ABSTRACT
In this retrospective cohort study, we evaluated the incidence of vascular events from carotid artery atherosclerosis after radiotherapy indication for laryngeal and hypopharyngeal cancer. From January 2007 to December 2016, we investigated 111 laryngeal/hypopharyngeal cancer patients who underwent curative radiotherapy and were followed up for ≥1 year (median follow-up duration, 60 months). We evaluated the incidence of vascular events from carotid artery atherosclerosis, defined as a transient ischemic attack or an atherothrombotic cerebral infarction, or from undergoing treatment such as carotid artery stenting for carotid artery stenosis. The median radiation dose was 66 Gy (range, 60–74); 48 patients (43.2%) received concurrent chemotherapy. The 5-year overall survival was 86.2%. Six patients required treatment for carotid artery disease. Carotid stenting was performed in three patients with carotid artery stenosis; three patients developed atherosclerotic cerebral infarction and received medical treatment, with a median of 51.7 months (range, 0.3–78.3) after radiotherapy initiation. The vascular event occurrence rate was 5.4% within 5 years and 10.7% within 8 years. In the univariate analysis, dyslipidemia, diabetes mellitus, and carotid calcification were significant factors for event occurrence. Because three out of six cases occurred out of the irradiated field, no carotid artery or carotid bulb dosimetric parameters showed significant correlation. As laryngeal/hypopharyngeal cancer patients, particularly with complications including dyslipidemia and diabetes mellitus, are at a high risk of carotid artery stenosis after radiotherapy, long-term carotid artery evaluation is necessary. Early intervention by stroke specialists can reduce the risk of fatal cerebral infarction.
Key Words: Laryngeal and hypopharyngeal cancer, Carotid artery atherosclerosis, Transient ischemic attack, Stroke, Carotid artery stenting
INTRODUCTION
Radiation-induced carotid artery disease (RCAD) was first described as early as 1959.1 Although it comprises accelerated atherosclerosis in addition to direct and indirect damage, its exact underlying mechanism remains unclear.2 Stenotic lesions progress over several years, often presenting as unstable plaques, and there is a high risk of developing atherosclerotic cerebral infarction after a few decades. In recent years, the prognosis of cancer has improved, and the onset of cerebral infarction caused by RCAD has become an issue in patients with long-term survival.
Several types of cerebral infarctions have been defined, including cardiac embolism, lacunar infarction, and atherothrombotic cerebral infarction.3 Although the types of cerebral infarctions caused by carotid artery atherosclerosis are most commonly observed in the Western population, the incidence of these types is gradually increasing in the Japanese population as Japan is now adopting a more Western diet.4,5 Cerebral infarction is a type of stroke that commonly occurs in people with many risk factors for arteriosclerosis, such as age, hypertension, dyslipidemia, and diabetes.6,7 Radiotherapy, in addition to other risk factors, has a combined effect on the RCAD progression.8
Regular checkups for the presence of carotid artery disease after radiotherapy are recommended. However, according to a questionnaire survey of the present status of doctors’ awareness of RCAD in Japan, only 9.4% of institutions perform regular examinations.9 Even our hospital has not routinely tested for carotid lesions after radiation treatment for head and neck cancer. To our knowledge, there are no reports about the risk of RCAD in a large series of Japanese head and neck cancer patients. Therefore, we performed this retrospective study to investigate the incidence of cerebrovascular events or treatment such as carotid artery stenting for carotid artery stenosis after the indication of radiotherapy. In addition, we examined the risk factor analysis of RCAD based on clinical and carotid dosimetric parameters.
MATERIALS AND METHODS
Patient population
From January 2007 to December 2016, we evaluated 123 patients who were treated for laryngeal cancer and hypopharyngeal cancer and underwent curative radiation therapy at Gifu Prefectural General Medical Center, Japan. We retrospectively investigated 111 cases that had been followed up for ≥1 year.
Target volume, dose prescription, and treatment planning
The gross tumor volume is defined as the gross tumor volume of the primary tumor. In cases of laryngeal cancer, the clinical target volume (CTV) in T1 disease is the entirety of the vocal cords, whereas the CTV in T2 disease includes a 1-cm margin surrounding the tumor in addition to the vocal cords. In cases with cervical lymph node metastasis, the CTV included the areas of levels II and III. In hypopharyngeal cancer, the CTV was defined as the gross tumor volume plus a 10-mm margin to cover microscopic disease in considering an anatomical feature and included the prophylactic lymph node areas of levels II and III. In cervical lymph node metastasis, the CTV included the areas covering the bilateral sites, including levels II–V, and the retropharyngeal nodes. The planning target volume is defined as the CTV plus a margin of 0.5–1 cm in the craniocaudal direction and 0.5 cm in the posterior–anterior direction. Radiotherapy was administered using 4-MV X-rays with a three-dimensional radiotherapy plan. In T1N0 and T2N0 laryngeal carcinoma cases, two parallel-opposed lateral fields were used with a pair of wedge filters. In node-positive cases, bilateral neck irradiation was performed. Intensity-modulated radiation therapy was not used for any cases. The total prescribed radiation dose ranged from 60 to 74 Gy. Conventional fractionation radiotherapy with 2 Gy/fr (1 fr/day and 5 fr/week) was performed 33–37 times for a total dose of 66–74 Gy in the patients. In the accelerated radiation arm, accelerated fractionation radiotherapy with 2.25 to 2.4 Gy (1 fr/day and 5 fr/week) was delivered 25–30 times for a total dose of 60 to 67.5 Gy. Twice-daily irradiation is prohibited, as is irradiation performed six or more times per week. Chemotherapy was considered in patients with T2 or greater or positive lymph node metastases, in which case conventional fractionation radiotherapy with 2 Gy/fr (1 fr/day and 5 fr/week) was delivered. A computed tomography-based treatment-planning system was mandatory to define the planning target volume. All patients were immobilized in the supine position with the use of an individually designed facial mask for reproducible positioning.
Chemotherapy
Concurrent chemoradiotherapy consisting of platinum was administered for high-risk cases in late T2 or node-positive cases. Chemotherapy regimens included triweekly cisplatin (CDDP) at 80–100 mg/m2, combined with the use of CDDP at 70 mg/m2 and 5-fluorouracil at 700 mg/m2, combined with the use of carboplatin and tegafur/uracil at 600 mg/day or combined with the use of nedaplatin at 140 mg/m2 and 5-fluorouracil at 800 mg/m2.
Follow-up
The patients were examined at least once a week during treatment. Once treatment ended, the frequency of follow-up was approximately once a month in the first year, every 2 months in the second year, every 4 months in the third year, and every 6 months thereafter. Radiological examinations, including CT, FDG-PET/CT, and/or magnetic resonance imaging (MRI), were performed at least once every 6 months.
Incidence of events from carotid artery atherosclerosis
The incidence of vascular events from carotid artery atherosclerosis defined as transient ischemic attack (TIA) or atherothrombotic cerebral infarction, or from undergoing treatment such as carotid artery stenting for carotid artery stenosis, was assessed. Cardiogenic cerebral embolism caused by arrhythmias, such as atrial fibrillation and lacuna infarction in which multiple thin blood vessels are clogged, were not included as vascular events. Cardiologists and stroke specialists participated in the diagnosis. Every event was diagnosed by a neurosurgeon using carotid ultrasonography and MRI and magnetic resonance angiography or digital subtraction angiogram. The participants were followed through December 2018 or were censored at death or last follow-up.
Identifying clinical risk factor and dose volume histogram analysis
Questionnaires were given to the patients to obtain data on demographics, smoking history, medical history, and prescription medications. Weight and height measurements were recorded. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of antihypertension medication.10 Diabetes mellitus was defined as a fasting blood glucose level ≥126 mg/dL or the use of diabetes mellitus medication.11 Dyslipidemia was defined as a total cholesterol level of ≥220 mg/dL, a low-density lipoprotein cholesterol level of ≥140 mg/dL, a fasting triglyceride level of ≥150 mg/dL, or the current use of antidyslipidemic drugs.12 Cardiovascular disease (CVD) was defined as disorders of the heart and blood vessels and included coronary heart disease, cerebrovascular disease, peripheral arterial disease, stroke, arrhythmia, and heart valve diseases.13-15 The carotid artery is defined as that extending from the clavicle to the entry point into the temporal bone, corresponding from the common carotid artery to the internal artery. The carotid bulb is defined as that present 2 cm above and below the carotid bifurcation.16 The following dosimetric parameters were calculated for each of the 222 carotid arteries V35 as the percentage of volume receiving a dose of ≥35 Gy. In addition, V45, V55, and V65, receiving doses of 45, 55, and 65 Gy, respectively, were defined in the same way, and the mean dose was calculated for the entire carotid artery and carotid bulb. Carotid calcifications were visually examined for the presence of calcium as structures with a density of >130 HU within the vessel wall on planning CT (4-MDCT scanner, Acteion, Toshiba).
Outcome measures and statistical analysis
Overall survival was defined as the time from the date of radiotherapy initiation to the date of death or from the date of radiotherapy initiation to the last follow-up (i.e., censor date). The cumulative incidence in the presence of competing risks were constructed using the Kaplan–Meier method. The differences in variables between groups were assessed by a univariate analysis with the use of Gray’s test. All two-sided p-values of <0.05 were considered statistically significant. All statistical analyzes were performed using R software (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria).
Ethical considerations
The study protocol was approved by the Ethics Committee of Gifu Prefecural General Medical Center (Permission number 457). A retrospective chart review was performed for patients who underwent radiotherapy for laryngeal or hypopharyngeal cancer.
RESULTS
A total of 111 patients with laryngeal and hypopharyngeal cancer were included in this study: 95 patients with laryngeal cancer and 16 patients with hypopharyngeal cancer (Table 1). The median follow-up duration of the entire cohort was 60.5 (range, 12.0–120.5) months. The median radiation dose was 66 Gy (range, 60–74), and 48 patients (43.2%) received concurrent chemotherapy. The 5-year overall survival of the entire cohort was 86.2% (95% confidence interval [CI], 76.6–92.0). Figure 1 shows the cumulative event occurrence rate from carotid atherosclerosis. The event occurred in six patients at 0.3 to 78.3 months from the date of the initiation of radiotherapy. The 5- and 8-year occurrence rates were 5.5% (95% CI, 0–10.5%) and 10.7% (95% CI,1.4–19.1%), respectively. Tables 2 and 3 show the results of the univariate analysis of positive risk factors. Dyslipidemia, diabetes mellitus, and carotid calcification were significantly associated with the development of a vascular event from carotid artery atherosclerosis. Carotid artery or carotid bulb dosimetric parameters did not show significant correlation via the univariate analysis.
Table 1.
Patients and treatment characteristics of the entire study cohort
| Factors | Groups | n | (%) |
| Gender | Male | 108 | (97.2) |
| Female | 3 | (2.7) | |
| Age (years) | Median (range) | 69 | (40–80) |
| PS | 0 | 93 | (83.8) |
| 1 | 17 | (15.3) | |
| 2 | 1 | (0.9) | |
| BMI (kg/m2) | Median (range) | 22.2 | (15.1–31.2) |
| Smoking status | Yes | 95 | (85.6) |
| No | 16 | (14.4) | |
| Pack-year | 40 | (0–100) | |
| Larynx | Glottis | 82 | (73.9) |
| Supraglottis | 8 | (7.2) | |
| Subglottis | 2 | (1.8) | |
| Hypopharynx | Piriform sinus | 15 | (13.5) |
| Posterior pharyngeal wall | 1 | (0.9) | |
| cTstage | 1 | 48 | (43.2) |
| 2 | 47 | (42.3) | |
| 3 | 10 | (9) | |
| 4 | 4 | (3.8) | |
| cNstage | 0 | 85 | (76.6) |
| 1 | 16 | (14.4) | |
| 2 | 10 | (8.1) | |
| Hypertension | Yes | 50 | (45.0) |
| No | 61 | (55.0) | |
| Diabetes mellitus | Yes | 28 | (25.2) |
| No | 83 | (74.8) | |
| Dyslipidemia | Yes | 37 | (33.3) |
| No | 74 | (66.7) | |
| Cardiovascular disease | Yes | 23 | (20.7) |
| No | 88 | (79.3) | |
| Antiplatelet therapy | Yes | 22 | (19.8) |
| No | 89 | (80.2) | |
| Radiation dose (Gy) | Total dose, Median (range) | 66 | (60–74) |
| Dose per fraction (Gy), Median (range) | 2 | (1.8–2.4) | |
| Radiation field | WN | 29 | (26.1) |
| Box | 82 | (73.9) | |
| Chemotherapy | Yes | 48 | (43.2) |
| CBDCA+UFT | 13 | (11.7) | |
| CDDP | 16 | (14.4) | |
| FP | 9 | (8.1) | |
| FN | 6 | (5.4) | |
| Others | 4 | (3.6) | |
| No | 63 | (56.8) |
PS: performance status
BMI: body mass index
WN: whole neck field including subclinical lymph node
Box; parallel-opposed fields with individualized wedge-filtered technique, 5 × 5 or 6 × 6 cm; CBDCA, carboplatin; UFT, tegafur/uracil; CDDP, cisplatin; FP, combined with the use of CDDP and 5-fluorouracil; FN, combined with the use of nedaplatin and 5-fluorouracil
Fig. 1.
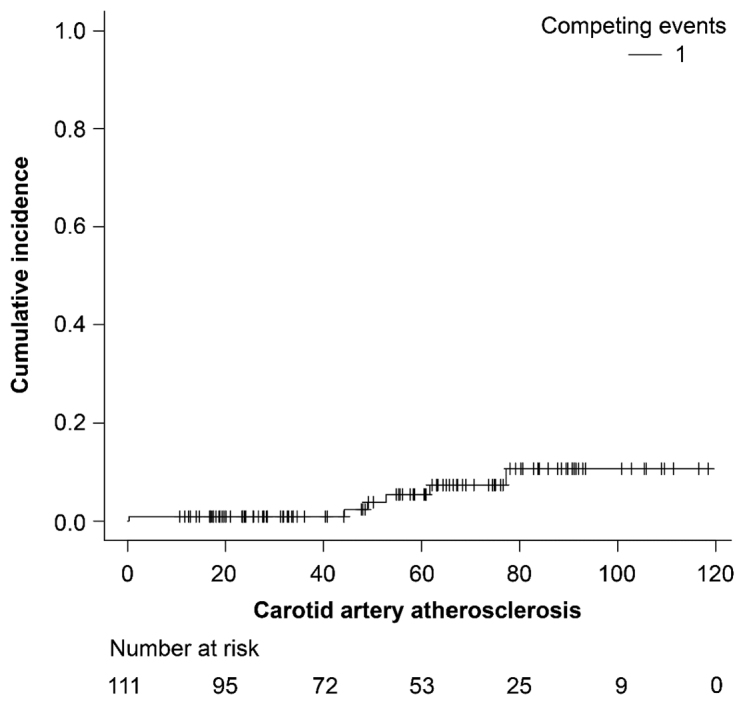
Cumulative incidence rate of a vascular event after the initiation of radiotherapy
Table 2.
Univariate analysis of variables per patient
| Factors | Variables | n | Event | 5 y % | P value |
| Age (years) | ≤70 | 58 | 2 | 6.1 | 0.43 |
| >70 | 53 | 4 | 4.8 | ||
| BMI (kg/m2) | ≤24 | 80 | 4 | 5.5 | 0.86 |
| >24 | 31 | 2 | 5.3 | ||
| Tobacco (pack years) | ≤20 | 34 | 0 | 0 | 0.08 |
| >20 | 77 | 6 | 7.9 | ||
| Primary | Larynx | 92 | 4 | 4.5 | 0.06 |
| Hypopharynx | 19 | 2 | 16.7 | ||
| Hypertension | No | 59 | 2 | 4.2 | 0.25 |
| Yes | 52 | 4 | 7.2 | ||
| Dyslipidemia | No | 74 | 0 | 0 | <0.01 |
| Yes | 37 | 6 | 15.8 | ||
| Diabetes mellitus | No | 82 | 1 | 2 | 0.03 |
| Yes | 29 | 5 | 14.3 | ||
| Cardiovascular disease | No | 88 | 4 | 5.1 | 0.59 |
| Yes | 23 | 2 | 6.2 | ||
| Antiplatelet therapy | No | 89 | 5 | 5.1 | 0.71 |
| Yes | 22 | 1 | 6.2 | ||
| Dose per fraction (Gy) | ≤2 | 62 | 2 | 3.7 | 0.65 |
| >2 | 49 | 4 | 7.1 | ||
| Radiation field | WN | 29 | 2 | 5.1 | 0.44 |
| Box | 82 | 4 | 7.7 | ||
| Chemotherapy | No | 63 | 4 | 6.5 | 0.81 |
| Yes | 48 | 2 | 9.1 |
BMI: body mass index
WN: whole neck field including subclinical lymph node
Box; parallel-opposed fields with individualized wedge-filtered technique, 5 × 5 or 6 × 6 cm; Bold value indicates a significance level pf p < 0.05.
Table 3.
Univariate analysis of variables per carotid artery
| Variables | Incidence of events from carotid artery
atherosclerosis |
P value | ||
| + (n = 6)
mean (range) |
− (n = 216)
mean (range) |
|||
| Entire Carotid | ||||
| Max dose (Gy) | 59.1 (52.9–70.0) | 58.8 (1.5–76.0) | 0.94 | |
| Mean dose (Gy) | 18.5 (4.6–49.0) | 14.8 (1.0–66.9) | 0.51 | |
| V35 (%) | 28.1 (2.7–78) | 22.2 (0–78) | 0.81 | |
| V45 (%) | 19.7 (1–74) | 13.0 (1–77) | 0.77 | |
| V55 (%) | 13.5 (0.5–65) | 5.6 (0–75) | 0.84 | |
| V65 (%) | 0 (0–48) | 0 (0–74) | 0.80 | |
| Carotid bulb | ||||
| Mean dose (Gy) | 31.7 (0.6–65) | 5.75 (1–70) | 0.48 | |
| Calcification | No | 1 | 159 | <0.01 |
| Yes | 5 | 57 | ||
Table 4 shows the details of the six patients who developed the event. All patients were male and had a history of smoking and dyslipidemia. In two patients with hypopharyngeal cancer, concurrent chemotherapy and bilateral neck irradiation of the lymph node area were performed. One patient developed TIA symptoms, and three patients developed cerebral infarction (Table 5). One case of cerebral infarction was observed on day 10 after treatment initiation. We recognized it as an event because it was triggered by chemoradiotherapy. Carotid artery stenosis was diagnosed in two patients during the follow-up contrast-enhanced CT; these patients were followed up by a neurosurgeon. They received carotid artery stenting at an appropriate time before symptoms appeared. One patient with TIA symptoms was stented (Fig. 2: Case 1 presentation), and three patients with stroke were treated by medication. The main stenosis area of the carotid artery was confirmed by digital subtraction angiogram examination. The main stenosis area was within the irradiation field in three patients but outside the irradiation field in three patients. Within the median observation period of 72.6 months, two patients were treated for lung cancer, one patient was treated for esophageal cancer, and one patient was treated for liver cancer; there were no cases of recurrence for primary disease.
Table 4.
Clinical characteristics of the six patients who were treated for RCAD
| Case | Age | Gender | Primary | Clinical stage | Smoking status
(pack-year) |
BMI
(kg/m2) |
Complications | Antiplatelet therapy | Carotid Calcifications (on the side where stenosis has occurred) |
| 1 | 67 | Male | Posterior pharyngeal wall | T2N1 | 40 | 23.1 | DM, DL | No | Yes |
| 2 | 69 | Male | Glottis | T1aN0 | 80 | 28.7 | HTN, CVD, DL | Yes | Yes |
| 3 | 72 | Male | Glottis | T1aN0 | 25 | 21.8 | DM, HTN, DL | No | Yes |
| 4 | 77 | Male | Piriform sinus | T2N0 | 75 | 25.1 | DM, HTN, CVD, DL | No | Yes |
| 5 | 70 | Male | Glottis | T1bN0 | 80 | 23.7 | DM, HTN, DL | No | No |
| 6 | 71 | Male | Glottis | T2N0 | 25 | 19.5 | DM, DL | No | Yes |
RCAD: Radiation-induced carotid artery disease
BMI: body mass index
DM: diabetes mellitus
HTN: hypertension
CVD: cardiovascular disease
DL: dyslipidemia
Table 5.
Treatment characteristics and follow-up of the six patients who were treated for RCAD
| Case | Total dose
(Gy) |
Dose/fraction
(Gy) |
Field | Chemotherapy | Location of stenosis | Duration of RT
administration (months) |
Vascular
Symptom |
Treatment | Current
status (months) |
|
| 1 | 60 | 2 | WN | FN | ICA | In-field | 44.9 | TIA | Stenting | NED (120) |
| 2 | 63 | 2.25 | Box | None | ICA | Out-of-field | 49.7 | None | Stenting | NED (103) |
| 3 | 63 | 2.25 | Box | None | ICA | Out-of-field | 78.3 | None | Stenting | NED (78.3) |
| 4 | 70 | 2 | WN | CDDP | ICA | In-field | 62.7 | Stroke | Medication | NED (66.9) |
| 5 | 63 | 2.25 | Box | None | CCA | In-field | 53.6 | Stroke | Medication | NED (55.7) |
| 6 | 70 | 2 | Box | None | ICA | Out-of-field | 0.3 | Stroke | Medication | NED (35) |
RCAD: Radiation-induced carotid artery disease
BMI: body mass index
WN: whole neck field including subclinical lymph node
Box; parallel-opposed fields with individualized wedge-filtered technique, 5 × 5 or 6 × 6 cm; FN, combined with the use of nedaplatin and 5-fluorouracil; CDDP, cisplatin
ICA: internal carotid artery
CCA: common carotid artery
RT: radiotherapy
TIA: transient ischemic attack
NED: no evidence of primary disease
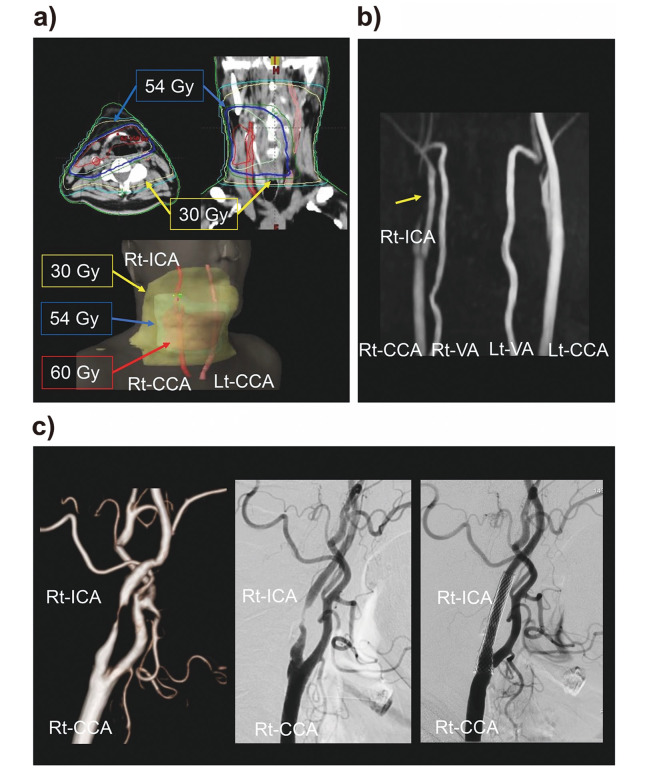
Fig. 2.
Case 1 presentation
Fig. 2a: Dose distribution
Fig. 2b: magnetic resonance angiography images 3 years after radiotherapy,
Fig. 2c: computed tomography angiography and digital subtraction angiography images before and after carotid stenting.
Abbreviations: Rt-ICA, right internal carotid artery; Rt-CCA, right common carotid artery; Rt-VA, right vertebral artery; Lt, Left
DISCUSSION
This study investigated the risk of events due to carotid artery stenosis in laryngeal and hypopharyngeal cancer patients treated with radiotherapy. Six of the 111 patients required treatment for atherothrombotic TIA or stroke and/or CAS, and the cumulative incidence rate was 5.5% within 5 years. The risk of vascular events due to carotid artery stenosis is high, particularly in patients with dyslipidemia, diabetes, and carotid calcifications. In two patients who underwent stenting, the stroke specialist intervened in the early timing and performed carotid stenting before symptoms appeared. Thus, the risk of fatal cerebral infarction might have been avoided, regardless of whether the carotid stenosis was within or outside of the radiation field.
A Dutch study by Dorresteijn et al compared stroke rates in patients who were treated for head and neck cancer with British patients reported in the Oxfordshire Community Stroke Project. They used the stroke rates from the British report as age- and sex-matched controls. In this study, 14 out of 367 patients (3.8%) had a stroke compared with 2.5 (0.7%).17 A study by Haynes et al compared stroke rates in American patients treated for head and neck cancer with the expected incidence based on population data from Stockholm. In this study, 20 out of 413 patients (4.8%) experienced a stroke. After matching for age, gender, and smoking status, this study showed an RR of 2.09 for stroke in patients with head and neck cancer who were treated with radiotherapy (p = 0.0007).18 Plummer et al reviewed 15 pieces of literature on the risk of TIA and stroke after head and/or neck radiotherapy and concluded that these risks may be at least doubled with head and neck radiation treatment.19 There are not yet any reports about the risk for a large series of Japanese patients. In our study, the incidence rate for vascular events was 6 of 111 (5.4%), which is similar to the results of these reports.
In this study, the risk of events was high, particularly in patients with dyslipidemia and diabetes. Cheng et al showed that age, smoking, and heart disease were significant risk factors for severe post-radiotherapy carotid stenosis.6 Silverberg suggested that significant differences in age, CVD incidence, dyslipidemia, and the angiographic incidence of atherosclerosis justify the description of RCAD as a clinical entity.20 Lam et al found that age and irradiation were important factors in carotid stenosis development.21 Chang et al also showed a positive correlation between the total plaque score, radiotherapy use, radiation dose, length of time after radiotherapy, hyperlipidemia, and age. In addition to the lifestyle risk factors, radiotherapy has multiple effects on the progression of atherosclerosis after irradiation.22
Several reports describing RCAD were characterized in the following studies.20,23-26 First, symptoms from radiotherapy appear several decades after treatment. Second, the location of stenosis is in-field of the radiotherapy. Third, it occurs in the common carotid artery. Fourth, it can occur even at doses as low as 35 Gy. In this study, carotid artery or carotid bulb dosimetric parameters did not show significant correlation on the univariate analysis, and carotid artery stenosis was also observed outside the radiation field, and one case experienced cerebral infarction during radiation treatment. Although these cases might not have been directly affected by radiotherapy, they were definitely diagnosed with atherothrombotic stroke. In addition, they were considered to be vascular events by the stroke specialist. Previously, only a few studies have conducted a detailed examination of the stenosis area of the carotid artery and the dose distribution of the radiation field.25 Atherosclerotic cerebral infarction/TIA should always be considered by physicians for patients with head and neck cancer, regardless of the radiation field and onset time.
The exact pathogenesis of RCAD remains unclear. According to the report by Lam et al, the common carotid artery or internal carotid artery were the most commonly involved stenotic sites, followed by the external carotid artery and the vertebral artery, although the most common sites of atherosclerotic carotid stenosis are the carotid bifurcation and the proximal segment of the internal carotid artery.27 Stenosis of the internal carotid artery was observed in five cases of this study. The primary mechanism may be a dysfunction of the endothelial cells, which are highly sensitive to radiation exposure.8 The increase in carotid intimal–medial thickness observed in irradiated carotid arteries also suggested the potential mechanism, indicating that radiation may accelerate the progression of atherosclerosis. Carotid artery stenosis and carotid intimal–medial thickness could be a surrogate endpoint of stroke/TIA in RCAD.28 According to a prospective study, the increase in carotid intimal–medial thickness is dominantly observed even after 1 year of radiotherapy.29 In this study, carotid calcification was one of the risk factors of RCAD. Although several studies have suggested that carotid artery calcification might be an indicative of carotid stenosis and stroke risk, it still remains controversial.30-32
Furthermore, regular checkups for the presence of carotid artery disease after radiotherapy are recommended. However, according to a questionnaire survey of the present status of doctors’ awareness of RCAD in Japan, only 9.4% of institutions perform regular examinations.9 Most laryngeal and hypopharyngeal cancer patients are smokers, and in patients with diabetes and/or dyslipidemia, the risk of carotid artery stenosis is high regardless of the radiation field size. Rosenthal et al reported that intensity-modulated radiation therapy significantly reduces the radiation dose to the carotid artery for T1-2 glottic cancer treatment.33 Although intensity-modulated radiation therapy should be considered, regular and long-term carotid artery evaluation is necessary especially for younger patients.
In the current study, carotid artery stenosis was identified by regular contrast-enhanced CT in two cases. A stroke specialist followed up those patients and performed stent placement despite the patients being asymptomatic. Although CT imaging is useful for diagnosing cancer recurrence, it sometimes obscures the condition of the carotid artery due to dental crown artifacts. Follow-up of the carotid artery is required for a long time even after 5 years of follow-up for recurrence. Non-invasive and simple ultrasonography is recommended.9 Magnetic resonance angiography should also be performed on a routine basis for cases in which ultrasonography is not suitable, for example, because the neck tissue hardens. Cancers that develop secondarily to head and neck cancers affect survival and reportedly have greater risks for survival than stroke.34 In our study, four of the six patients who experienced a vascular event have been treated for secondary cancer. There are no clear guidelines on how often and how long follow-up for RCAD should be conducted.
Although our study has some limitations such as the retrospective analysis, small number of cases, and short observation period, the results highlight the risks after radiotherapy in Japanese laryngeal and hypopharyngeal cancer patients.
In conclusion, laryngeal and hypopharyngeal cancer patients with complications who were treated by radiotherapy are at a high risk of carotid artery stenosis, and long-term and regular evaluation of the carotid artery is desirable. Early intervention by a stroke specialist may avoid the risk of fatal stroke.
FUNDING
None.
ACKNOWLEDGEMENTS
The authors would like to thank Enago (www.enago.jp) for the English language review.
CONFLICTS OF INTEREST
None declared.
Abbreviations
- CTV
Clinical target volume
- ICA
Internal carotid artery
- MRA
Magnetic resonance angiography
- RCAD
Radiation-induced carotid artery disease
- TIA
Transient ischemic attack
REFERENCES
- 1.Thomas E, Forbus WD. Irradiation injury to the aorta and the lung. AMA Arch Pathol. 1959;67(3):256–263. [PubMed]
- 2.Steele SR, Martin MJ, Mullenix PS, Crawford JV, Cuadrado DS, Andersen CA. Focused high-risk population screening for carotid arterial stenosis after radiation therapy for head and neck cancer. Am J Surg. 2004;187(5):594–578. [DOI] [PubMed]
- 3.Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21(4):637–676. [DOI] [PubMed]
- 4.Kimura K, Kazui S, Minematsu K, Yamaguchi T, Japan Multicenter Stroke Investigator’s Collaboration. Analysis of 16,922 patients with acute ischemic stroke and transient ischemic attack in Japan. A hospital-based prospective registration study. Cerebrovasc Dis. 2004;18(1):47–56. [DOI] [PubMed]
- 5.Kimura K, Kazui S, Minematsu K, Yamaguchi T, Japan Multicenter Stroke Investigators’ Collaboration (J-MUSIC). Hospital-based prospective registration of acute ischemic stroke and transient ischemic attack in Japan. J Stroke Cerebrovasc Dis. 2004;13(1):1–11. [DOI] [PubMed]
- 6.Cheng SW, Ting AC, Lam LK, Wei WI. Carotid stenosis after radiotherapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126(4):517–521. [DOI] [PubMed]
- 7.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American heart association/American stroke association council on stroke - co-sponsored by the council on cardiovascular radiology and intervention. The American academy of neurology affirms the value of this guideline. Circulation. 2006;113(10):e409–e449. [PubMed]
- 8.Xu J, Cao Y. Radiation-induced carotid artery stenosis: A comprehensive review of the literature. Interv Neurol. 2014;2(4):183–192. [DOI] [PMC free article] [PubMed]
- 9.Taguchi Y, Takashima S, Tanaka K. Present status of doctors’ awareness for radiation-induced carotid artery disease in Japan. Japanese J Strok. 2013;35(4):269–273.
- 10.Otani K, Haruyama R, Gilmour S. Prevalence and correlates of hypertension among Japanese adults, 1975 to 2010. Int J Environ Res Public Health. 2018;15(8):1645. [DOI] [PMC free article] [PubMed]
- 11.Haneda M, Noda M, Origasa H, et al. Japanese Clinical Practice Guideline for Diabetes 2016. J Diabetes Investig. 2018;9(3):657–697. [DOI] [PMC free article] [PubMed]
- 12.Teramoto T, Sasaki J, Ishibashi S, et al. Diagnostic criteria for dyslipidemia. J Atheroscler Thromb. 2013;20(8):655–660. [DOI] [PubMed]
- 13.Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25(9):846–984. [DOI] [PMC free article] [PubMed]
- 14.Teramoto T, Sasaki J, Ishibashi S, et al. Other high-risk conditions. J Atheroscler Thromb. 2013;20(11):785–789. [DOI] [PubMed]
- 15.Reamy BV, Williams PM, Kuckel DP. Prevention of cardiovascular disease. Prim Care. 2018;45(1):25–44. [DOI] [PubMed]
- 16.Carpenter D, Mowery G, Broadwater G, et al. The risk of carotid stenosis in head and neck cancer patients after radiation therapy. Oral Oncol. 2018;80(5):9–15. [DOI] [PMC free article] [PubMed]
- 17.Dorresteijn LDA, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20(1):282–288. [DOI] [PubMed]
- 18.Haynes JC, Machtay M, Weber RS, Weinstein GS, Chalian AA, Rosenthal DI. Relative risk of stroke in head and neck carcinoma patients treated with external cervical irradiation. Laryngoscope. 2002;112(10):1883–1887. [DOI] [PubMed]
- 19.Plummer C, Henderson RD, O’Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: A review. Stroke. 2011;42(9):2410–2418. [DOI] [PubMed]
- 20.Silverberg GD, Britt RH, Goffinet DR. Radiation-induced carotid artery disease. Cancer. 1978;41(1):130–137. [DOI] [PubMed]
- 21.Lam WW, Leung SF, So NM, et al. Incidence of carotid stenosis in nasopharyngeal carcinoma patients after radiotherapy. Cancer. 2001;92(9):2357–2363. [DOI] [PubMed]
- 22.Chang YJ, Chank TC, Lee, TH, Ryu SJ. Predictors of carotid artery stenosis after radiotherapy for head nad neck cancers. J Vasc Surg. 2009;50(2):280–285. [DOI] [PubMed]
- 23.Horimoto M, Kodama N, Takamatsu H. Bilateral internal carotid artery disease secondary to cervical radiation. A case report. Angiology. 1996;47(6):609–613. [DOI] [PubMed]
- 24.Loftus CM, Biller J, Hart MN, Cornell SH, Hiratzka LF. Management of radiation-induced accelerated carotid atherosclerosis. Arch Neurol. 1987;44(7):711–714. [DOI] [PubMed]
- 25.Nishi K, Uno M, Ueda S, et al. Carotid endarterectomy for radiation-induced carotid artery stenosis. Neurol Med Chir (Tokyo). 1997;37(11):844–848. [DOI] [PubMed]
- 26.Martin JD, Buckley AR, Graeb D, Walman B, Salvian A, Hay JH. Carotid artery stenosis in asymptomatic patients who have received unilateral head-and-neck irradiation. Int J Radiat Oncol Biol Phys. 2005;63(4):1197–1205. [DOI] [PubMed]
- 27.Lam WW, Liu KH, Leung SF, et al. Sonographic characterisation of radiation-induced carotid artery stenosis. Cerebrovasc Dis. 2002;13(3):168–173. [DOI] [PubMed]
- 28.Gujral DM, Chahal N, Senior R, Harrington KJ , Nutting CM. Radiation-induced carotid artery atherosclerosis. Radiother Oncol. 2014;110(1):31–38. [DOI] [PubMed]
- 29.Muzaffar K, Collins SL, Labropoulos N, Baker WH. A prospective study of the effects of irradiation on the carotid artery. Laryngoscope. 2000;110(11):1811–1814. [DOI] [PubMed]
- 30.Hollander M, Hak AE, Koudstaal PJ, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003;34(10):2367–2372. [DOI] [PubMed]
- 31.Constantine S, Roach D, Liberali S, et al. Carotid artery calcification on orthopantomogram (CACOstudy) – is it indicative od carotid stenosis? Aust Dent J. 2019;64(1):4–10. [DOI] [PubMed]
- 32.Fanning NF, Walters TD, Fox AJ, Symons SP. Association between calcification of the cervical carotid artery bifurcation and white matter ischemia. AJNR Am J Neuroradiol. 2006;27(2):378–383. [PMC free article] [PubMed]
- 33.Rosenthal D, Fuller C, Barker J, et al. Simple carotid-sparing Intensity-modulated radiotherapy technique and preliminary experience for T1-2 glottic cancer. Int J Radiat Oncol Biol Phys. 2010;77(2):455–461. [DOI] [PMC free article] [PubMed]
- 34.Marcel M, Leys D, Mounier-Vehier D, et al. Clinical outcome in patients with high-grade internal carotid artery stenosis after irradiation. Neurology. 2005;65(6):959–961. [DOI] [PubMed]