ABSTRACT
The standard chemotherapy regimen for unresectable or recurrent biliary tract cancer is gemcitabine combined with cisplatin (GC). To evaluate the effectiveness and safety of chemotherapy in patients with unresectable or recurrent biliary tract cancer in the real world, we retrospectively analyzed the clinical courses of patients who underwent chemotherapy with GC from January 2015 to November 2019. Forty-eight patients underwent the GC regimen. One patient (2.1%) achieved a complete response, seven patients (14.6%) achieved a partial response, 26 patients (54.2) achieved stable disease, 11 patients (22.9%) achieved progressive disease, and 3 patients (6.3%) were not evaluable. The overall response rate was 16.7%. The median overall survival was 14.2 months (95% CI: 13.8–14.6), and the median progression-free survival was 7.7 months (95% CI: 4.2–11.2). Thirty-nine patients (81.3%) experienced grade 3 or higher severe adverse events as follows: 54.2% experienced neutropenia, 20.8% experienced anemia, 12.5% experienced thrombocytopenia and 20.8% experienced biliary tract infection. As a second-line chemotherapy, S-1 was used in seventeen patients, and stable disease was achieved in three patients (17.6%). The GC regimen for biliary tract cancer is effective and safe for unresectable or recurrent biliary tract cancer in routine clinical practice.
Key Words: biliary tract cancer, cisplatin, gemcitabine, S-1, cholangiocarcinoma
INTRODUCTION
According to Vital Statistics Japan (Ministry of Health, Labour and Welfare), the number of deaths due to biliary tract cancer was 18,179 in 2017, making this cancer the sixth leading cause of cancer death in Japan. Standard chemotherapy for unresectable or recurrent biliary tract cancer is gemcitabine combined with cisplatin (GC) because the superiority of GC in terms of survival compared with gemcitabine monotherapy has been shown in a phase III trial, the ABC-02 trial.1 A phase II trial (BT22) with the same regimen was performed in Japan, and the result was consistent with that of the ABC-02 trial.2,3 Although the GC regimen is used worldwide, including in Japan, its feasibility in routine clinical practice is not fully elucidated. Nagoya University Hospital is a high-volume center for biliary tract cancer4 where many patients receive treatment, including surgery and chemotherapy. The purpose of the present study is to evaluate the effectiveness and safety of chemotherapy in patients with unresectable or recurrent biliary tract cancer in the real world.
In the present study, we analyzed the clinical courses of patients with biliary tract cancer who underwent chemotherapy with the GC regimen in our institute.
PATIENTS AND METHODS
Patients
The subjects were 48 patients who received the GC regimen as first-line chemotherapy for unresectable or recurrent biliary tract cancer (intrahepatic or extrahepatic cholangiocarcinoma, gallbladder cancer, or ampullary carcinoma) in Nagoya University Hospital from January 2015 to November 2019. The eligibility criteria are the minimum requirements for chemotherapy, including unresectable or recurrent biliary tract cancer, an Eastern Cooperative Oncology Group (ECOG) performance status 0–2, neutrophil count ≥ 1,000/μL, platelet count ≥ 100,000/μL and no prior chemotherapy, except for adjuvant chemotherapy. Patients with obstructive jaundice needed biliary drainage to decrease total bilirubin to ≤ 3 mg/dL. This study does not include patients who did not receive the GC regimen according to the physicians’ choice; these patients received gemcitabine alone or the best supportive care alone. This study was approved by the review boards of Nagoya University Hospital.
Treatment
The treatment regimen, which consisted of 25 mg/m2 cisplatin followed by 1,000 mg/m2 gemcitabine on days 1 and 8, was administered every three weeks. To prevent chemotherapy-induced nausea and vomiting, a 5-HT3 receptor antagonist and corticosteroid were routinely used. The chemotherapeutic agents, antiemetics and hydration were unified as a registered regimen. When the neutrophil count decreased to <500/μL, granulocyte colony-stimulating factor was administered. If the neutrophil count was <1,000/μL or the platelet count was < 100,000/μL on the scheduled date of administration, the dose was postponed. The treatment was continued until the disease progressed, the patients experienced intolerable side effects, or curative resection was expected.
Evaluation of efficacy and safety
Radiological tumor assessments were conducted approximately every 2 months using computed tomography or magnetic resonance imaging (MRI) in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, and the best response during the treatment was evaluated. Overall survival (OS) and progression-free survival (PFS) were evaluated from the initiation of the GC regimen, and the endpoints were analyzed using Kaplan–Meier curves and the log-rank test. Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE v5.0). All statistical analyses were performed using IBM SPSS 26 (IBM Japan, Ltd., Tokyo, Japan).
RESULTS
Patient characteristics
The patient characteristics are shown in Table 1. The tumor sites of 23 patients (47.9%) were the hilar bile duct. Thirty-two patients (66.7%) had disease recurrence after surgery. Biliary drainage was performed at the initiation of the GC regimen in nine patients (18.8%). Thirty-four patients (70.8%) started the GC regimen during hospitalization.
Table 1.
Patient characteristics
| Characteristics | n | ( | % | ) | ||
| Sex | ||||||
| Male | 36 | ( | 75.0 | ) | ||
| Female | 12 | ( | 25.0 | ) | ||
| Age, years | ||||||
| Median | 67 | |||||
| Range | 20–85 | |||||
| PS | ||||||
| 0 | 39 | ( | 81.3 | ) | ||
| 1 | 8 | ( | 16.7 | ) | ||
| 2 | 1 | ( | 2.1 | ) | ||
| Primary tumor sites | ||||||
| Intrahepatic | 7 | ( | 14.6 | ) | ||
| Hilar | 23 | ( | 47.9 | ) | ||
| Gallbladder | 6 | ( | 12.5 | ) | ||
| Extrahepatic | 7 | ( | 14.6 | ) | ||
| Ampulla | 5 | ( | 10.4 | ) | ||
| Histological type | ||||||
| Adenocarcinoma | 39 | ( | 81.3 | ) | ||
| Adenosquamous carcinoma | 1 | ( | 2.1 | ) | ||
| Poorly differentiated carcinoma | 1 | ( | 2.1 | ) | ||
| Unknown | 7 | ( | 14.6 | ) | ||
| Extent of disease | ||||||
| Initial onset | 16 | ( | 33.3 | ) | ||
| Locally advanced | 8 | ( | 16.7 | ) | ||
| Metastatic | 8 | ( | 16.7 | ) | ||
| Recurrent | 32 | ( | 66.7 | ) | ||
| Previous therapy | ||||||
| Surgery | 32 | ( | 66.7 | ) | ||
| Adjuvant chemotherapy | 11 | ( | 22.9 | ) | ||
| Surgery and radiotherapy | 3 | ( | 6.3 | ) | ||
| Biliary drainage | ||||||
| Internal | 5 | ( | 10.4 | ) | ||
| External | 4 | ( | 8.3 | ) | ||
| Hospitalized or outpatient at the initiation of GC | ||||||
| Hospitalized | 14 | ( | 20.2 | ) | ||
| Outpatient | 34 | ( | 70.8 | ) | ||
Treatment
One course of the GC regimen includes two doses of GC, and the median times of the doses was 12.5 (range 2–39), which is equivalent to 6.25 courses. The median duration from the initiation of GC to the last dose was 7.4 months (95% confidence interval [CI]: 5.3–9.5), and the maximum duration was 36.1 months. Administration was delayed and/or the dose was reduced due to toxicities in 39 patients (81.3%).
Effectiveness and safety of the GC regimen
The overall responses are shown in Table 2. The response rate was 16.7%, and the disease control rate was 70.1%. The median OS and PFS were 14.2 months (95% CI: 13.8–14.6) and 7.7 months (95% CI: 4.2–11.2), respectively (Figure 1). The median OS of the patients with initial onset (n=16) was 14.3 months (95% CI: 13.6–15.0), and that of the patients with recurrence (n=32) was 14.2 months (95% CI: 7.7–20.6).
Table 2.
Overall responses
| Response | n | ( | % | ) |
| Complete response (CR) | 1 | ( | 2.1 | ) |
| Partial response (PR) | 7 | ( | 14.6 | ) |
| Stable disease (SD) | 26 | ( | 54.2 | ) |
| Progressive disease (PD) | 11 | ( | 22.9 | ) |
| Not evaluable (NE) | 3 | ( | 6.3 | ) |
Fig. 1.
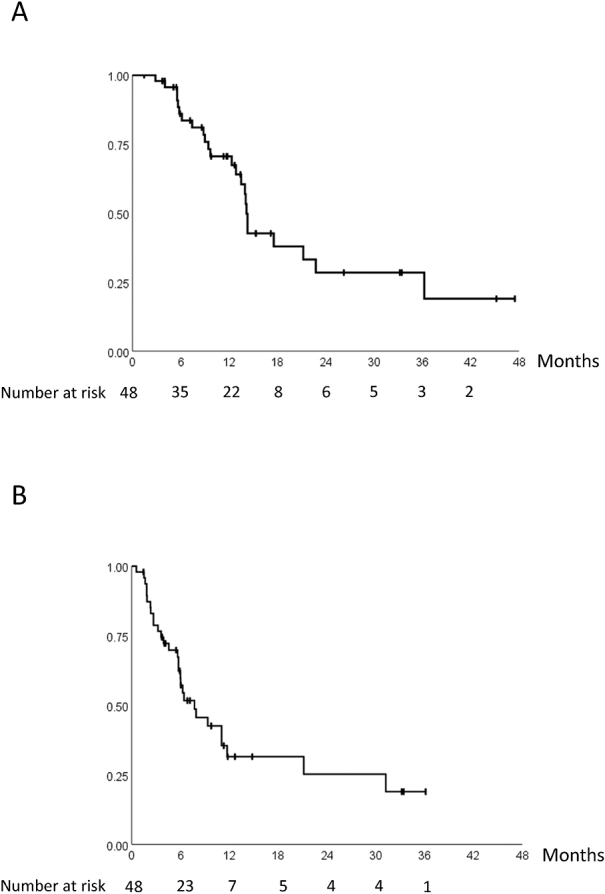
Kaplan–Meier curve of overall survival and progression-free survival
Fig. 1A:Overall survival
Fig. 1B: Progression-free survival
Thirty-nine (81.3%) patients experienced grade ≥ 3 adverse events. The adverse events are summarized in Table 3. Biliary enzyme elevation grade ≥3 was observed in 11 (22.9%) patients. Biliary tract infection was observed in 11 (22.9%) patients, and 10 (20.8%) of them were grade ≥3. Seven patients (14.6%) needed emergency admission due to biliary tract infection and/or obstructive jaundice.
Table 3.
Summary of maximum toxicity grades
| Grade 3 (%) | Grade 4 (%) | All grades (%) | |
| Hematological | |||
| Leukopenia | 27.1 | 6.3 | 85.4 |
| Neutropenia | 33.3 | 20.8 | 81.3 |
| Anemia | 20.8 | 0 | 97.9 |
| Thrombocytopenia | 10.4 | 2.1 | 83.3 |
| Febrile neutropenia | 2.1 | 0 | 2.1 |
| AST increased | 6.3 | 0 | 72.9 |
| ALT increased | 2.1 | 0 | 62.5 |
| ALP increased | 6.3 | 0 | 62.5 |
| GGT increased | 18.8 | 4.2 | 62.5 |
| Bilirubin increased | 0 | 0 | 31.3 |
| Creatinine increased | 2.1 | 0 | 6.3 |
| Nonhematological | |||
| Fatigue | 2.1 | 0 | 52.1 |
| Anorexia | 0 | 0 | 52.1 |
| Nausea | 0 | 0 | 43.8 |
| Fever | 0 | 0 | 27.1 |
| Constipation | 0 | 0 | 25.0 |
| Biliary tract infection | 20.8 | 0 | 22.9 |
| Edema | 0 | 0 | 16.7 |
| Oral stomatitis | 0 | 0 | 14.6 |
| Vomiting | 0 | 0 | 12.5 |
| Diarrhea | 0 | 0 | 10.4 |
| Dysgeusia | 0 | 0 | 10.4 |
| Skin rash | 2.1 | 0 | 4.2 |
| Peripheral sensory neuropathy | 0 | 0 | 4.2 |
| Pneumonitis | 2.1 | 0 | 2.1 |
| Hearing impairment | 0 | 0 | 2.1 |
| Hiccups | 0 | 0 | 2.1 |
| Alopecia | 0 | 0 | 2.1 |
Subsequent treatment
Twenty-eight patients received any subsequent treatment after cessation of the GC regimen. Seventeen patients who had disease progression received oral fluoropyrimidine S-1 (tegafur, gimeracil and oteracil combination), and three patients who discontinued the GC regimen due to adverse events received gemcitabine alone as a second-line chemotherapy. Two underwent curative surgery. One patient who had poorly differentiated carcinoma with neuroendocrine differentiation underwent chemotherapy with carboplatin plus etoposide. Five patients received subsequent treatment in other hospitals, and the details are unknown.
Regarding S-1 as second-line chemotherapy, three patients of 17 (17.6%) achieved stable disease (SD), and no patients achieved complete response (CR) or partial response (PR). The median OS and PFS were 5.9 months (95% CI: 5.2–6.6) and 2.3 months (95% CI: 1.5–3.1), respectively.
Case presentations
A case achieved a complete response with GC. A 74-year-old woman with hilar cholangiocarcinoma who underwent surgery five years ago was diagnosed with liver metastases (Figure 2). After three courses of GC, the liver metastases were undetectable on MRI. Four months after the cessation of GC, the liver metastases reappeared. Gemcitabine alone was administered, and the patient is alive three years and nine months after the initiation of GC.
Fig. 2.
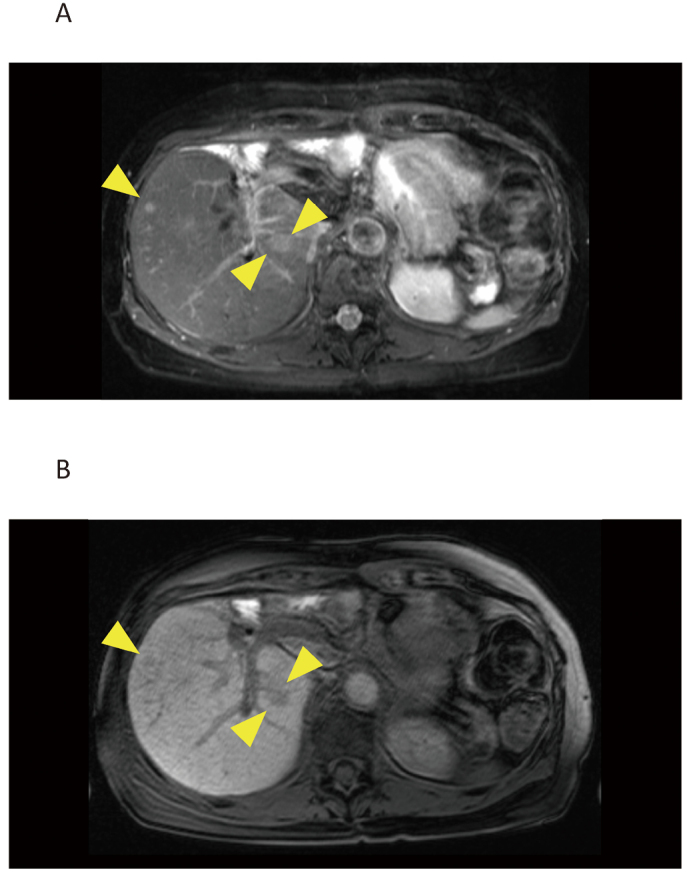
MRI of liver metastases in a 74-year-old woman who received surgery for hilar cholangiocarcinoma five years ago
Fig. 2A: A fat suppression T2-weighted image
Fig. 2B: A gadolinium-EOB-DTPA-enhanced T1-weighted image in the hepatocyte phase
Two patients received surgery after GC. A 53-year-old man with gallbladder cancer with lymph node metastasis received three courses (six doses) of GC and achieved tumor shrinkage that was evaluated SD with RECIST. The patient underwent surgery, and the pathological findings revealed tumor necrosis of approximately 50%. Two and a half years after surgery, the patient was alive without recurrence. The other patient was a 49-year-old man with hilar cholangiocarcinoma. This patient received 23 doses (equivalent to 11.5 courses) of GC and achieved a PR. The patient underwent surgery, and the pathological findings revealed necrosis of approximately 90% of the tumor. Five months after surgery, the patient was alive without recurrence.
DISCUSSION
Because cisplatin is usually administered during hospitalization in Japan and hydration is necessary to prevent cisplatin-induced nephrotoxicity, short hydration has recently been developed for outpatient chemotherapy. The GC regimen in our hospital was administered during hospitalization at the beginning and was able to shift to outpatient chemotherapy because a large volume of hydration was not necessary with a smaller dose of cisplatin than the standard dose used for other cancers.
In the ABC-02 trial1 and BT22 trial,2 the response rate (RR) was 32.1% and 19.5%, OS was 11.7 and 11.2 months, and PFS was 8.0 and 5.8 months, respectively. In the present study, the RR, OS, and PFS were 16.7%, 14.2 months, and 7.7 months, respectively, which were comparable to those in the pivotal clinical trials. In addition, in a retrospective analysis by Kim et al in Korea,5 the RR, OS, and PFS were 12.9%, 10.4 months, and 5.2 months, respectively.
The patients’ characteristics and inclusion criteria are similar to those in previous studies, including the ABC-02 trial1 and BT22 trial,2 except for the high rate of patients with recurrent disease after surgery in the present study. The rate of patients with previous curative surgery in the GC arm in the ABC-02 trial and BT22 trial was 18.2% and 26.8%, respectively, whereas in the present study, two-thirds of the patients experienced recurrence after surgery. Therefore, we compared OS between patients with initial onset and those with recurrence, and the survival duration was not significantly different. In addition, two patients in the present study received conversion surgery after the GC regimen. Performing conversion surgery is expected to improve prognosis.
In the ABC-02 trial,1 BT22 trial2 and the present study, the incidence of neutropenia ≥ grade 3 was 25.3%, 56.1% and 54.2% and that of thrombocytopenia ≥ grade 3 was 8.6%, 39% and 12.5%, respectively. The profile of hematological toxicity in the present study was similar to that in previous clinical trials. The frequency of severe nonhematological toxicities in the present study was low, which was similar to that in previous trials.
Recently, in the FUGA-BT (JCOG1113) randomized phase III clinical trial, the combination of gemcitabine plus S-1 was proven to be noninferior to the GC regimen in terms of OS.6 Furthermore, a phase III study (KHBO1401-MITSUBA) demonstrated the significant survival benefits of the combination of gemcitabine, cisplatin and S-1 (GCS) over GC treatment and indicated that GCS could be a new standard treatment.7
With regard to second-line chemotherapy, Kobayashi et al retrospectively analyzed 55 patients who received S-1.8 The overall response rate was 4.0%, and the disease control rate was 38.0%. The OS and PFS were 6.0 months and 2.3 months, respectively. According to a systematic review of second-line chemotherapy by Lamarca et al,9 the RR, OS and PFS were 7.7%, 7.2 months and 3.2 months, respectively. Forano et al reported that the RR with second-line therapy was 3.4%, with a median OS and PFS of 6.6 months and 3.0 months, respectively,10 and concluded that current second-line treatment has limited value.
In a preliminary report of the randomized ABC-06 trial,11 which directly compared short-term infusional fluorouracil plus leucovorin and oxaliplatin (FOLFOX) with active symptom control alone in patients progressing after a first-line GC regimen, FOLFOX improved OS at 6 months (61% versus 36%) and at 12 months (26% versus 11%). FOLFOX could be a standard of care in second-line chemotherapy.
CONCLUSION
In conclusion, the GC regimen is effective and safe for patients with unresectable or recurrent biliary tract cancer in the real world.
CONFLICTS OF INTEREST
Osamu Maeda has received research funding from Eli Lilly Japan K.K. Yuichi Ando has received research funding from Yakult Honsya Co., Ltd.; Mochida Pharmaceutical Co., Ltd.; Taiho Pharmaceutical Co., Ltd.; Eli Lilly Japan K.K.; and Nippon Kayaku Co., Ltd.
REFERENCES
- 1.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. [DOI] [PubMed]
- 2.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469–474. [DOI] [PMC free article] [PubMed]
- 3.Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25(2):391–398. [DOI] [PubMed]
- 4.Chaudhary RJ, Higuchi R, Nagino M, et al. Survey of preoperative management protocol for perihilar cholangiocarcinoma at 10 Japanese high-volume centers with a combined experience of 2,778 cases. J Hepatobiliary Pancreat Sci. 2019;26(11):490–502. [DOI] [PubMed]
- 5.Kim BJ, Hyung J, Yoo C, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol. 2017;80(1):209–215. [DOI] [PubMed]
- 6.Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) Randomized Phase III Clinical Trial. Ann Oncol. 2019;30(12):1950–1958. [DOI] [PubMed]
- 7.Sakai D, Kanai M, Kobayashi S, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). Ann Oncol. 2018;29(Supplement 8):viii205. [DOI] [PMC free article] [PubMed]
- 8.Kobayashi S, Ueno M, Ohkawa S, et al. A retrospective study of S-1 monotherapy as second-line treatment for patients with advanced biliary tract cancer. Jpn J Clin Oncol. 2012;42(9):800–806. [DOI] [PubMed]
- 9.Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328–2338. [DOI] [PubMed]
- 10.Fornaro L, Vivaldi C, Cereda S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res. 2015;34:156. [DOI] [PMC free article] [PubMed]
- 11.Lamarca A, Palmer DH, Wasan HS, et al. ABC-06 vertical bar A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC plus mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. Journal of Clinical Oncology. 2019;37(15_suppl):4003.


