to ensure that participants were familiar with the use of smartphones and would be able to play the PA-promoting smartphone game without additional assistance beyond the in-game tutorial. Exclusion criteria were health risks that contraindicate exercise testing,12 impaired physical mobility, and acute infections or injuries. There was no racial or gender bias in the selection of participants. Eligible participants received detailed information about purpose and procedures of this study and gave written informed consent before participation.
Intervention and Control Condition
Participants in the intervention group received a novel, self-developed smartphone. The game includes individualized multidimensional (strength, endurance, balance, flexibility) exercise and daily PA (walking) promotion following established PA guidelines.13 A key component of the game’s PA-related content is the integration of exercise tests such as the 1-min Sit-to-Stand Test (STS)14 and the Six Minute Walk Test (6MWT).15 These tests assess the fitness level of each individual user at baseline and periodically during play and allow building tailored exercise regimens with appropriate entry levels and individualized rates of intensity progression. For the STS, the phone is placed in the pants pocket and repetitions are counted via the phone’s accelerometer and predefined threshold values for the seated and standing position. For the 6MWT (to be conducted outdoors), the distance is measured via GPS. Both measuring techniques have been validated before (unpublished data). Execution of in-game exercises (130 exercise variations) and daily PA are tracked via the phone’s sensors (accelerometer/pedometer, camera, and audio sensor). Camera tracking can be used for certain strength exercises in which the phone is placed in front of the player and the repetitions of the exercise are auto-counted via movement recognition by the front-facing camera. Audio tracking requires the player to count the repetitions out aloud. Key elements of the game’s storyline are the restoration of a garden (used as a metaphor for the own body) and the taming of the Schweinehund (in German, a self-depreciating idiom denoting one’s weaker self, often referring to the lazy procrastination regarding PA), both of which can be achieved through regular in-game PA and the related in-game rewards. The game mechanics are anchored in the CALO-RE taxonomy of behavior change techniques,16 whereby the progression in the storyline is independent of the player’s initial fitness level but depends rather on the regularity of game use and the meeting of personalized PA goals. The behavior change techniques incorporated into the game design are among others ‘action planning/goal setting’, ‘instruction on how to perform the behavior’, ‘prompts/cues’, ‘graded tasks’, and ‘feedback/rewards’, which have been found to be particularly effective in changing PA behavior in recent meta-analyses.17,18 By combining manageable challenges with appealing rewards, the game aims to increase the players’ game- and PA-related self-efficacy and enjoyment – key factors for a long-term adherence.7,19 Because the game is self-explanatory, providing an extensive in-game tutorial explaining all features and mechanics of the game and because the game was designed to encourage regular use through the motivating character of the game design, participants in the intervention group only received very basic verbal instructions from the study personnel on how to use and control the game but not on how often or when to use it.
Participants in the control group received a one-time individual lifestyle counseling (60 min) by health professionals that included the promotion of baseline activities of daily life,20 as well as a structured exercise plan including strength and endurance exercises with moderately increasing intensity and duration, essentially comparable to the content of the game, that was to be implemented autonomously.
Outcome Measures
Participant Characteristics
At baseline and after the 24-week intervention, all participants underwent a clinical examination including medical history and a physical examination consisting of measurements of height, body mass, body fat content via bioelectrical impedance analysis (InBody 720, JP Global Markets GmbH, Eschborn, Germany), resting blood pressure (BP), and resting electrocardiography (ECG). To verify that participants met the PA-related inclusion criteria, habitual PA was assessed at the first measurement appointment using the Freiburg Questionnaire of PA.21
Glycemic Control and Circulating Cardiovascular Risk Factors
During the clinical examination and after an overnight fast of ≥8 hours, blood samples were drawn by trained medical staff and transported to the laboratory of the University Hospital Basel, Switzerland for further analysis. Analyzed parameters included HbA1c, total cholesterol, low- (LDL) and high-density lipoprotein (HDL), and triglycerides.
Aerobic Capacity
Following the clinical examination, participants underwent a cardiorespiratory fitness test on a bicycle ergometer (ergoselect 200, ergoline GmbH, Bitz, Germany) to assess the maximum oxygen uptake (V̇O2peak) and the first ventilatory threshold (VT1).22 After a 3-minute warm-up phase at 25 W, workload increased linearly (ramp protocol) by 15 W/min until participants’ exhaustion. Respiratory gas parameters were analyzed breath-by-breath (MetaMax 3B, Cortex Biophysik GmbH, Leipzig, Germany). Maximal exhaustion was accepted if at least two of the following four criteria were met: (I) respiratory exchange ratio ≥1.1; (II) blood lactate concentration >8 mmol/l; (III) RPE ≥18; and (IV) maximum heart rate (HRmax) >95% of predicted HRmax [208 − 0.7 × age (years)].23
Daily Physical Activity
After the cardiorespiratory fitness test, participants received a Vivofit 2 accelerometer wristband (Garmin International Inc., Olathe, KS, USA) with the instruction to wear it continuously during the following week for the assessment of the daily PA. The Garmin Vivofit has previously been shown to be valid for step detection under various walking conditions with constant as well as varying walking speeds.24,25 To eliminate any impact of a step count feedback on the participants’ PA behavior, the devices’ displays were irreversibly blackened out. In addition to the accelerometer wristbands, participants received a diary to record any non-wear time during the monitoring period. Only days with uninterrupted wear were considered for data analyses and a minimum of four complete days was required. Day 0 (the day participants received the device) of the monitoring period was not considered for data analyses, as is common in the assessment of PA, because participants are known to change their activity pattern on the initial day of data recording.26
Statistical Analysis
Analyses were performed in May/June 2018. Summary statistics were calculated to characterize the study sample and for pre- and post-intervention data as appropriate. Continuous data were summarized using the mean (standard deviation, SD) or the median (interquartile range, IQR). The primary outcome of daily PA (steps/d) after the intervention and further outcomes were analyzed by analysis of covariance.27 Results are presented as differences in outcome (with 95% confidence intervals, CI) between participants in the intervention group and those in the control group, adjusted for the corresponding values at baseline. Correlations between total in-game training (min) and outcome measures were analyzed using Spearman’s rank correlation coefficient. R 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses and graphics with the significance level set to 0.05 (2-sided).
Sample Size
It was hypothesized that the expected difference in daily PA (primary outcome) after 24 weeks between the two groups would be 2,500 steps/d with an SD in either group of 3,000 steps/d.28 By including daily PA (steps/d) at baseline as a covariate in the analysis, it was further aimed to reduce error variability and therefore hypothesized that the correlation between baseline and outcome daily PA would be 0.7. With a significance level of 0.05 (2-sided), the sample size needed to attain a targeted power of 90% for showing superiority of the experimental intervention over control was determined as a total of 34 participants (17 in each group).
Results
Participant Flow and Characteristics
Sixty-eight participants were assessed for eligibility (Figure 1). Thirty-two subjects were excluded because they did not meet inclusion criteria (n=19) or declined to participate (n=13). All remaining participants (n=36) were randomly assigned to either the intervention group (n=18) or the control group (n=18). Baseline characteristics of study participants were balanced between both groups (Table 1). All participants received antidiabetic drug treatment prior to enrollment in the study and did not change medication during the intervention period. One participant was lost to follow up due to medical reasons not related to the study. Thirty-five participants completed the study and were included in the analysis of the primary outcome.
Figure 1:
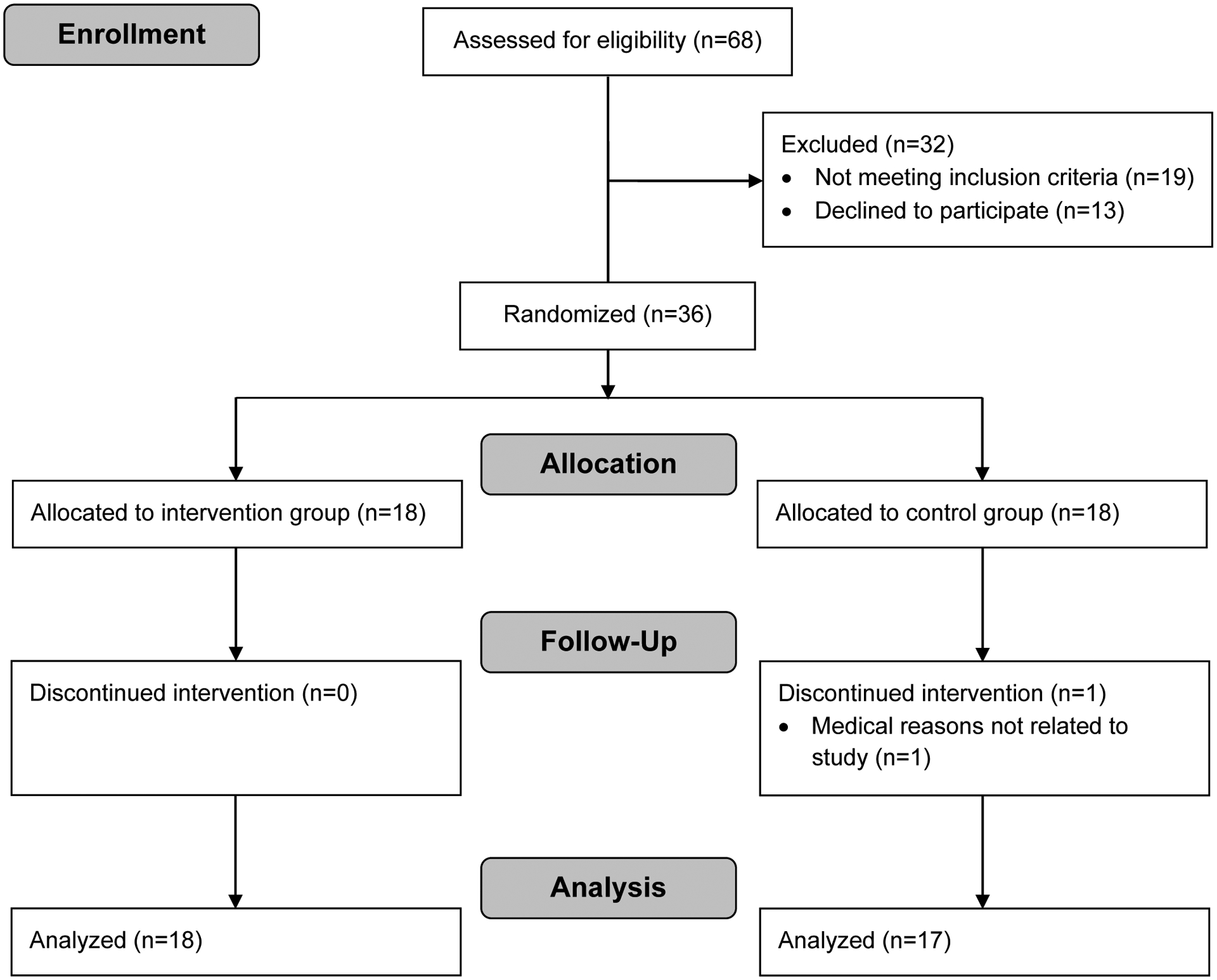
Flow diagram of study participants.
Table 1:
Baseline characteristics of study participants
| Intervention (n=18) | Control (n=18) | ||||
|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | ||
| BASIC | |||||
| Sex | Female | 8 | 9 | ||
| Male | 10 | 9 | |||
| Age (years) | 18 | 57 (53, 60) | 18 | 60 (54, 63) | |
| Height (m) | 18 | 172 (166, 177) | 18 | 172 (165, 177) | |
| Weight (kg) | 18 | 93 (89, 96) | 18 | 98 (88, 111) | |
| BMI (kg/m2) | 18 | 31 (29, 34) | 18 | 33 (30, 36) | |
| Fat mass (%) | 18 | 39 (35, 43) | 18 | 35 (33, 43) | |
| MVPA (min/week) | 18 | 45 (0, 60) | 18 | 38 (0, 68) | |
| MEDICAL RECORD | |||||
| Diabetes duration (years) | 18 | 3.3 (1.4, 4.3) | 18 | 4.1 (1.5, 7.0) | |
| Antidiabetic drug intake | 18 | 18 | |||
| Metformin | 18 | 18 | |||
| DPP-4-inhibitor | 7 | 6 | |||
| Thiazolidinedione | - | 1 | |||
| SGLT2 inhibitor | - | 1 | |||
| GLP-1 receptor agonist | 1 | 2 | |||
| Sulfonylurea | 2 | 1 | |||
| Antihypertensive drug intake | 12 | 11 | |||
| Lipid-lowering drug intake | 4 | 7 | |||
IQR, interquartile range; BMI, body mass index; MVPA, moderate-to-vigorous physical activity; DPP-4, dipeptidyl peptidase 4; SGLT2, sodium-glucose cotransporter 2; GLP-1, glucagon-like peptide 1
Changes in Daily Physical Activity
All participants wore the accelerometer wristband continuously for a minimum of five full days. Daily PA increased by an average of 3,998 (SD 1,293) steps/d in the intervention group and by an average of 939 (SD 1,156) steps/d in the control group during the 24-week intervention (Table 2). The adjusted difference of the increase in daily PA between both groups was 3,128 steps/d (95% CI 2,313, 3,943; p<0.001) in favor of the intervention group.
Table 2:
Effects of game use compared to a one-time lifestyle counseling on daily physical activity, aerobic capacity and anthropometric, metabolic and physiological parameters
| Outcome | Intervention (n=18) | Control (n=17) | Adjusted differencea (95% CI) |
P value | ||
|---|---|---|---|---|---|---|
| Pre-intervention (mean (SD)) |
Post-intervention (mean (SD)) |
Pre-intervention (mean (SD)) |
Post-intervention (mean (SD)) |
|||
| Steps per day | 5,785 (793) | 9,783 (1,334) | 5,612 (1,192) | 6,552 (1,280) | 3,128 (2,313, 3,943) | <0.001 |
| Total body fat mass (kg) | 35.1 (8.7) | 32.4 (9.1) | 38.7 (10.6) | 37.9 (9.8) | −2.1 (−4.2, 0.0) | 0.045 |
| Skeletal muscle mass (kg) | 32.1 (5.7) | 32.7 (5.8) | 34.1 (6.6) | 34.5 (7.0) | 0.2 (−1.0, 1.5) | 0.710 |
| HbA1c (%) | 6.2 (0.6) | 6.2 (0.7) | 6.9 (0.7) | 7.0 (1.0) | −0.9 (−1.5, −0.2) | 0.016 |
| Total cholesterol (mmol/l) | 4.9 (0.9) | 5.1 (0.8) | 4.6 (1.0) | 4.8 (0.9) | 0.2 (−0.4, 0.7) | 0.546 |
| HDL cholesterol (mmol/l) | 1.2 (0.2) | 1.2 (0.3) | 1.3 (0.3) | 1.3 (0.2) | 0.0 (−0.1,0.2) | 0.463 |
| LDL cholesterol (mmol/l) | 2.8 (0.9) | 3.0 (0.9) | 2.5 (0.9) | 2.7 (0.8) | 0.1 (−0.4, 0.6) | 0.740 |
| Triglycerides (mmol/l) | 2.0 (0.9) | 1.9 (0.8) | 1.9 (1.0) | 1.8 (0.9) | 0.0 (−0.4, 0.4) | 0.951 |
| HR at rest (bpm) | 66 (10) | 64 (10) | 68 (11) | 69 (9) | −3 (−7, 1) | 0.099 |
| SBP at rest (mmHg) | 136 (14) | 133 (15) | 134 (12) | 134 (14) | −3 (−9, 4) | 0.384 |
| DBP at rest (mmHg) | 88 (8) | 85 (8) | 86 (8) | 87 (9) | −3 (−7, 1) | 0.180 |
| V̇O2peakb (ml/(kg·min)) | 24.0 (4.3) | 25.5 (3.5) | 23.2 (4.1) | 22.9 (4.0) | 1.9 (0.7, 3.0) | 0.002 |
| V̇O2 at VT1c (ml/(kg·min)) | 15.4 (2.5) | 17.2 (2.6) | 15.0 (1.7) | 14.9 (2.2) | 1.9 (0.9, 2.9) | <0.001 |
| Workload at VT1c (W) | 82.2 (17.3) | 95.6 (15.7) | 84.8 (15.8) | 87.1 (15.9) | 10.8 (7.1, 14.5) | <0.001 |
SD, standard deviation; CI, confidence interval; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HR, heart rate; bpm, beats per minute; SBP, systolic blood pressure; DBP, diastolic blood pressure; V̇O2peak, peak oxygen uptake; V̇O2, oxygen uptake; VT1, first ventilatory threshold.
Analysis of covariance comparing post-intervention values between the intervention group and the control group adjusted for the corresponding pre-intervention values.
Pre- and post-intervention data available in 17/18 participants in the intervention group and in 13/17 participants in the control group.
Pre- and post-intervention data available in 13/17 participants in the control group.
Changes in Glycemic Control
HbA1c remained unchanged at 6.2% (SD 0.7) in the intervention group and increased by 0.1 percentage points (SD 1.3) in the control group during the intervention period with an adjusted difference of −0.9 percentage points (95% CI −1.5, −0.2; p=0.016) in favor of the intervention group. There were no apparent changes in total, LDL-, and HDL-cholesterol, or triglycerides over the course of the intervention in either group.
Changes in Aerobic Capacity
Relative V̇O2peak increased by 1.4 ml/(kg·min) (SD 2.0) in the intervention group and decreased by 0.3 ml/(kg·min) (SD 1.1) in the control group during the intervention period with an adjusted difference of 1.9 ml/(kg·min) (95% CI 0.7, 3.0; p=0.002) in favor of the intervention group. Absolute values did not change significantly in either group with an adjusted difference of 0.10 l/min (95% CI, −0.02, 0.23; p=0.110) between both groups. Relative and absolute V̇O2 at VT1 increased by 1.8 ml/(kg·min) (SD 1.2) and 0.13 l/min (SD 0.09) in the intervention group and decreased by 0.1 ml/(kg·min) (SD 1.4) and 0.03 l/min (SD 0.15) in the control group. The adjusted difference between the two groups was 1.9 ml/(kg·min) (95% CI 0.9, 2.9; p<0.001) and 0.14 l/min (95% CI 0.05, 0.23; p=0.003) in favor of the intervention group. Increases in workload at VT1 were observed for both groups post-intervention, with an adjusted difference between both groups of 10.8 W (95% CI 7.1, 14.5; p<0.001) in favor of the intervention group.
Changes in Anthropometric and Further Physiological Parameters
Total body fat mass decreased by 2.7 kg (SD 2.5) in the intervention group and by 0.9 kg (SD 3.4) in the control group. The adjusted difference between the two groups was −2.1 kg (95% CI −4.2, 0.0; p=0.045) in favor of the intervention group. Skeletal muscle mass did not change significantly in either group during the intervention with an adjusted difference of 0.2 kg (95% CI −1.0, 1.5; p=0.710). No apparent changes were observed for resting HR as well as for systolic or diastolic BP at rest.
Strong positive correlations were found between total time of in-game training and change in daily PA (steps/d) (r=0.91) as well as change in workload at VT1 (r=0.66), indicating that those who used the game as a training tool more, increased their daily PA and aerobic capacity more than those who used the game less (Figure 2).
Figure 2:
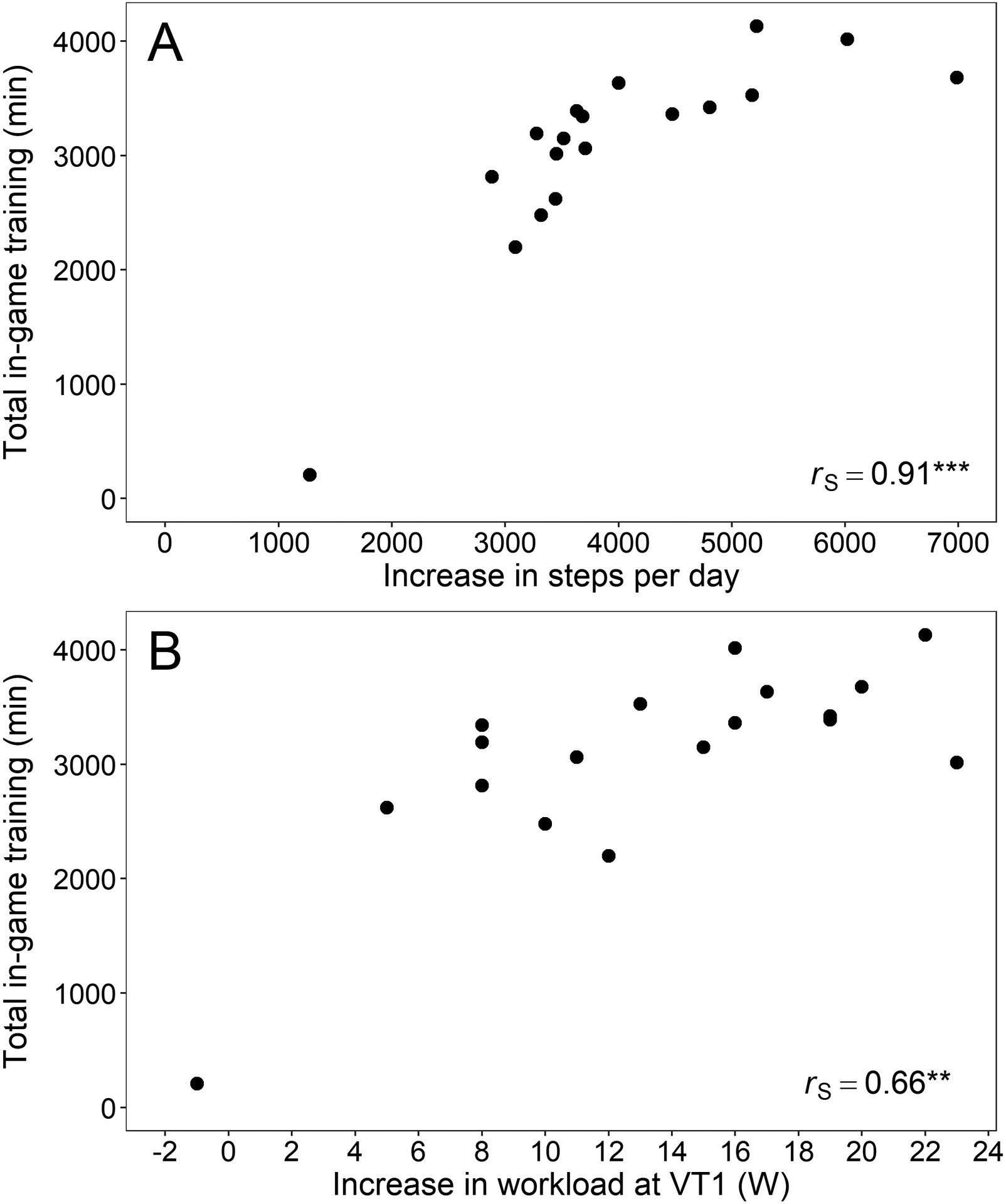
Correlation between total in-game training (min) and (A) increase in steps per day and (B) increase in workload at VT1 (W). ** p<0.01 and *** p<0.001 (Spearman’s rank correlation coefficient.
Discussion
In this RCT, the use of a novel, self-developed smartphone game that included individualized exercise and daily PA promotion led to a significantly higher increase in daily PA (+3,000 steps) after 24 weeks than a control intervention consisting of a one-time lifestyle counseling. The magnitude of the shown increase in steps/d is comparable to that of a 24-week pedometer-based behavioral modification program including a face-to-face session and regular telephone follow-ups (+2,800 steps),28 providing strong evidence that a smartphone game that incorporates personalized PA recommendations and step count goals in the storyline can generate meaningful and sustained improvements in daily PA in a previously inactive target group. Increases of 2,000 steps/d in daily life over a 12-month period have been shown to be of clinical relevance in patients with an impaired glucose tolerance as they are associated with an 8% lower cardiovascular event rate.29 The strong positive correlation (r=0.91) between the total time of in-game training and the increase in steps/d in the intervention group suggests that indeed the game played a decisive role in motivating participants to become and remain more physically active.
The increased amount of daily PA in this study was accompanied by an improvement in aerobic capacity. Relative V̇O2 (ml/(kg·min)) at VT1 increased by 11.7% and absolute V̇O2 (l/min) by 9.2%, indicating a de facto improvement in aerobic capacity. The increased V̇O2 enabled participants to generate an 18% higher workload (+14.7 W) at VT1. A higher V̇O2 at VT1 has been shown to be inversely and independently associated with fatal cardiovascular and all-cause mortality events,30 underlining the clinical relevance of the found improvements in aerobic capacity. Relative V̇O2peak (ml/(kg·min)) increased as well (+6.3%) but was not accompanied by significant increases in absolute V̇O2peak (l/min). This is not surprising, as the game was not designed to improve V̇O2peak, but rather to improve basic endurance through increases in daily PA. The slight change in relative V̇O2peak was likely due to a decrease in body weight caused by the significant reduction in total body fat mass of 2.7 kg (7.7%) during the intervention. The reduction in body fat mass in this study was modest when compared to an intensive 12-week lifestyle intervention consisting of dietary changes, exercise, cognitive-behavioral modifications, and medication adjustments, which showed an average reduction in body fat mass of 15%.31 It is important to note however, that the reduction in body fat mass in the aforementioned study and congruent with other large lifestyle interventions targeting weight loss,32 was accompanied by a loss in lean body mass of 2.3 kg (3.5%), while in this study skeletal muscle mass was preserved. Because type 2 diabetes is associated with a 3-fold increased risk of sarcopenia,33 preservation of muscle mass is crucial and should be the focus of any lifestyle intervention to prevent an accelerated functional decline and maintain independent functioning, a central component of health-related quality of life.34 It is therefore advisable to design lifestyle interventions to target weight loss more moderately and incorporate sufficient amounts of regular PA to prevent possible diet and inactivity-related losses of skeletal muscle mass.
Despite the strong increase in daily PA and the associated improvement in aerobic capacity, no improvements in glycemic control were found. While significant decreases in HbA1c of 0.5 percentage points following walking interventions of durations between 8 and 36 weeks have been found in a recent meta-analysis,35 an inconclusive glycemic control benefit of step goal/pedometer use in type 2 diabetes, similar to the findings of this study, has also been reported before.36 This recent meta-analysis found no association between step goal/pedometer-mediated increases in daily PA and improvements in glycemic control despite average increases in daily PA of 3,200 steps/d.36 A recent RCT that promoted daily PA through pedometers and physician-prescribed step count goals did find reductions in HbA1c of 0.38 percentage points following a 1,200-step-per-day increase in daily PA (60% lower increase than this study) after one year.37 It is possible that the longer intervention duration of one year in that study (30) played a role in eliciting the improvements in HbA1c and that the present study would have yielded similar improvements in a comparable timeframe. This assumption is supported by the findings of a recent study,38 investigating the effects of a novel online game delivering diabetes self-management education content to patients with diabetes that showed the greatest impact on HbA1c in the six months after completing the intervention (12 months after baseline). It is thus conceivable that a similar time lag applies to the present study through which it may take more time than 24 weeks before the gradual adoption of health-improving PA-behaviors induced by the game leads to glycemic improvements that are reflected in a lower HbA1c. It can further be conjectured that a higher exercise intensity, as may be the case in certain console-based exergames,39 would have elicited (more detectable) improvements in glycemic control, comparable to the 0.3 percentage point reduction in HbA1c found after only 12 weeks of autonomous exergame use.8 However, because this may have then potentially affected the adherence to the game over 24-weeks and subsequently compromised the shown increases in PA, the game was deliberately designed to be mainly of low-intensity character to lower the threshold for regular use in an inactive, low-motivation target group and to support a sustained change in PA behavior. In addition, it is noteworthy, that the participants of the studies included in the aforementioned meta-analysis35 and those of the exergame intervention8 had a markedly higher average HbA1c at baseline than did the participants in the intervention group of this study. Average HbA1c values of 6.9–8.1%35 and 7.1%8 leave more room to improve glycemic control through increases in daily PA than does a baseline value of 6.2% that indicates a medically already well-controlled HbA1c. The fact that HbA1c actually slightly increased in the control group despite unchanged medication, along with the significant adjusted difference of −0.9 percentage points in favor of the intervention group, further suggests that the PA promotion through the game is more effective than a one-time lifestyle counseling and at the very least contributes to a successful stabilization of a well-adjusted glycemic control.
This study has a number of strengths, including the novelty of the smartphone game-based intervention, the longer-term intervention of 24 weeks, and the objective one-week assessment of daily PA. The individualized fitness-level-adjusted training regimen and PA promotion were administered remotely via the game and without the need for physical contact between study personnel and participants. This may set a potential precedent for future PA-promoting interventions with the potential for a wide and easy dissemination even in geographically dispersed healthcare settings. As initial increases in daily steps have been shown to diminish during the subsequent weeks with a return to baseline values after six weeks in other PA-promoting game apps such as Pokémon GO,40 the fact that this study found significantly increased daily PA after 24 weeks, measured objectively by accelerometers, is a major strength that suggests a longer-term sustainability of the game-induced increases in daily PA.
Limitations
A limitation of this study is that it remains uncertain, which conceptual ideas and behavior change mechanics incorporated into the game have caused the increase in daily PA and more general, whether it was the behavior change theme, the cell phone modality, or both that drove the increase in daily PA. This would be important knowledge for the design of future game applications and interventions targeting changes in PA behavior in unmotivated, inactive target groups. It remains to be determined if the general theme of taming the Schweinehund would work in other cultures/countries, particularly those that are sensitive to self-depreciating language. Further, future studies should incorporate a one-year follow-up to assess how many study participants continue to play the game after the intervention period and to which extent. It will be insightful to see if the game is able to serve as a stepping-stone towards increased physical activity levels beyond the game particularly in those who cease to play after the intervention or if participants who quit playing the game after the intervention relapse into physical inactivity.
Conclusions
In conclusion, this RCT demonstrates that a novel, behavior change technique-based smartphone game delivering multidimensional home-based exercise and PA-promotion significantly increases daily PA (steps/d) and aerobic capacity after 24 weeks and seems to contribute to the stabilization of a medication-supported diabetes treatment. The playful approach of the game seems to elicit a sustained PA motivation that would be beneficial to other inactive target populations with and without chronic diseases. Future studies with preferable larger sample sizes are needed to examine and confirm the effectiveness of the game in different target populations and help identify those populations that are most responsive to a PA promoting smartphone game.
Supplementary Material
Acknowledgments
We thank Promotion Software GmbH for providing technical support in programming the game application “Mission: Schweinehund” as well as during the intervention period. We further are grateful to Novartis Pharma GmbH for the financial support during the development of the game application, as well as to RMIT University for having provided support, and thank all participants for their time and effort, which were critical to the successful completion of this research. This research is funded by the Swiss National Science Foundation (SNSF grant no. 166214).
Footnotes
Conflict of Interest and Funding
This research is funded by the Swiss National Science Foundation (SNSF grant no. 166214). CH is supported by an NIH NIDDK Award (grant: T32 DK064584). The authors declare no conflict of interest.
References
- 1.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56–64. doi: 10.1016/S2213-8587(13)70112-8 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO | Global Report on Diabetes.; 2016. http://www.who.int/diabetes/global-report/en/. Accessed January 18, 2018.
- 3.Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. CDC | National Diabetes Statistics Report. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Published July 17, 2017. Accessed January 18, 2018.
- 5.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–2905. doi: 10.1007/s00125-012-2677-z [DOI] [PubMed] [Google Scholar]
- 6.Korkiakangas EE, Alahuhta MA, Laitinen JH. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: a systematic review. Health Promot Int. 2009;24(4):416–427. doi: 10.1093/heapro/dap031 [DOI] [PubMed] [Google Scholar]
- 7.Munson S, Poole E, Perry D, Peyton T. Gamification and Health In: Walz SP, Deterding S, eds. The Gameful World: Approaches, Issues, Applications. The MIT Press; 2015:597–623. [Google Scholar]
- 8.Kempf K, Martin S. Autonomous exercise game use improves metabolic control and quality of life in type 2 diabetes patients - a randomized controlled trial. BMC Endocr Disord. 2013;13(1):57. doi: 10.1186/1472-6823-13-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Höchsmann C, Schüpbach M, Schmidt-Trucksäss A. Effects of Exergaming on Physical Activity in Overweight Individuals. Sports Med. 2016;46(6):845–860. doi: 10.1007/s40279-015-0455-z [DOI] [PubMed] [Google Scholar]
- 10.Höchsmann C, Walz SP, Schäfer J, Holopainen J, Hanssen H, Schmidt-Trucksäss A. Mobile Exergaming for Health-Effects of a serious game application for smartphones on physical activity and exercise adherence in type 2 diabetes mellitus-study protocol for a randomized controlled trial. Trials. 2017;18(1):103. doi: 10.1186/s13063-017-1853-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Published October 19, 2013. Accessed March 16, 2016. [PubMed]
- 12.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 9th Ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 13.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 14.Strassmann A, Steurer-Stey C, Lana KD, et al. Population-based reference values for the 1-min sit-to-stand test. Int J Public Health. 2013;58(6):949–953. doi: 10.1007/s00038-013-0504-z [DOI] [PubMed] [Google Scholar]
- 15.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 16.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–1498. doi: 10.1080/08870446.2010.540664 [DOI] [PubMed] [Google Scholar]
- 17.Howlett N, Trivedi D, Troop NA, Chater AM. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Transl Behav Med. 2018. doi: 10.1093/tbm/iby010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien N, McDonald S, Araújo-Soares V, et al. The features of interventions associated with long-term effectiveness of physical activity interventions in adults aged 55–70 years: a systematic review and meta-analysis. Health Psychol Rev. 2015;9(4):417–433. doi: 10.1080/17437199.2015.1012177 [DOI] [PubMed] [Google Scholar]
- 19.Mueller F, Khot RA, Gerling K, Mandryk R. Exertion Games. Found Trends® Human–Computer Interact. 2016;10(1):1–86. doi: 10.1561/1100000041 [DOI] [Google Scholar]
- 20.U.S. Department of Health and Human Services. 2008. Physical Activity Guidelines for Americans Be Active, Happy, and Healthy. Washington (DC) http://www.health.gov/paguidelines/. [Google Scholar]
- 21.Frey I, Berg A, Grathwohl D, Keul J. [Freiburg Questionnaire of physical activity--development, evaluation and application]. Soz- Präventivmedizin. 1999;44(2):55–64. [DOI] [PubMed] [Google Scholar]
- 22.Binder RK, Wonisch M, Corra U, et al. Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur J Cardiovasc Prev Rehabil. 2008;15(6):726–734. doi: 10.1097/HJR.0b013e328304fed4 [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156. doi: 10.1016/S0735-1097(00)01054-8 [DOI] [PubMed] [Google Scholar]
- 24.Wahl Y, Düking P, Droszez A, Wahl P, Mester J. Criterion-Validity of Commercially Available Physical Activity Tracker to Estimate Step Count, Covered Distance and Energy Expenditure during Sports Conditions. Front Physiol. 2017;8:725. doi: 10.3389/fphys.2017.00725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höchsmann C, Knaier R, Eymann J, Hintermann J, Infanger D, Schmidt‐Trucksäss A. Validity of activity trackers, smartphones, and phone applications to measure steps in various walking conditions. Scand J Med Sci Sports. 2018;28(7):1818–1827. doi: 10.1111/sms.13074 [DOI] [PubMed] [Google Scholar]
- 26.Mattocks C, Ness A, Leary S, et al. Use of accelerometers in a large field-based study of children: protocols, design issues, and effects on precision. J Phys Act Health. 2008;5 Suppl 1:S98–111. [DOI] [PubMed] [Google Scholar]
- 27.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323(7321):1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Greef KP, Deforche BI, Ruige JB, et al. The effects of a pedometer-based behavioral modification program with telephone support on physical activity and sedentary behavior in type 2 diabetes patients. Patient Educ Couns. 2011;84(2):275–279. doi: 10.1016/j.pec.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 29.Yates T, Haffner SM, Schulte PJ, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet Lond Engl. 2014;383(9922):1059–1066. doi: 10.1016/S0140-6736(13)62061-9 [DOI] [PubMed] [Google Scholar]
- 30.Kunutsor SK, Kurl S, Khan H, Zaccardi F, Rauramaa R, Laukkanen JA. Oxygen uptake at aerobic threshold is inversely associated with fatal cardiovascular and all-cause mortality events. Ann Med. 2017;49(8):698–709. doi: 10.1080/07853890.2017.1367958 [DOI] [PubMed] [Google Scholar]
- 31.Hamdy O, Mottalib A, Morsi A, et al. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 5-year longitudinal study. BMJ Open Diabetes Res Care. 2017;5(1):e000259. doi: 10.1136/bmjdrc-2016-000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pownall HJ, Bray GA, Wagenknecht LE, et al. Changes in Body Composition over Eight Years in a Randomized Trial of a Lifestyle Intervention: The Look AHEAD Study. Obes Silver Spring Md. 2015;23(3):565–572. doi: 10.1002/oby.21005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010;33(7):1497–1499. doi: 10.2337/dc09-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Exp Gerontol. 2013;48(9):888–897. doi: 10.1016/j.exger.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu S, Cai X, Schumann U, Velders M, Sun Z, Steinacker JM. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta-analysis. PloS One. 2014;9(10):e109767. doi: 10.1371/journal.pone.0109767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu S, Cai X, Chen X, Yang B, Sun Z. Step counter use in type 2 diabetes: a meta-analysis of randomized controlled trials. BMC Med. 2014;12:36. doi: 10.1186/1741-7015-12-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasgupta K, Rosenberg E, Joseph L, et al. Physician step prescription and monitoring to improve ARTERial health (SMARTER): A randomized controlled trial in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2017;19(5):695–704. doi: 10.1111/dom.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerfoot BP, Gagnon DR, McMahon GT, Orlander JD, Kurgansky KE, Conlin PR. A Team-Based Online Game Improves Blood Glucose Control in Veterans With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2017;40(9):1218–1225. doi: 10.2337/dc17-0310 [DOI] [PubMed] [Google Scholar]
- 39.Höchsmann C, Zürcher N, Stamm A, Schmidt-Trucksäss A. Cardiorespiratory Exertion While Playing Video Game Exercises in Elderly Individuals With Type 2 Diabetes. Clin J Sport Med Off J Can Acad Sport Med. 2016;26(4):326–331. doi: 10.1097/JSM.0000000000000258 [DOI] [PubMed] [Google Scholar]
- 40.Howe KB, Suharlim C, Ueda P, Howe D, Kawachi I, Rimm EB. Gotta catch’em all! Pokémon GO and physical activity among young adults: difference in differences study. BMJ. 2016;355:i6270. doi: 10.1136/bmj.i6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


