Abstract
Purpose
Expectation affects pain experience in humans. Numerous studies have reported that pre-stimulus activity in the anterior insular cortex (aIC), together with prefrontal and limbic regions, integrated pain intensity and expectations. However, it is unclear whether the resting-state functional connectivity (rs-FC) between the aIC and other brain regions affects chronic pain. The purpose of this study was to examine the rs-FC between the aIC and the whole brain regions in female patients with severe knee osteoarthritis (OA).
Patients and Methods
Nineteen female patients with chronic severe knee OA and 15 matched controls underwent resting-state functional magnetic resonance imaging. We compared the rs-FC from the aIC seed region between the two groups. A disease-specific measurement of knee OA was performed.
Results
The aIC showed stronger rs-FC with the right orbitofrontal cortex (OFC), subcallosal area, and bilateral frontal pole compared with controls. The strength of rs-FC between the left aIC and the right OFC was positively correlated with the knee OA pain score (r = 0.49, p = 0.03). The strength of rs-FC between the right aIC and right OFC was positively correlated with the knee OA total score (r = 0.48, p = 0.036) and pain score (r = 0.46, p = 0.049). The OFC, subcallosal area, and frontal pole, together with the aIC, were activated during anticipation of pain stimulus. These areas have been reported as representative pain-related expectation regions.
Conclusion
This was the first study to show the stronger rs-FCs between the aIC and other pain-related expectation regions in female patients with severe knee OA. Female sex and preoperative pain intensity are risk factors of persistent postoperative pain after total knee arthroplasty. It is suggested that the functional relationship between pain-related expectation regions affects the formation of severe knee OA and persistent postoperative pain following total knee arthroplasty.
Keywords: functional magnetic resonance imaging, chronic pain, insular cortex, orbitofrontal cortex
Introduction
Expectation strongly modulates pain experience in humans. In our brain, the insular cortex (IC) plays an important role in pain processing. Particularly, the anterior insular cortex (aIC) is involved in pain processing, such as expectation,1–14 cognitive control (attention, value, reward, decision-making),3,4,8–11 salience,10,11 and prediction errors (PEs).12–14 Recently, magnetic resonance imaging (MRI) studies have revealed that the aIC integrated pain intensity and expectation in healthy subjects. Moreover, the aIC is activated with the prefrontal cortex (PFC) and limbic regions during anticipation of a pain stimulus.1–13 The IC, PFC, and limbic regions are activated by a pain stimulus even in patients with chronic pain.15 In addition, the IC activity was correlated with pain intensity in patients with knee osteoarthritis (OA) during anticipation of pain.16 Therefore, functional coupling between the aIC and the other pain-related expectation regions may be involved in the mechanism of chronic pain and occur even at rest without a pain stimulus. However, thus far, there are no studies examining the relationship of resting-state activity between the aIC and other pain-related expectation regions in patients with chronic pain.
Knee OA is a chronic pain disease, with a predominance in female patients.17,18 Total knee arthroplasty for patients with severe knee OA is a common procedure applied to improve pain and disability; however, this procedure is occasionally associated with persistent postoperative pain.19–21 Female sex19,22 and preoperative pain intensity19,21,22 are recognized as risk factors of persistent postoperative pain. The pathology of knee OA differs depending on sex and pain severity. Therefore, in this study, we targeted only female patients with severe knee OA. In a resting-state functional MRI (rs-fMRI) study, Baliki et al and Cottam et al23,24 identified that resting-state functional connectivity (rs-FC) between the aIC and the default mode network was altered in patients with chronic knee OA. These studies focused on the salience role of the aIC. As a result, the region of interest (ROI) of the aIC used by Baliki et al and Cottam et al was involved in the salience network. However, the ROI of the aIC involved in expectation can be larger and close to the anatomical size.5,12,13
The objective of this study was to examine the characteristics of rs-FC between the anatomically defined aIC and the whole brain regions in female patients with severe knee OA using rs-fMRI. We hypothesized that the rs-FC between the aIC and other pain-related expectation regions would be altered in patients versus healthy controls, and the strength of the rs-FC of aIC would be associated with pain scores in female patients with chronic severe knee OA.
Patients and Methods
Participants
We recruited 19 female patients diagnosed with chronic knee OA and 15 age- and gender-matched controls. The 19 patients with Kellgren–Lawrence25 grade 3 (N = 6) and grade 4 (N = 13) knee OA underwent knee arthroplasty. Five of the 15 controls had knee pain. Considering that their degree of pain using a visual analog scale (VAS) was <30/100, and the duration of pain was <3 months, these patients were not diagnosed with chronic pain. Two patients and two controls received medication. One patient received tramadol and acetaminophen, while the other patient received tramadol and pregabalin. Two controls received celecoxib, as required.
The exclusion criteria were history of neurological disease, brain injury, psychiatric disorders, rheumatoid arthritis, claustrophobia, and insertion of metal or electronic devices. This study was conducted in accordance with the tenets of the Declaration of Helsinki, and approved by the Hiroshima University Ethics Committee (E-302). Written informed consent was provided by all participants.
Clinical Measurements
Participants underwent the following measurements prior to MRI scans. The VAS was used to measure current pain intensity (range: 0–100). A disease-specific measurement of knee OA was performed using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).26 The WOMAC consists of 24 questions (five for pain, two for stiffness, and 17 for physical function), which are rated using an intensity scale of 0–4. The pain score (range: 0–20), stiffness score (range: 0–8), physical function score (range: 0–68), and the total score (range: 0–96) are determined based on this index. The Pain-DETECT27 was used to measure elements of neuropathic pain (range: 0–35). The Pain Catastrophizing Scale (PCS)28 was used to measure the degree of catastrophic thoughts regarding pain (range: 0–52). High scores indicate worse outcome in all measurements. In patients with bilateral knee OA, the side most affected by pain was evaluated.
Acquisition of Neuroimaging Data
The fMRI procedure was performed using a 3.0-Tesla MRI scanner (Ingenia Elition; Philips, Amsterdam, Netherlands) equipped with a 32-channel head coil. Functional images were captured using a single-shot T2*-weighted echo-planar imaging sequence (time repetition: 2500 ms; time echo: 30 ms; flip angle: 80°; matrix: 64 × 64; field of view: 212 × 212 mm2; 40 slices, slice thickness: 0.8 mm without gap; no oblique axial images; voxel size: 3.3 mm × 3.3 mm × 3.2 mm; 240 volumes; scan time: 10 min). During the functional scan, the patients were requested to keep their eyes open, relax, and look at a cross mark in front of them without moving or falling asleep. A three-dimensional high-resolution anatomical image was captured using a T1-weighted gradient echo pulse sequence (time repetition: 6.8 ms; time echo: 3.1 ms; flip angle: 9°; field of view: 256 × 240 mm2; voxel size: 1 mm × 1 mm × 1.2 mm; scan time: 6 min 58 s).
Statistical Analysis
Statistical analysis was performed using the Stata/MP, version 15.1 (Stata Corporation, College Station, TX, USA). A nonparametric statistic test (Mann–Whitney U-test) was used for the age, VAS, WOMAC, Pain-DETECT, and PCS.
FC Analysis
Rs-fMRI data were analyzed using the SPM12 (Welcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), CONN (Functional Connectivity Toolbox; http://www.nitrc.org/projects/conn),29 and Matlab version 8.5 (R2017b; MathWorks, Natick, MA, USA). After discarding the first 10 images to eliminate any signal decay, preprocessing was performed including realignment, slice-timing correction, outlier detection, co-registration to the anatomical image, segmentation of the anatomical image (gray matter, white matter, and cerebrospinal fluid), normalization with the standard Montreal Neurological Institute brain, and smoothing with an 8-mm Gaussian kernel. After preprocessing, signal and motion artefacts (global signal z-value threshold ≥5; composite motion threshold ≥0.9 mm) were removed from the data using a CompCor strategy,30 and the data were band-pass filtered (0.02–0.08 Hz) to reduce the influence of noise.
A seed-to-voxel analysis was performed using the left and right aIC as the region of ROI. The ROIs of the left and right aIC were generated using the Neuromorphometrics atlas in SPM12, provided by Neuromorphometrics, Inc. (http://neuromorphometrics.com) under academic subscription.
The within-group imaging analysis was performed using a one-sample test, with age as a covariate. The threshold for statistical significance was determined at p < 0.001 for the uncorrected peak-level and p < 0.05 for the cluster-level after family-wise error correction.
Spearman’s rank correlation coefficients were calculated to investigate the relationship between the strength of rs-FC with the aIC and clinical measurements in female patients with chronic severe knee OA.
Results
Demographic and Clinical Measurements
The demographic and clinical measurements of the participants are shown in Table 1. Compared with the controls, patients with chronic knee OA exhibited significantly higher scores in the VAS, WOMAC, pain-DETECT, and PCS; however, they did not differ in age.
Table 1.
Demographic and Clinical Measurements of the Participants
| Data | Patients with Knee OA | Controls | p |
|---|---|---|---|
| (N = 19) | (N = 15) | ||
| Age, year | 73.2 ± 5.1 | 74.9 ± 4.6 | 0.25 |
| Duration, month | 102.9 ± 88.6 | ||
| VAS | 64.5 ± 15.1 | 5.1 ± 8.1 | < 0.001 |
| WOMAC | |||
| total | 61.1 ± 12.4 | 7.9 ± 14.7 | < 0.001 |
| pain | 12.3 ± 3.0 | 1.1 ± 1.6 | < 0.001 |
| stiffness | 5.1 ± 2.5 | 0.9 ± 1.8 | < 0.001 |
| physical activity | 43.2 ± 9.9 | 5.9 ± 11.6 | < 0.001 |
| Pain-DETECT | 9.2 ± 5.4 | 2.6 ± 2.9 | < 0.001 |
| PCS | 26.3 ± 10.8 | 9.2 ± 13.0 | < 0.001 |
Note: Data are presented as the means ± SD.
Abbreviations: OA, osteoarthritis; PCS, Pain Catastrophizing Scale; SD, standard deviation; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
The rs-FC of aIC
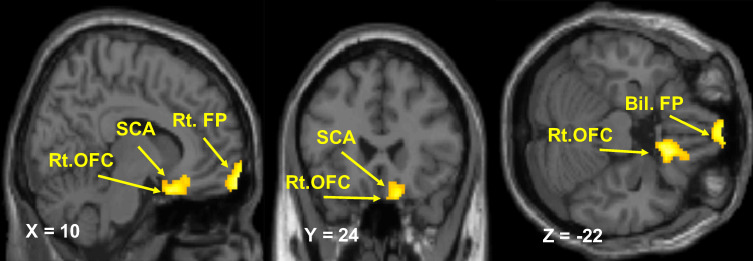
In female patients with chronic severe knee OA, the left aIC showed stronger rs-FC with the right orbitofrontal cortex (OFC) and the subcallosal area (SCA) compared with controls (Table 2; Figure 1). The right aIC showed stronger rs-FC with the right OFC, SCA, and the bilateral frontal pole (FP) compared with controls (Table 2; Figure 2).
Table 2.
Brain Regions Showing Increased Functional Connectivity with the Left and Right Anterior Insular Cortex in Patients with Knee Osteoarthritis Relative to Control Subjects
| Anatomical Location | MNI Coordinates | Cluster Size | p-FWE | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left | |||||
| Rt. orbitofrontal cortex | 18 | 14 | −30 | 150 | < 0.005 |
| subcallosal area | 87 | ||||
| Right | |||||
| Rt. orbitofrontal cortex | 14 | 20 | −22 | 171 | < 0.005 |
| subcallosal area | 96 | ||||
| Bil. frontal pole | 8 | 68 | 20 | 519 | < 0.001 |
Note: The X, Y, and Z coordinates accord with the MNI atlas.
Abbreviations: Rt, right; Bil, bilateral; MNI, Montreal Neurological Institute; FWE, family-wise error corrected.
Figure 1.
Resting-state functional connectivity of the left anterior insular cortex. Brain regions showing a significant increase in the functional connectivity of the left anterior insular cortex in patients with knee osteoarthritis relative to controls (p < 0.001 uncorrected, peak-level and p < 0.05 cluster-level, after FWE correction).
Abbreviations: FWE, family-wise error; Rt, right; OFC, orbitofrontal cortex; SCA, subcallosal area.
Figure 2.
Resting-state functional connectivity of the right anterior insular cortex. Brain regions showing a significant increase in the functional connectivity of the right anterior insular cortex in patients with knee osteoarthritis relative to controls (p < 0.001 uncorrected, peak-level and p < 0.05 cluster-level, after FWE correction).
Abbreviations: FWE, family-wise error; Rt, right; Bil, bilateral; OFC, orbitofrontal cortex; SCA, subcallosal area; FP, frontal pole.
Correlation Between FC and Clinical Measurements
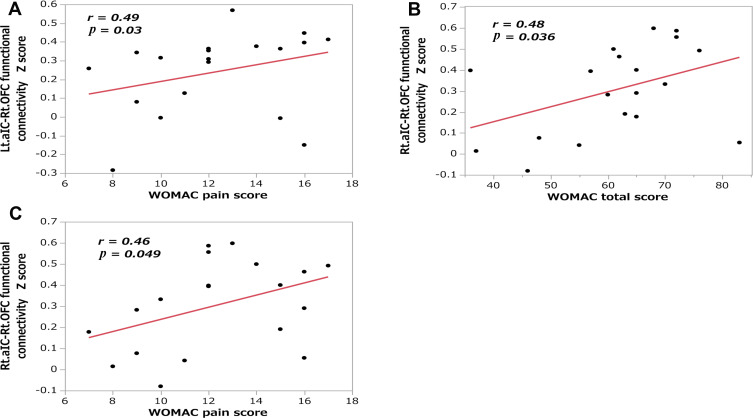
In female patients with chronic severe knee OA, the correlation analyses revealed that the strength of rs-FC between the left aIC and right OFC was correlated with the WOMAC pain score (r = 0.49, N = 19, p = 0.03; Figure 3A). Moreover, the strength of rs-FC between the right aIC and right OFC was correlated with the WOMAC total score (r = 0.48, N = 19, p = 0.036; Figure 3B) and WOMAC pain score (r = 0.46, N = 19, p = 0.049; Figure 3C). We did not detect any significant relationship between the strength of FC and other clinical measurements (Table 3).
Figure 3.
Correlations between the anterior insular cortex-right orbitofrontal cortex functional connectivity and clinical measurements. Scatter plots showing the correlations of functional connectivity with the (A) WOMAC pain score in the Lt. aIC and Rt. OFC; (B) WOMAC total score in the Rt. aIC and Rt. OFC; and (C) WOMAC pain score in the Rt. aIC and Rt. OFC.
Abbreviations: aIC, anterior insular cortex; Lt, left; Rt, right; OFC, orbitofrontal cortex; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Table 3.
Correlation Between the Anterior Insular Cortex-Right Orbitofrontal Cortex Functional Connectivity and Clinical Measurements
| Lt.aIC-Rt.OFC | WOMAC total | WOMAC pain | WOMAC stiffness | WOMAC physical |
|---|---|---|---|---|
| cc | 0.29 | 0.49* | 0.15 | 0.23 |
| p | 0.22 | 0.03 | 0.52 | 0.34 |
| VAS | Pain-DETECT | PCS | ||
| cc | 0.26 | −0.02 | 0.05 | |
| p | 0.28 | 0.92 | 0.84 | |
| Rt.aIC-Rt.OFC | WOMAC total | WOMAC pain | WOMAC stiffness | WOMAC physical |
| cc | 0.48* | 0.46* | 0.24 | 0.37 |
| p | 0.04 | 0.049 | 0.31 | 0.12 |
| VAS | Pain-DETECT | PCS | ||
| cc | 0.26 | −0.01 | 0.02 | |
| p | 0.28 | 0.97 | 0.94 |
Note: *p < 0.05 denotes significant correlation.
Abbreviations: Lt, left; Rt, right; aIC, anterior insular cortex; cc, Spearman’s rank correlation coefficients; OFC, orbitofrontal cortex; PCS, Pain Catastrophizing Scale; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Discussion
Using rs-fMRI, we investigated the abnormality of rs-FCs between bilateral aIC and other brain regions in female patients with chronic severe knee OA. We found that the patients exhibited an increased rs-FC of left aIC to right OFC, and SCA. In addition, the rs-FC with the right aIC, SCA, right OFC, and bilateral FP was increased. Furthermore, we showed that the strength of rs-FC between the left aIC and right OFC was significantly associated with the WOMAC pain score. Moreover, the strength of rs-FC between the right aIC and right OFC was significantly associated with the WOMAC total score and WOMAC pain score.
Previous studies suggested that healthy subjects perceived a noxious stimulus as more painful or fearful when they expected aversiveness or experienced a nocebo effect prior to the stimulus. In addition, the FC of the aIC and OFC was increased prior to or during the stimulus.4,7,31,32 During anticipation of dental treatment and hyperventilation, an increased activation of the aIC and OFC was also found in healthy subjects.8,33 The OFC represents and retains the affective value of past comfort-discomfort.34–36 Atlas et al4 suggested that the aIC modulates pain perception by integrating aversive expectation from the OFC during anticipation. On the other hand, the expectation of pleasure mediates pain. The FC of the aIC and OFC was decreased when a pain stimulus and monetary wins occurred simultaneously.37 In summary, the OFC is an expectation region of value and reward for pain, and involved in the modulation of pain processing. Our results suggested that the increased rs-FC of the aIC and OFC affect subjective pain by increasing the aversive expectation for pain. In fact, we found that the strength of rs-FC between the aIC and OFC exhibited a positive correlation with the disease-specific measurement of knee OA. Approximately 15–30% of patients experience persistent postoperative pain after undergoing total knee arthroplasty.19–21 This may be due to the aversive expectation of pain remaining after total knee arthroplasty, which completely removes the sensory aspects of pain.
The SCA is connected to the medial OFC and subgenual anterior cingulate cortex (ACC). Moreover, the amygdala, ventral striatum, and hippocampus are involved in emotion experience and processing.38 The aIC, SCA, OFC, and ACC are composed of agranular visceromotor cortices. These areas contribute to generating predictions for somatosensory inputs, such as pain stimulus, and indirectly enabling the creation of PEs.39,40 In fact, PEs to a cued pain stimulus have been found in the aIC, PFC, and limbic regions.12,13 Geuter et al12 indicated that the predictions and PEs played an important role in our pain perception, and brain processes of the predictions and PEs may contribute to chronic pain. Therefore, it is suggested that the increased rs-FC with the aIC, SCA, and OFC may be involved in our pain perception by increasing the emotional experiences and creating the predictions and PEs without a stimulus.
The SCA, medial FP, and medial OFC are included in medial PFC (mPFC), together with pregenual ACC. In female patients with chronic pelvic pain, the rs-FC between the aIC and mPFC (medial FP) increased, and the increased rs-FC between these regions was positively correlated with pain intensity, anxiety, and depression scale.41 In nonspecific chronic low back pain patients with a high clinical sign, activation of the aIC and mPFC (medial FP, pregenual ACC) showed a positive covariance in anxiety and depression scales in response to expected pain.42 In major depressive disorder, an increased rs-FC of the aIC/ventrolateral PFC and FP with the SCA was associated with remission and treatment failure.43 Although we did not evaluate anxiety and depression scores in this study, it has been reported that chronic pain patients with knee OA have higher scores versus healthy controls.24,44 Moreover, older knee OA patients with depressive symptoms are at elevated risk for incidental major depression or anxiety disorders.45 On the other hand, it was reported that decreased rs-FC between the aIC and mPFC (medial FP) was negatively associated with pain score in females with primary dysmenorrhea during menstruation.46 The role of connectivity between the aIC and mPFC in chronic pain is inconsistent. However, our results suggest that the increased rs-FC between the aIC, SCA, and FP may be a neural network change, indicating the psychological aspects of female patients with chronic severe knee OA.
The present study had several limitations. Firstly, owing to the cross-sectional design of this study, we could not determine the causal relationships between chronic pain and cerebral abnormalities. Further longitudinal studies would be necessary to answer this question. In fact, it was reported that structures in the brain were altered following surgery in patients with chronic knee and hip OA.47,48 Secondly, two subjects received medicines, which could affect the central nervous system. In a sub-analysis excluding those two subjects, the stronger rs-FC between the aIC and pain-related expectation regions remained. However, it is possible that medicines may have influenced the results. Thirdly, the number of participants was small. In this study, we targeted only females and showed a significant correlation between the strength of rs-FC and pain scores. For the generalization of the present findings, it would be important to also examine males and increase the number of participants. Fourthly, we did not directly measure pain expectation. It would be necessary to investigate the effect of expectation on pain and subsequently evaluate its relationship with rs-FC. Recently, pain-free rs-FC has been reported to predict individual pain sensitivity or the placebo effect.49,50 In fact, Wagner et al50 identified that placebo-induced pain reduction (positive expectation effect) was associated with pain-free rs-FC networks, including the aIC. Finally, it has been reported that the activity of the ventral aIC was different from that of the dorsal aIC in the expectation or PEs.12,13 The ventral aIC contributed to both expectation and PEs, while the dorsal aIC did only to the former.13 Future studies should investigate the subdivisions of ROIs in the aIC.
Conclusion
Using rs-fMRI analysis, this was the first study to show that the rs-FC between the aIC and other pain-related expectation regions was already increased in female patients with chronic severe knee OA. The extent of this increase was significantly associated with disease-specific measurement. These findings enhance our understanding of the neuropathology and treatment of female patients with chronic severe knee OA.
Acknowledgments
We thank Yoshiko Matsuoka, Yuji Takahashi, Shougo Kamioka, Ryo Nishikori, and Yutaka Misezaki, at the Department of Clinical Radiology (Hiroshima University Hospital, Hiroshima, Japan) for their assistance in the magnetic resonance imaging procedures.
Abbreviations
ACC, anterior cingulate cortex; aIC, anterior insular cortex; FP, frontal pole; IC, insular cortex; MRI, magnetic resonance imaging; OA, osteoarthritis; OFC, orbitofrontal cortex; PCS, Pain Catastrophizing Scale; PEs, prediction errors; PFC, prefrontal cortex; ROI, region of interest; rs-FC, resting-state functional connectivity; rs-fMRI, resting-state functional magnetic resonance imaging; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proce Nat Acad Sci. 2005;102(36):12950–12955. doi: 10.1073/pnas.0408576102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown CA, Seymour B, El-Deredy W, Jones AKP. Confidence in beliefs about pain predicts expectancy effects on pain perception and anticipatory processing in right anterior insula. Pain. 2008;139(2):324–332. doi: 10.1016/j.pain.2008.04.028 [DOI] [PubMed] [Google Scholar]
- 3.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex. 2011;21(3):719–726. doi: 10.1093/cercor/bhq146 [DOI] [PubMed] [Google Scholar]
- 4.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30(39):12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharvit G, Corradi-DellʼAcqua C, Vuilleumier P. Modality-specific effects of aversive expectancy in the anterior insula and medial prefrontal cortex. Pain. 2017;13(1):1529–1542. doi: 10.1371/journal.pcbi.1005142 [DOI] [PubMed] [Google Scholar]
- 6.Atlas LY, Wager TD. How expectations shape pain. Neurosci Lett. 2012;520(2):140–148. doi: 10.1016/j.neulet.2012.03.039 [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Jackson T, Huang C. Neural activation during anticipation of near pain-threshold stimulation among the pain-fearful. Front Neurosci. 2016;10:342. doi: 10.3389/fnins.2016.00342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CS, Wu SY, Wu LT. The anterior insula and anterior cingulate cortex are associated with avoidance of dental treatment based on prior experience of treatment in healthy adults. BMC Neurosci. 2015;16:88. doi: 10.1186/s12868-015-0224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristofori I, Harquel S, Isnard J, Mauguière F, Sirigu A. Monetary reward suppresses anterior insula activity during social pain. Soc Cogn Affect Neurosci. 2015;10(12):1668–1676. doi: 10.1093/scan/nsv054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage. 2013;64:538–546. doi: 10.1016/j.neuroimage.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiech K, Lin C-S, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci. 2010;30(48):16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geuter S, Boll S, Eippert F, Büchel C. Functional dissociation of stimulus intensity encoding and predictive coding of pain in the insula. Elife. 2017;6:e24770. doi: 10.7554/eLife.24770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazeli S, Büchel C. Pain-related expectation and prediction error signals in the anterior insula are not related to aversiveness. J Neurosci. 2018;38(29):6461–6474. doi: 10.1523/JNEUROSCI.0671-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabor A, Thacker MA, Moseley GL, Körding KP, Blohm G. Pain: A statistical account. PLoS Comput Biol. 2017;13(1):e105142. doi: 10.1371/journal.pcbi.1005142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):436–484. doi: 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 16.Brown CA, Deredy W, Jones AKP. When the brain expects pain: common neural responses to pain anticipation are related to clinical pain and distress in fibromyalgia and osteoarthritis. Eur J Neurosci. 2014;39(4):663–672. doi: 10.1111/ejn.12420 [DOI] [PubMed] [Google Scholar]
- 17.Muraki S, Oka H, Akune T, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage. 2009;17(9):1137–1143. doi: 10.1016/j.joca.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 18.Tang X, Wang S, Zhan S, et al. The Prevalence of Symptomatic Knee Osteoarthritis in China: results From the China Health and Retirement Longitudinal Study. Arthritis Rheumatol. 2016;68(3):648–653. doi: 10.1002/art.39465 [DOI] [PubMed] [Google Scholar]
- 19.Schug SA, Bruce J. Risikostratifizierung bezüglich der Entwicklung von chronischem postoperativem Schmerz [Risk stratification for the development of chronic postsurgical pain]. Der Schmerz. 2018;32(6):471–476. doi: 10.1007/s00482-018-0332-4 [DOI] [PubMed] [Google Scholar]
- 20.Vina ER, Ran D, Ashbeck EL, Kwoh CK. Widespread pain is associated with increased risk of no clinical improvement after TKA in women. Clin Orthop Relat Res. 2019;00:1–13. doi: 10.1097/CORR.0000000000001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gungor S, Fields K, Aiyer R, Valle AGD, Su EP. Incidence and risk factors for development of persistent postsurgical pain following total knee arthroplasty: A retrospective cohort study. Medicine. 2019;98(28):e16450. doi: 10.1097/MD.0000000000016450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435. doi: 10.1136/bmjopen-2011-000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baliki MN, Mansour AR, Baria AT, Apkarian AV, Zang Y-F. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9(9):e106133. doi: 10.1371/journal.pone.0106133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottam WJ, Iwabuchi SJ, Drabek MM, Reckziegel D, Auer DP. Altered connectivity of the right anterior insula drives the pain connectome changes in chronic knee osteoarthritis. Pain. 2018;159(5):929–938. doi: 10.1097/j.pain.0000000000001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellamy N. WOMAC® Osteoarthritis Index User Guide XI. Queensland: Brisbane; 2015. [Google Scholar]
- 27.Freynhagen R, Baron R, Gockel U, Tölle TR. pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488 [DOI] [PubMed] [Google Scholar]
- 28.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9(5):745–758. doi: 10.1586/ERN.09.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 30.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong J, Gollub RL, Polich G, et al. A Functional Magnetic Resonance Imaging Study on the Neural Mechanisms of Hyperalgesic Nocebo Effect. J Neurosci. 2008;28(49):13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman S, Yu R, Egorova N, et al. Distinct neural representations of placebo and nocebo effects. Neuroimage. 2015;112:197–207. doi: 10.1016/j.neuroimage.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holtz K, Pané-Farré CA, Wendt J, Lotze M, Hamm AO. Brain activation during anticipation of interoceptive threat. Neuroimage. 2012;61(4):857–865. doi: 10.1016/j.neuroimage.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 34.Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81(2):267–279. doi: 10.1016/j.neuron.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison SE, Salzman CD. Representations of appetitive and aversive information in the primate orbitofrontal cortex. Ann N Y Acad Sci. 2011;1239(1):59–70. doi: 10.1111/j.1749-6632.2011.06255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plassmann H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30(32):10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker S, Gandhi W, Pomares F, Wager TD, Schweinhardt P. Orbitofrontal cortex mediates pain inhibition by monetary reward. Soc Cogn Affect Neurosci. 2017;12(4):651–661. doi: 10.1093/scan/nsw173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29(4):1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419–429. doi: 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seth AK, Friston KJ. Active interoceptive inference and the emotional brain. Phil Trans R Soc. 2016;371(1708):20160007. doi: 10.1098/rstb.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1–13. doi: 10.1016/j.jpain.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd DM, Helbig T, Findlay G, Roberts N, Nurmikko T. Brain areas involved in anticipation of clinically relevant pain in low back pain populations with high levels of pain behavior. J Pain. 2016;17(5):577–587. doi: 10.1016/j.jpain.2016.01.470 [DOI] [PubMed] [Google Scholar]
- 43.Dunlop BW, Rajendra JK, Craighead WE, et al. Functional Connectivity of the Subcallosal Cingulate Cortex And Differential Outcomes to Treatment With Cognitive-Behavioral Therapy or Antidepressant Medication for Major Depressive Disorder. Am J Psychiatry. 2017;174(6):533–545. doi: 10.1176/appi.ajp.2016.16050518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao X, Mao C, Wang Y, et al. Brain gray matter alterations in Chinese patients with chronic knee osteoarthritis pain based on voxel-based morphometry. Medicine. 2018;97(12):e0145. doi: 10.1097/MD.0000000000010145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karp JF, Dew MA, Wahed AS, et al. Challenges and solutions for depression prevention research: methodology for a depression prevention trial for older adults with knee arthritis and emotional distress. Am J Geriatr Psychiatry. 2016;24(6):433–443. doi: 10.1016/j.jagp.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dun W-H, Yang J, Yang L, et al. Abnormal structure and functional connectivity of the anterior insula at pain-free periovulation is associated with perceived pain during menstruation. Brain Imaging and Behavior. 2017;11(6):1787–1795. doi: 10.1007/s11682-016-9646-y [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis GN, Parker RS, Sharma S, Rice DA, McNair PJ. Structural brain alterations before and after total knee arthroplasty: A longitudinal assessment. Pain Med. 2018;19(11):2166–2176. doi: 10.1093/pm/pny108 [DOI] [PubMed] [Google Scholar]
- 49.Spisak T, Kincses B, Schlitt F, et al. Pain-free resting-state functional brain connectivity predicts individual pain sensitivity. Nat Commun. 2005;102(36):187. doi: 10.1073/pnas.0408576102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner IC, Rütgen M, Hummer A, Windischberger C, Lamm C. Placebo-induced pain reduction is associated with negative coupling between brain networks at rest. NeuroImage. 2020;219:117024. doi: 10.1016/j.neuroimage.2020.117024 [DOI] [PubMed] [Google Scholar]