ABSTRACT
Solitary fibrous tumor/hemangiopericytoma (SFT/HPC) is a rare tumor derived from mesenchymal tissue. Although standard chemotherapy for SHT/HPC has not been established, temozolomide plus bevacizumab (TMZ+Bev) therapy for SFT/HPC has been reported. The effectiveness and safety of TMZ+Bev (temozolomide 150 mg/m2 orally on days 1–7 and days 15–21 and bevacizumab 5 mg/kg intravenously on day 8 and day 22 on a 28-day cycle), which was administered from December 2013 until April 2019 to four patients with SFT/HPC, were retrospectively analyzed. Four patients with SFT/HPC received TMZ+Bev. The age of the patients ranged from 41 to 75 years. Two were male, and the primary tumor sites were the meninges in three patients and the pleura in one. One achieved partial response; the others, stable disease (SD). The progression-free survival time ranged from 9.4 to 29.6 months according to RECIST v1.1. One patient died 59 months after using TMZ+Bev, and the others survived for 17 to 64 months. All patients experienced Grade 3 or higher lymphopenia, and three had Grade 3 or higher leukopenia and neutropenia. One patient subsequently received doxorubicin; another, pazopanib. TMZ+Bev therapy for SFT/HPC is safe and effective for maintaining long-term SD.
Key Words: solitary fibrous tumor, hemangiopericytoma, temozolomide, bevacizumab, chemotherapy
INTRODUCTION
Solitary fibrous tumors (SFTs) and hemangiopericytomas (HPCs) are rare mesenchymal neoplasms that may occur in any organ, including the pleura and central nervous system (CNS). Nuclear atypia, hypercellularity, more than 4 mitoses/10 HPFs, and necrosis are associated with aggressive clinical behavior.1 Extracranial metastatic disease of CNS SFT/HPC can occur in the bone, lung, and liver.2 Rarely, patients present with hyperglycemia (Doege-Potter Syndrome),3,4 which is associated with the production of a form of insulin-like growth factor, IGF-2.5,6
Classically, SFT is characterized by CD34-positive spindle cells in a collagenous background with an elaborate vasculature that includes staghorn blood vessels.7 However, approximately 5 to 10% of SFTs are negative for CD34 expression. In addition, Ki-67 is a nuclear protein that is expressed in proliferating cells and is thus a surrogate indicator of the cellular proliferation rate. Ki-67 expression is reported to be related to the disease-free survival time of patients with SFT of the pleura.8
In the World Health Organization Classification of Tumors of Soft Tissue and Bone 2013, the subcategory of HPC was removed from the classification of extrapleural SFT because HPC included tumors that represented cellular examples of SFT, as well as other distinct tumor types that may histologically resemble SFT.9 On the other hand, neuropathologists retained the term HPC given its historical understanding and distinct clinicopathologic correlations, such as high recurrence rates and long-term risk of systemic metastasis.10
Recently, both SFT and HPC have become to be considered variants of a single pathologic entity characterized by the presence of an NGFI-A Binding Protein 2 (NAB2) and Signal Transducer and Activator of Transcription 6 (STAT6) fusion protein.11-13 Therefore, in the World Health Organization Classification of Tumors of the Central Nervous System 2016, the combined term SFT/HPC was created to describe such lesions.10 Nuclear STAT6 signals in immunohistochemistry are reported to be useful for distinguishing HPC/SFT from other mesenchymal neoplasms.14-16
Although various chemotherapeutic agents were tried to treat SFT/HPC, most of them showed insufficient effectiveness. Park et al reported the efficacy of combination chemotherapy with temozolomide plus bevacizumab (TMZ+Bev).17 Temozolomide is an imidazotetrazine derivative of the alkylating agent dacarbazine and is approved for the treatment of malignant glioma and Ewing sarcoma in Japan, and bevacizumab is an antibody that targets vascular endothelial growth factor (VEGF) and inhibits angiogenesis. In the present study, we analyzed the clinical course of patients with SFT/HPC who received TMZ+Bev in our hospital.
PATIENTS AND METHODS
Patientsl
This study was a retrospective analysis of the clinical course of patients with SFT/HPC who received TMZ+Bev from December 2013 until April 2019 at Nagoya University Hospital. Because the administration of these two drugs for this disease is not approved and is off-label in Japan, we conducted this study after approval of the institutional review board. This retrospective observational study was approved by the Ethics Review Board of Nagoya University Hospital (approval number 2019-0053).
Diagnosis of SFT/HPC
Histopathological diagnoses of SFT/HPC were made by expert pathologists using HE staining and immunohistochemical analyses for markers including CD34 and Ki-67. Additional immunohistochemical analysis of STAT6 was performed to confirm the diagnosis.
Chemotherapy
All patients received temozolomide 150 mg/m2 orally on days 1–7 and days 15–21 and bevacizumab 5 mg/kg intravenously on day 8 and day 22 on a 28-day cycle. The response was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) and Choi criteria.18 The toxicity profile was evaluated according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0).
RESULTS
The histopathological findings of SFT/HPC in all four patients revealed malignant characteristics including high cell density and/or mitosis (Fig. 2A, 4A, 6A and 8A). Strong nuclear staining with an anti-STAT6 antibody was observed in the tumors of all four cases (Fig. 2D, 4D, 6D and 8D). All four patients received TMZ+Bev. Patient characteristics and therapeutic effectiveness are summarized in Table 1, and adverse events are described in Table 2. The clinical course of each patient is as follows.
Fig. 1.
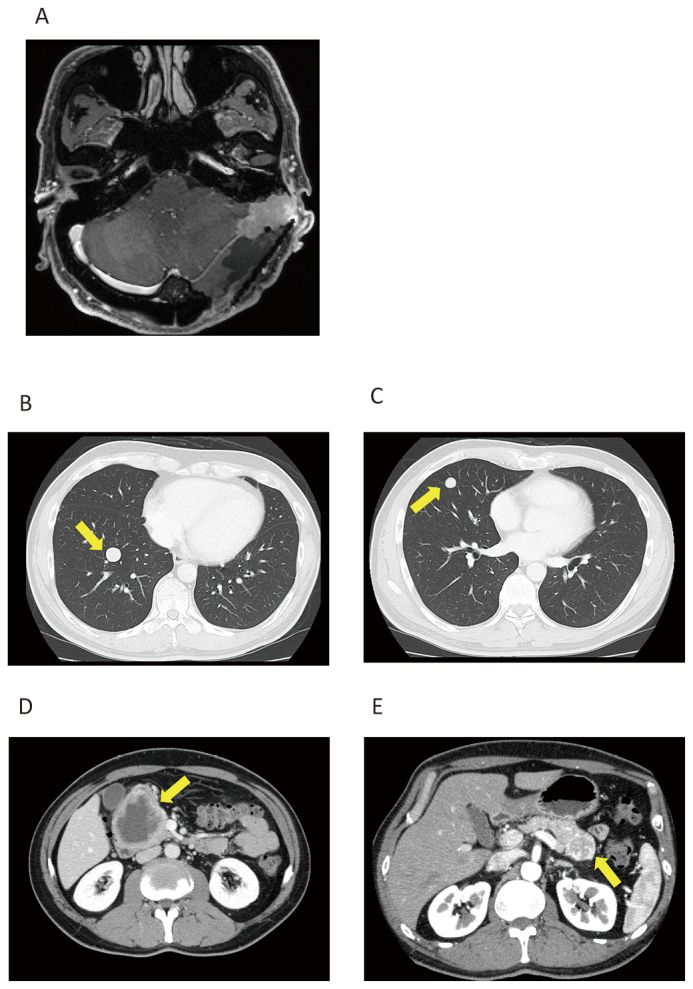
Case 1
Fig. 1A: Magnetic resonance imaging (MRI) of brain tumor at tentorium cerebelli.
Fig. 1B and 1C: Computed tomography (CT) of lung metastases.
Fig. 1D and 1E: CT of pancreatic metastases.
Fig. 2.
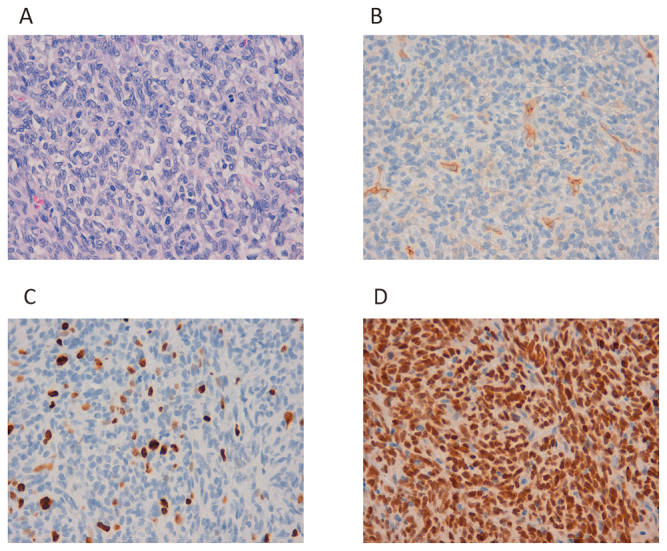
Histopathology of Case 1.
Fig. 2A: Growth of swollen spindle cells with necrosis and mitosis.
Fig. 2B: Partial CD34 positivity is observed.
Fig. 2C: The Ki-67 index is 20%.
Fig. 2D: Strong nuclear staining with anti-STAT6 antibody can be observed.
Table 1.
Patient characteristics and effectiveness of TMZ+Bev
| Best overall
response |
Progression-free
survival (months) |
Overall survival (months) |
Prognosis | ||||||||
| Age | Sex | Primary organ | Metastases | Choi | RECIST | Choi | RECIST | ||||
| 41 | M | meninges | pancreas, lung | SD | SD | 26.4 | 29.6 | 64.4+ | alive | ||
| 47 | F | meninges | pelvis, bone | SD | SD | 8.9 | 18.6 | 20.7+ | alive | ||
| 52 | F | meninges | liver, lung | SD | SD | 9.4 | 9.4 | 59.0 | died | ||
| 75 | M | pleura | lymph nodes, spleen, liver |
PR | PR | 15.8 | 15.8 | 17.3+ | alive | ||
Table 2.
Adverse events of the four patients who received TMZ+Bev
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Leukopenia | 1 | 3 | ||
| Neutropenia | 1 | 3 | ||
| Lymphopenia | 3 | 1 | ||
| Thrombocytopenia | 3 | 1 | ||
| Anemia | 2 | 1 | ||
| Hemolysis | 1 | |||
| Anorexia | 2 | |||
| Nausea | 2 | |||
| Vomiting | 1 | |||
| Diarrhea | 2 | |||
| Stomatitis | 1 | |||
| Skin rash | 1 | |||
| Fatigue | 1 | |||
| Proteinuria | 2 | 2 | ||
| Hypoalbuminemia | 4 | |||
| AST increased | 2 | |||
| ALT increased | 3 | |||
| γ GTP increased | 2 | |||
| ALP increased | 2 | |||
| LDH increased | 2 |
Case 1
A 41-year-old man underwent surgery for brain tumor nine years ago. He received stereotactic radiation therapy for local recurrence (Fig. 1A). Metastases in the lung (Fig. 1B, 1C) and pancreas (Fig. 1D, 1E) were diagnosed, and TMZ+Bev was started. Three months after starting TMZ+Bev, he underwent surgical resection for a recurrent brain tumor (Fig. 2). Although the disease progressed in nine months before starting TMZ+Bev, the patient maintained stable disease (SD) for over 24 months. He experienced autoimmune hemolytic anemia, which resolved after corticosteroid administration and was not exacerbated despite restarting TMZ+Bev after two months of cessation. Therefore, the cause of the hemolytic anemia was unlikely to be related to TMZ+Bev. Because his metastatic tumors grew very slowly and the adverse effects were tolerable, TMZ+Bev was continued for over five years.
Case 2
A 47-year-old woman underwent surgery and radiation therapy for brain tumors 22 years ago (Fig. 3A). She experienced buttock pain, and multiple bone metastases including in the right pelvis (Fig. 3B) and vertebrae (Fig. 3C) were diagnosed five years ago. Biopsy from the right pelvis was performed to confirm that the metastasis was SFT/HPC (Fig. 4). She underwent palliative radiation therapy (30 Gy in 10 fractions) for metastases in the 6th to 8th thoracic vertebrae. TMZ+Bev was initiated, and the patient maintained SD for approximately nine months and 19 months according to the Choi criteria and RECIST, respectively. Metastatic progression occurred gradually, and thus, TMZ+Bev was continued for 20 months. She experienced minimal adverse effects.
Fig. 3.
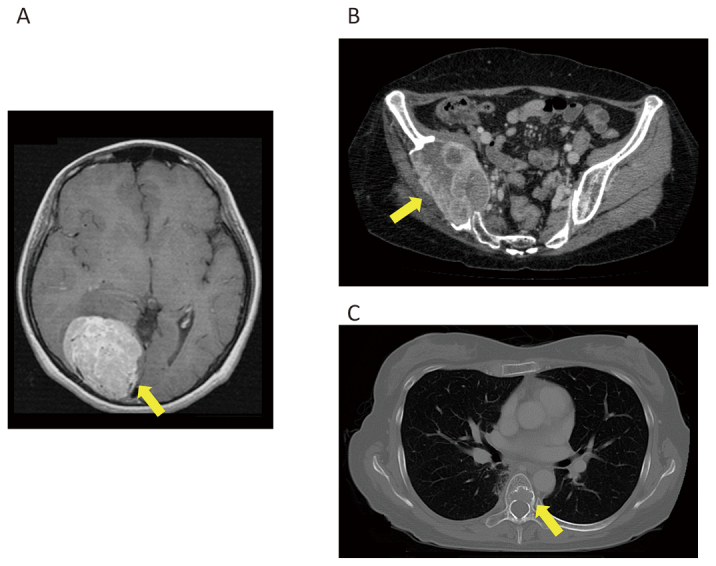
Case 2
Fig. 3A: MRI of brain tumor at the right occipital lobe.
Fig. 3B: CT of metastasis at the right ilium.
Fig. 3C: CT of metastasis at the seventh thoracic vertebra.
Fig. 4.
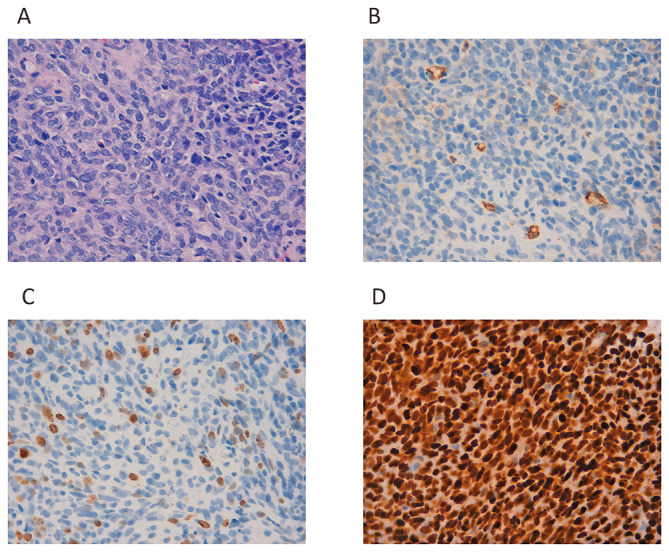
Histopathology of Case 2.
Fig. 4A: Dense growth of spindle-shaped cells with a round nuclear shape.
Fig. 4B: Slight CD34 positivity can be observed.
Fig. 4C: The Ki-67 index is 15%.
Fig. 4D: Strong nuclear staining with anti-STAT6 antibody can be observed.
Case 3
A 52-year-old woman underwent surgery and radiation therapy for brain tumors 18 years ago. She was diagnosed with liver metastasis five years ago (Fig. 5A) and underwent partial hepatectomy (Fig. 6). Four months ago, local recurrence (Fig. 5B) and metastases in the liver (Fig. 5C) and the lung (Fig. 5D) were diagnosed and progressed over approximately three months. TMZ+Bev was initiated, and the patient maintained SD for nine months. One year and nine months after the start of TMZ+BEV, liver metastasis progressed. Although pazopanib was administered, it was not effective. TMZ+Bev was restarted, but the disease continued to progress. She died four years and eleven months after the initial administration of TMZ+Bev.
Fig. 5.
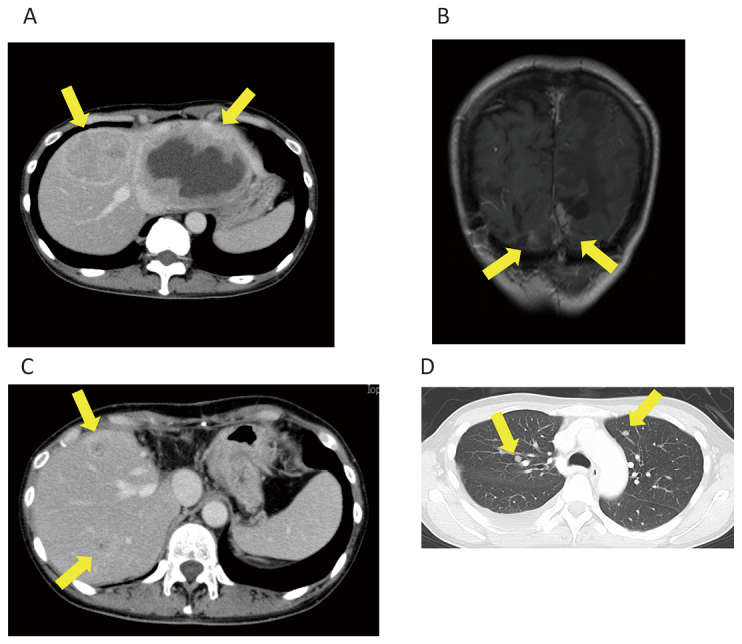
Case 3
Fig. 5A: Liver metastases before hepatectomy.
Fig. 5B: Recurrence of brain tumor contact to the falx cerebri and the tentorium cerebelli.
Fig. 5C: Recurrence of liver metastasis.
Fig. 5D: Lung metastases.
Fig. 6.
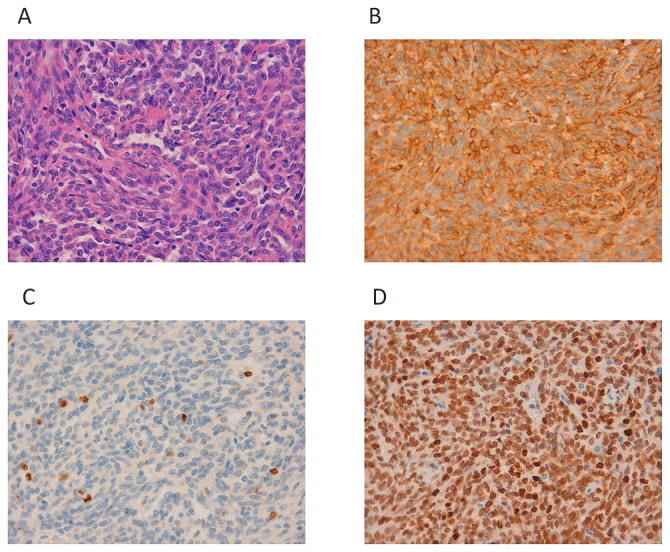
Histopathology of Case 3.
Fig. 6A: Growth of spindle-shaped cells with oval nuclei.
Fig. 6B: CD34 positivity can be observed.
Fig. 6C:The Ki-67 index is 3–4%.
Fig. 6D: Strong nuclear staining with anti-STAT6 antibody can be observed.
Case 4
A 75-year-old man underwent surgery for a left pleural tumor one year and one month ago (Fig. 7A, 8). Metastases in lymph nodes (Fig. 7B), the spleen (Fig. 7D) and the liver (Fig. 7E) were diagnosed, and TMZ+Bev was initiated. The paratracheal lymph nodes (Fig. 7B) and subcarinal lymph nodes shrank (Fig. 7C), and splenic metastasis (Fig. 7D) was undetectable after TMZ+Bev treatment. The liver metastases (Fig. 7E) were unchanged. He achieved partial response (PR) five months after the initiation of TMZ+Bev. However, liver metastasis progressed 16 months after the initial administration TMZ+Bev (Fig. 7F). He received doxorubicin as a second-line chemotherapy regimen.
Fig. 7.
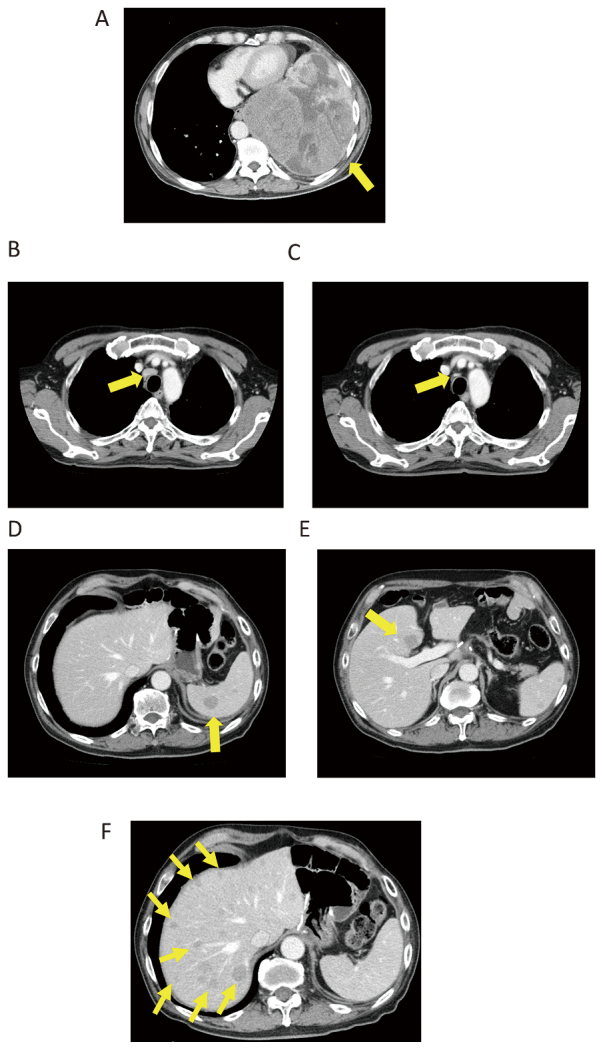
Case 4
Fig. 7A: Left intrathoracic tumor before surgery.
Fig. 7B and 7C: Paratracheal lymph node metastases before (B) and after (C) TMZ+Bev.
Fig. 7D: Splenic metastasis before TMZ+Bev
Fig. 7E: Liver metastases before TMZ+Bev
Fig. 7F: Liver metastases progressed 16 months after the initiation of TMZ+Bev.
Fig. 8.
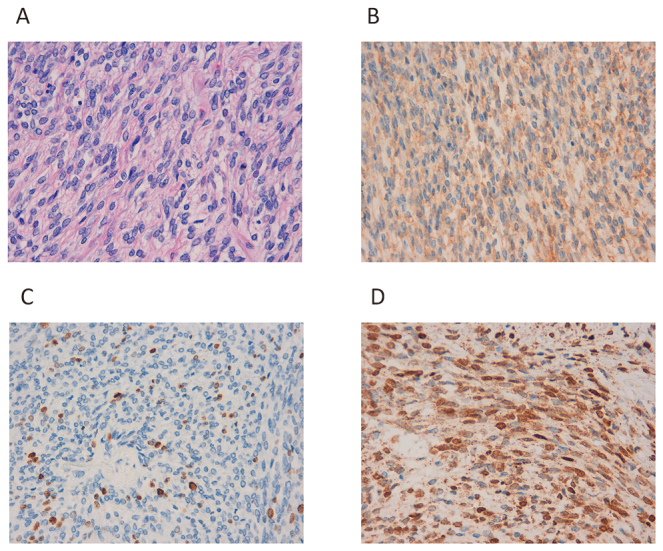
Histopathology of Case 4.
Fig. 8A: Growth of spindle-shaped cells with oval nuclei.
Fig. 8B: CD34 positivity can be observed.
Fig. 8C: The Ki-67 index is 3–4%.
Fig. 8D: Strong nuclear staining with anti-STAT6 antibody can be observed.
DISCUSSION
Out of four patients who received TMZ+Bev, one (Case 4) achieved PR. According to the CT and/or MRI findings for postoperative surveillance before TMZ+Bev, the metastatic progression of two patients (Cases 1 and 3) seemed to be suppressed because the time to progression was prolonged after the administration of TMZ+Bev. Because the toxicities due to TMZ+Bev were tolerable, the treatment can be continued for years in some cases.
Although only one patient achieved PR, all four patients maintained SD for a long duration. The reason for the discrepancy is not clear. The number of cases in this study is too small to analyze the issue. However, one possible explanation is the heterogeneity of the characteristics. The size of tumor, duration between diagnosis and the initiation of TMZ+Bev, and locations of the primary lesion and metastases were different for the patients. Tumor shrinkage and the duration of effectiveness might depend on such characteristics. In addition, no patients in the present study showed a significant decrease in the tumor density on contrast-enhanced CT. Although we analyzed the CT images according to previously reported methods, the conditions including the selection of the regions of interest (ROI), the timing of the scan and the method of injection of the contrast media might not be exactly the same as in the institutions that reported the effectiveness according to the Choi criteria.
The precise pharmacological mechanisms of TMZ+Bev have not been fully elucidated.17 Bevacizumab may enhance the cytotoxic activity of temozolomide. The VEGF–VEGF receptor pathway could be a potential key therapeutic target in HPC/SFT. As described below, tyrosine kinase inhibitors, of which the VEGF receptor is a target, have also demonstrated evidence of activity in HPC/SFT.
Recent reports of SFT/HPC adopt both RECIST and Choi criteria to evaluate the treatment efficacy. Choi criteria are developed as an evaluation method of treatment efficacy for gastrointestinal stromal tumors (GISTs) with imatinib, which measures tumor diameter and tumor density in computed tomography (CT) with contrast enhancement and is reported to have good correlation according to [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET).18 Of the four patients presented here, none showed a significant decrease in density on contrast-enhanced CT.
There is no established standard chemotherapy regimen for SFT/HPC. Previous reports of chemotherapy for SFT/HPC are summarized in Table 3.
Table 3.
Previous reports of chemotherapy regimens for SFT/HPC
| Response | ||||||||||||||||
| Number
of patients |
Evaluation | Number
of evaluable patients |
PR (%) | SD (%) | PD (%) | PFS
(months) |
OS
(months) |
Reference | ||||||||
| Chemotherapy regimen | ||||||||||||||||
| anthracycline-based *1 | 17 | RECIST | 17 | 1 | (5.9) | 13 | (76.5) | 3 | (17.6) | 4.2 | 14.6 | 19 | ||||
| doxorubicin-based *2 | 15 | RECIST | 15 | 0 | (0) | 14 | (93.3) | 1 | (6.7) | 4.6 | 22.8 | 20 | ||||
| gemcitabine-based *3 | 2 | RECIST | 2 | 0 | (0) | 1 | (50) | 1 | (50) | |||||||
| paclitaxel | 1 | RECIST | 1 | 0 | (0) | 1 | (100) | 0 | (0) | |||||||
| anthracycline-based *4 | 30 | RECIST | 30 | 6 | (20) | 8 | (26.7) | 17 | (56.7) | 4 | – | 21 | ||||
| bevacizumab monotherapy | 7 | RECIST | 7 | 2 | (28.6) | 4 | (57.1) | 1 | (14.3) | 9 | 32.8 | 22 | ||||
| temozolomide plus
bevacizumab |
14 | RECIST | 14 | 2 | (14.3) | 12 | (85.7) | 0 | (0) | 10.8 | 24.3 | 17 | ||||
| Choi | 14 | 11 | (78.6) | 2 | (14.3) | 1 | (7.1) | 9.7 | ||||||||
| dacarbazine | 8 | RECIST | 8 | 3 | (37.5) | 4 | (50) | 1 | (12.5) | 7 | – | 23 | ||||
| trabectedin | 11 | RECIST | 11 | 1 | (9.1) | 8 | (72.7) | 2 | (18.2) | 11.6 | 22.3 | 25 | ||||
| pazopanib | 13 | RECIST | 13 | 1 | (7.7) | 8 | (61.5) | 2 | (15.4) | 4.7 | 13.3 | 26 | ||||
| Choi | 11 | 5 | (45.5) | 4 | (36.4) | 2 | (18.2) | |||||||||
| pazopanib | 36 | RECIST | 36 | 2 | (5.6) | 21 | (58.3) | 12 | (33.3) | 5.6 | not reached | 27 | ||||
| Choi | 35 | 18 | (51.4) | 9 | (25.7) | 8 | (22.9) | 5.6 | ||||||||
| sorafenib | 5 | RECIST | 5 | 0 | (0) | – | – | – | 19.7 | 28 | ||||||
| sunitinib | 35 | RECIST | 31 | 2 | (6.5) | 16 | (51.6) | 13 | (41.9) | 6 | 16 | 29 | ||||
| Choi | 29 | 14 | (48.3) | 5 | (17.2) | 10 | (34.5) | 7 | ||||||||
| axitinib | 17 | RECIST | 17 | 1 | (5.9) | 14 | (82.4) | 2 | (11.8) | 9.4 | 25.3 | 30 | ||||
| Choi | 17 | 7 | (41.2) | 6 | (35.3) | 4 | (23.5) | 5.1 | ||||||||
*1: Doxorubicin (Dox) monotherapy for 14 patients, ifosfamide (Ifos) + epirubicin (Epi) for 1 patient, Ifos + Dox for 1 patient and dacarbazine (DTIC) + Ifos + Dox for 1 patient.
*2: Dox + Ifos for 12 patients, Dox + DTIC for 1 patient, Dox + cisplatin for 1 patient and Dox monotherapy for 1 patient.
*3: Gemcitabine (Gem) monotherapy for 1 patient and Gem + docetaxel for 1 patient.
*4: Epi + Ifos for 22 patients, Dox for 7 patients and Epi for 1 patient.
Constantinidou et al reported that conventional anthracycline-based chemotherapy has limited efficacy.19 Of 24 patients, 17 received anthracycline-based chemotherapy, three received axitinib, two received TMZ+Bev, one received trabectedin and one received an experimental drug. The overall survival (OS) time from the time of diagnosis for these 24 patients was 46 months.
According to a report by Park et al,20 of 21 patients, 4 received more than 1 regimen of chemotherapy for a total of 25 treatments. Of 18 patients who received first-line chemotherapy, no patients achieved complete response (CR) or PR, and 16 (89%) experienced SD. Five patients maintained SD for at least 6 months after first-line treatment. The median OS time from diagnosis was 10.3 years.
Stacchiotti et al reported 42 patients treated with chemotherapy.21 Among the 31 patients who received anthracycline-based chemotherapy, 30 patients could be evaluated. The overall PFS time was 4 months, and 20% of patients were progression-free at 6 months.
The effectiveness of bevacizumab monotherapy was reported by Agulnik et al22 Of the seven patients, two (29%) experienced PR, while only one (14%) experienced PD at the first evaluation. Combination treatment with TMZ+Bev was reported by Park et al17 for the first time. Of 14 patients, 11 (79%) achieved PR according to Choi criteria, with a median time to response of 2.5 months. The most frequently observed toxic effect was myelosuppression. Both dacarbazine and temozolomide are triazene compounds. The effectiveness of dacarbazine has also been reported.23
Eribulin and trabectedin, cytotoxic drugs that are used for soft tissue sarcoma, have been reported as treatment agents for SFT/HPC. Of 128 patients treated with eribulin,24 there was one patient with SFT in whom drug activity was reported. The efficacy of trabectedin was reported in eleven patients with advanced SFT.25 Moreover, it has been used as second-line therapy in 8 patients (73%) and as at least third-line therapy in another 3 (27%).
As there are angiogenesis inhibitors other than bevacizumab, the efficacy of several small molecule compounds has been reported. In a prospective study with 13 patients treated with pazopanib as a first-line treatment for metastatic disease,26 the median OS time was 13.3 months, and the median PFS time was 4.7 months. Using the Choi criteria, 5 achieved PR (46%) and 4 maintained SD (36%). In a phase II trial of pazopanib,27 36 were enrolled (34 with malignant SFT and two with dedifferentiated SFT). Of the 35 patients evaluated, 18 (51%) achieved PR, and nine (26%) experienced SD according to the Choi criteria. The administration of sorafenib has also been reported.28 Two of five patients achieved disease control with sorafenib for 9 months, while disease progression occurred within the month preceding their inclusion. For sunitinib,29 of 31 patients, two achieved PR, and 16 experienced SD. A <30% decrease in size was observed in three patients. Fourteen of 29 patients evaluated according to the Choi criteria achieved PR. The median PFS by RECIST was 6 months. In two of six patients, sunitinib resistance was overcome by increasing the dose to 50 mg/day. The use of axitinib has also been reported.30 Of 17 patients, the best responses according to the Choi criteria were PR in seven patients and SD in six patients. The best responses according to RECIST were PR in one patient and SD in 14 patients. The median Choi-PFS time was 5.1 months. The median Choi-PFS times were 14.8 and 2.8 months for responsive and nonresponsive patients, respectively. The median OS time was 25.3 months.
As described above, various chemotherapies for SFT/HPC have been reported. These chemotherapeutic regimens can be divided into three groups. One is traditional chemotherapy with cytotoxic agents for soft tissue sarcoma such as doxorubicin, ifosfamide, and taxanes. Another is temozolomide + bevacizumab or bevacizumab monotherapy. The third is tyrosine kinase inhibitors including pazopanib, sorafenib and sunitinib. Since traditional cytotoxic chemotherapies have limited effectiveness, other options have been developed.
There are some limitations in the present study. First, because the incidence of SFT/HPC is very rare, the number of cases is small. Second, since this is a retrospective analysis, the treatment plan was dependent on each physician. Third, the tumors’ primary lesions were located in the meninges and thorax. Since tumors with different primary lesions might have different biological characteristics, the therapeutic effect and clinical courses of various tumors might not be the same.
CONCLUSION
TMZ+Bev for SFT/HPC is safe, effective and can be continued long-term. An optimized treatment strategy for SFT/HPC should be established.
CONFLICTS OF INTEREST
Yuichi Ando received research funding from Chugai Pharmaceutical Co., Ltd., outside the submitted work. The other authors have no conflicts of interest to disclose.
Abbreviations
- Bev
Bevacizumab
- CR
Complete response
- HPC
Hemangiopericytoma
- OS
Overall survival
- PD
Progressive disease
- PFS
Progression-free survival
- PR
Partial response
- RECIST criteria
Response Evaluation Criteria in Solid Tumors
- SD
Stable disease
- SFT
Solitary fibrous tumor
- STAT6
Signal Transducer and Activator of Transcription 6
- TMZ
Temozolomide
REFERENCES
- 1.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22(12):1501–1511. [DOI] [PubMed]
- 2.Metellus P, Bouvier C, Guyotat J, et al. Solitary fibrous tumors of the central nervous system: clinicopathological and therapeutic considerations of 18 cases. Neurosurgery. 2007;60(4):715–722; discussion 722. DOI: 10.1227/01.NEU.0000255418.93678.AD [DOI] [PubMed]
- 3.Chamberlain MH, Taggart DP. Solitary fibrous tumor associated with hypoglycemia: an example of the Doege-Potter syndrome. J Thorac Cardiovasc Surg. 2000;119(1):185–187. [DOI] [PubMed]
- 4.Balduyck B, Lauwers P, Govaert K, Hendriks J, De Maeseneer M, Van Schil P. Solitary fibrous tumor of the pleura with associated hypoglycemia: Doege-Potter syndrome: a case report. J Thorac Oncol. 2006;1(6):588–590. [PubMed]
- 5.de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14(4):979–993. DOI: 10.1677/ERC-07-0161 [DOI] [PubMed]
- 6.Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab. 2014;99(3):713–722. DOI: 10.1210/jc.2013-3382 [DOI] [PMC free article] [PubMed]
- 7.Cheah AL, Billings SD, Goldblum JR, Carver P, Tanas MZ, Rubin BP. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology. 2014;46(5):389–395. DOI: 10.1097/PAT.0000000000000122 [DOI] [PubMed]
- 8.Franzen D, Diebold M, Soltermann A, et al. Determinants of outcome of solitary fibrous tumors of the pleura: an observational cohort study. BMC Pulm Med. 2014;14:138. DOI: 10.1186/1471-2466-14-138 [DOI] [PMC free article] [PubMed]
- 9.Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer. 2014;120(12):1763–1774. DOI: 10.1002/cncr.28657 [DOI] [PubMed]
- 10.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. DOI: 10.1007/s00401-016-1545-1 [DOI] [PubMed]
- 11.Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45(2):131–132. DOI: 10.1038/ng.2522 [DOI] [PMC free article] [PubMed]
- 12.Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45(2):180–185. DOI: 10.1038/ng.2509 [DOI] [PMC free article] [PubMed]
- 13.Mohajeri A, Tayebwa J, Collin A, et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer. 2013;52(10):873–886. DOI: 10.1002/gcc.22083 [DOI] [PubMed]
- 14.Schweizer L, Koelsche C, Sahm F, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125(5):651–658. DOI: 10.1007/s00401-013-1117-6 [DOI] [PubMed]
- 15.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27(3):390–395. DOI: 10.1038/modpathol.2013.164 [DOI] [PubMed]
- 16.Fritchie KJ, Jin L, Rubin BP, et al. NAB2-STAT6 Gene Fusion in Meningeal Hemangiopericytoma and Solitary Fibrous Tumor. J Neuropathol Exp Neurol. 2016;75(3):263–271. DOI: 10.1093/jnen/nlv026 [DOI] [PubMed]
- 17.Park MS, Patel SR, Ludwig JA, et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer. 2011;117(21):4939–4947. DOI: 10.1002/cncr.26098 [DOI] [PMC free article] [PubMed]
- 18.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753–1759. DOI: 10.1200/JCO.2006.07.3049 [DOI] [PubMed]
- 19.Constantinidou A, Jones RL, Olmos D, et al. Conventional anthracycline-based chemotherapy has limited efficacy in solitary fibrous tumour. Acta Oncol. 2012;51(4):550–554. DOI: 10.3109/0284186X.2011.626450 [DOI] [PubMed]
- 20.Park MS, Ravi V, Conley A, et al. The role of chemotherapy in advanced solitary fibrous tumors: a retrospective analysis. Clin Sarcoma Res. 2013;3(1):7. DOI: 10.1186/2045-3329-3-7 [DOI] [PMC free article] [PubMed]
- 21.Stacchiotti S, Libertini M, Negri T, et al. Response to chemotherapy of solitary fibrous tumour: a retrospective study. Eur J Cancer. 2013;49(10):2376–2383. DOI: 10.1016/j.ejca.2013.03.017 [DOI] [PubMed]
- 22.Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24(1):257–263. DOI: 10.1093/annonc/mds237 [DOI] [PubMed]
- 23.Stacchiotti S, Tortoreto M, Bozzi F, et al. Dacarbazine in solitary fibrous tumor: a case series analysis and preclinical evidence vis-a-vis temozolomide and antiangiogenics. Clin Cancer Res. 2013;19(18):5192–5201. DOI: 10.1158/1078-0432.CCR-13-0776 [DOI] [PubMed]
- 24.Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes. Lancet Oncol. 2011;12(11):1045–1052. DOI: 10.1016/S1470-2045(11)70230-3 [DOI] [PubMed]
- 25.Khalifa J, Ouali M, Chaltiel L, et al. Efficacy of trabectedin in malignant solitary fibrous tumors: a retrospective analysis from the French Sarcoma Group. BMC Cancer. 2015;15:700. DOI: 10.1186/s12885-015-1697-8 [DOI] [PMC free article] [PubMed]
- 26.Maruzzo M, Martin-Liberal J, Messiou C, et al. Pazopanib as first line treatment for solitary fibrous tumours: the Royal Marsden Hospital experience. Clin Sarcoma Res. 2015;5:5. DOI: 10.1186/s13569-015-0022-2 [DOI] [PMC free article] [PubMed]
- 27.Martin-Broto J, Stacchiotti S, Lopez-Pousa A, et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20(1):134–144. DOI: 10.1016/S1470-2045(18)30676-4 [DOI] [PubMed]
- 28.Valentin T, Fournier C, Penel N, et al. Sorafenib in patients with progressive malignant solitary fibrous tumors: a subgroup analysis from a phase II study of the French Sarcoma Group (GSF/GETO). Invest New Drugs. 2013;31(6):1626–1627. DOI: 10.1007/s10637-013-0023-z [DOI] [PubMed]
- 29.Stacchiotti S, Negri T, Libertini M, et al. Sunitinib malate in solitary fibrous tumor (SFT). Ann Oncol. 2012;23(12):3171–3179. DOI: 10.1093/annonc/mds143 [DOI] [PubMed]
- 30.Stacchiotti S, Simeone N, Lo Vullo S, et al. Activity of axitinib in progressive advanced solitary fibrous tumour: Results from an exploratory, investigator-driven phase 2 clinical study. Eur J Cancer. 2019;106:225–233. DOI: 10.1016/j.ejca.2018.10.024 [DOI] [PubMed]


