Abstract
Integrase strand transfer inhibitors (INSTIs) are important components of drug formulations that are used to treat people living with HIV, and second-generation INSTIs dolutegravir and bictegravir impart high barriers to the development of drug resistance. Reported 10 years ago, X-ray crystal structures of prototype foamy virus (PFV) intasome complexes explained how INSTIs bind integrase to inhibit strand transfer activity and provided initial glimpses into mechanisms of drug resistance. However, comparatively low sequence identity between PFV and HIV-1 integrases limited the depth of information that could be gleaned from the surrogate model system. Recent high resolution structures of HIV-1 intasomes as well as intasomes from a closely related strain of simian immunodeficiency virus (SIV), which were determined using single particle cryogenic electron microscopy, have overcome this limitation. The new structures reveal the binding modes of several advanced INSTI compounds to the HIV/SIV integrase active site and critically inform the structural basis of drug resistance. These findings will help guide the continued development of this important class of antiretroviral therapeutics.
Keywords: HIV/AIDS, integrase, integrase strand transfer inhibitor, DNA integration, single particle cryo-EM
Introduction
HIV-1 infects activated CD4+ T cells to expand rapidly within the first few weeks of virus exposure. Unchecked, virus replication in the majority of cases outpaces host cellular and humoral immune responses, eventually decimating CD4+ T cell homeostasis and culminating in death due to AIDS-related complications [1]. The advent of combinatorial antiretroviral therapy (ART), which has in large part shifted HIV/AIDS from an almost certain death sentence to a chronically manageable disease, is a milestone of modern day infectious disease research and development. Small molecule inhibitors of the HIV-1 enzymes reverse transcriptase (RT), protease (PR), and integrase (IN) comprise the majority of today’s ART formulations [2]. After the virus enters a susceptible cell, RT converts the two copies of positive-strand genomic RNA into linear double-strand viral DNA (vDNA), which IN subsequently inserts into host chromosomal DNA. IN harbors two distinct magnesium-dependent activities: 3′-processing and strand transfer. IN initially processes the vDNA ends adjacent to conserved CA dinucleotides, yielding recessed CAOH-3′ termini. During strand transfer, IN employs the 3′-OH groups as nucleophiles to cut target DNA (tDNA) in staggered fashion, joining the vDNA CA-3′ ends to host DNA 5′-phosphates via transesterification. The resulting hemi-integrant, with unjoined vDNA 5′ ends, is converted by host DNA repair machinery into stably integrated provirus. Readers may consult [3] and [4] for recent in-depth reviews of retroviral integration.
IN activity requires a pair of magnesium ions bound to the enzyme active site, which are coordinated by the carboxylate side chains of invariant Asp and Glu residues that collectively comprise the DDE catalytic triad. The magnesium cofactors vastly enhance the reactivities of chemical nucleophiles (water in the case of 3′-processing and CAOH for strand transfer) and destabilize scissile phosphodiester bonds [5]. Clinical compounds selectively inhibit strand transfer activity, and thus are known as IN strand transfer inhibitors (INSTIs). Resistance to first generation INSTI compounds raltegravir (RAL) and elvitegravir (EVG) emerged relatively quickly [6, 7]. Second-generation compounds dolutegravir (DTG) and bictegravir (BIC) impart comparatively high barriers to the development of drug resistance [8, 9], and are accordingly preferred components of current frontline and drug-switch ART formulations [10]. Other advanced compounds, not yet approved for clinical applications, similar to DTG and BIC display drug resistance profiles that are superior to the first-generation compounds [11–16].
INSTIs do not appreciably bind apo HIV-1 IN protein and instead target the intasome nucleoprotein complex composed of IN and vDNA after completion of 3′-processing [17]. Over the past decade, intasomes from several retroviruses have been characterized by X-ray crystallography [18, 19] or single-particle cryogenic electron microscopy (cryo-EM) [20–22]. Due to favorable biochemical properties of its IN [18, 23], pioneering studies with prototype foamy virus (PFV, a spumavirus that is only distantly related to its lentiviral cousins) provided initial snapshots of retroviral intasome structure and function. In particular, crystals of PFV intasome-drug complexes revealed essential mechanistic details of INSTI action.
INSTIs comprise two commonalities among various pharmacophores, including a central metal-chelating core of coplanar heteroatoms and a terminal halobenzyl group connected to the core via a flexible linker (Fig. 1A) [3, 4]. The compounds display broad antiretroviral activities, inhibiting infection by a variety of retroviruses including spumavirus [23, 24], which validated the PFV model for initial INSTI studies. The structures confirmed, as anticipated from prior solution measurements [25], that INSTI heteroatoms engage the active site magnesium ions. The structures additionally revealed stacking contacts of the halobenzyl group with the penultimate dC nucleotide of the processed CAOH end of vDNA, replacing the adenine base of the terminal dAOH nucleotide and deactivating the intasome by dislocating the 3′-OH nucleophile required for strand transfer activity [18].
Figure 1.
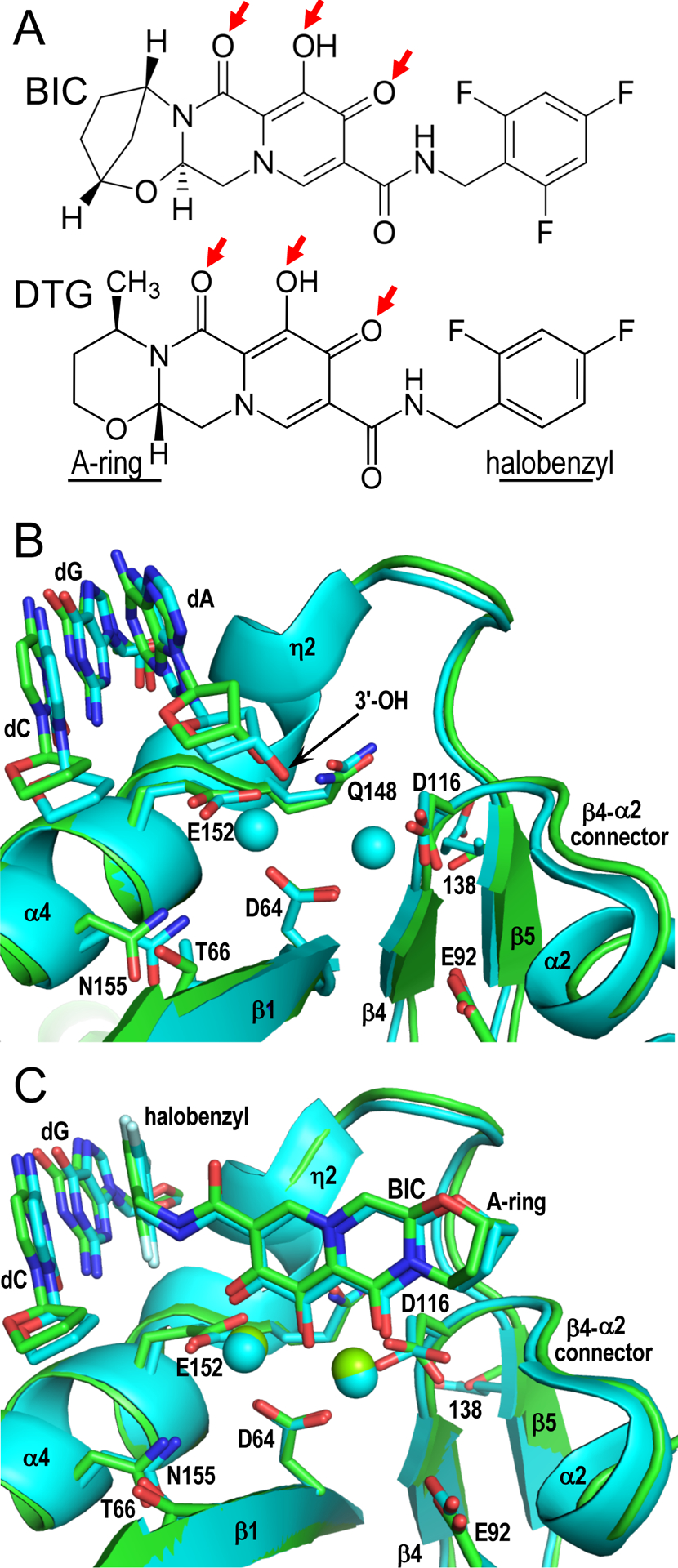
Primate lentiviral intasome structures and INSTI binding. (A) Chemical structures of licensed second-generation INSTIs BIC and DTG highlight heteroatoms that interact with IN active site metal ions (red arrows) as well as divergent A-rings and halobenzyl groups. (B) SIVrcm (green; Protein Data Base [PDB] code 6RWL) and HIV-1 (cyan; PDB code 6PUT) intasomes were superimposed via Cα positions of active site residues Asp64, Asp116, and Glu152 (respective side chains shown as sticks). Residues that when changed confer INSTI resistance and terminal CA nucleotides of the vDNA plus-strand as well as the dG of the opposing strand that pairs with dC are also shown as sticks; position 138 is Glu in HIV-1 IN and Thr in SIVrcm IN. Red and blue, oxygen and nitrogen, respectively. Cyan spheres, calcium atoms in the HIV-1 model. Local resolutions of the active site regions of the SIVrcm and HIV-1 intasomes reached 2.8 Å and 2.7 Å, respectively. (C) Superposition of BIC-bound SIVrcm (PDB code 6RWM) and HIV-1 (PDB code 6PUW) intasomes, oriented as in panel B. Displaced 3′-dAOH nucleotides were omitted for clarity. Green and cyan spheres, magnesium ions in respective SIVrcm and HIV-1 structures; white, fluorine atoms of INSTI halobenzyl groups. Other labeling same as in panel B; local resolutions of these SIVrcm and HIV-1 active site regions were 2.4 Å and 2.7 Å, respectively. PyMOL [40] was used to generate panel B and C structures.
Despite these insights, a key limitation for understanding mechanisms of drug resistance and furthering INSTI development programs was the limited amino acid identity between PFV and HIV-1 INs, which is ~15% [26]. Importantly, INSTIs select for resistance in HIV-1 IN at residues that for the most part differ in PFV IN [16, 18]. The field accordingly required intasome structures based on IN from HIV-1 or a highly-related lentiviral surrogate. Herein we review the highlights of recent single-particle cryo-EM structures of primate lentiviral intasomes in both unbound and INSTI-bound states determined to near atomic resolution [16, 26].
Results and Discussion
Cryo-EM structures of primate lentiviral intasomes and INSTI binding
Using a hyperactive Sso7d-IN fusion construct, Lyumkis and colleagues described the first structure of a primate lentiviral intasome, which was of the HIV-1 strand transfer complex that forms upon completion of the strand transfer reaction [22]. INSTIs compete with tDNA for binding to HIV-1 IN [17]. Because the strand transfer complex contains tDNA and the Sso7d-IN protein harbored an active site mutation to preclude IN catalysis, this particular intasome construct was incompatible with INSTI studies.
Because the recent papers utilized nucleoprotein complexes with processed vDNA substrates, both approaches were amenable for INSTI binding studies [16, 26]. While Lyumkis and colleagues employed Sso7d-HIV-1 IN fusion protein with an intact catalytic triad [16], we screened ~20 wild-type primate lentiviral IN proteins for biochemical and biophysical properties conducive for intasome structural biology, which led us to red-capped mangabey simian immunodeficiency virus (SIVrcm) [26]. The 5′-half of the HIV-1 genome, which encodes IN, descended from SIVrcm [27]; SIVrcm and HIV-1 IN share 75% sequence identity and 88% homology considering substitutions of chemically related amino acid residues. The active site regions of the SIVrcm and HIV-1 intasomes in the absence (Fig. 1B) and presence (Fig. 1C) of bound inhibitors are accordingly highly similar. As established from the pioneering work with PFV intasomes [18, 28], INSTI binding dramatically disrupted vDNA end structure, disabling the strand transfer capabilities of the IN enzymes [16, 26].
Insights into INSTI drug resistance mechanisms
The recent papers reported several INSTI-bound intasome structures [16, 26]. In addition to BIC (Fig. 1C), Lyumkis and colleagues determined structures of three experimental naphthyridine-based INSTIs in complex with the HIV-1 intasome [16], while we refined DTG bound to the SIVrcm intasome [26]. To gain insight into the mechanisms of drug resistance, we additionally determined the structure of BIC bound to IN mutant Q148H/G140S intasomes. The double mutation, which arose initially in response to RAL treatment [29], was subsequently shown to confer significant (>150-fold) cross resistance to EVG, yet overall milder, approximate 2 to 10-fold, resistance to DTG and BIC [7, 30–33].
The IN active site region is bracketed on one side by the invariant CAOH dinucleotide of vDNA and, on the opposing side, by the region of IN that links secondary structural elements β4 and α2 (referred to as the β4-α2 connector; Fig. 1B). Crucially, the second-generation INSTIs more fully span the active site than do first-generation compounds. In particular, the A-rings of DTG and BIC make multiple close contacts with the β4-α2 connector [26]. To assess the role of these contacts in INSTI potency and drug resistance, we used an analog of DTG lacking the A-ring. Similar concentrations of DTG and its truncated analog inhibited wild-type HIV-1 infection. While Q148H/G140S changes rendered the virus 4-fold resistant to DTG, they conferred much greater 80-fold resistance to the truncated analog. Moreover, loss of the A-ring dramatically increased the dissociation rate of the compound from Q148H/G140S IN-vDNA complexes [26]. These experimental observations established the importance of the A-ring to the superior activities of the second-generation INSTIs to inhibit Q148H/G140S HIV-1.
Detailed analysis of the elements that contribute to magnesium ion coordination at the wild-type versus Q148H/G140S IN active sites yielded additional unexpected insights into the mechanism of INSTI resistance. One water molecule in the active site (designated W5) bridges Gln148 and DDE triad residues Asp116 and Glu152 via stable hydrogen bond interactions, and thus takes part in the secondary coordination shell of the magnesium atoms (Fig. 2A). Crucially, the bulkier His148 sidechain in the INSTI-resistant mutant precludes W5 positioning. Partial disruption of the secondary coordination shell is expected to destabilize the metal ion cluster and accordingly the affinity of INSTI binding, which strongly depends on optimized interactions with the magnesium ions [25]. This affect is exacerbated by the associated G140S change; the mutant Ser140 sidechain forms a stable hydrogen bond with His148 Nδ1, thereby increasing the acidity of His148 Nε2 (Fig. 2B, δ+) and thus enhancing metal ion cluster destabilization. Canonical enzymes, including retroviral RT and PR, must turnover to catalyze multiple successive chemical reactions. By contrast, IN promotes one pair of consecutive reactions, which are not rate limiting steps in the viral replication cycle [34]. Therefore, a subtle detuning of the IN active site may not appreciably affect viral fitness, yet still allow the pathogen to reduce its susceptibility to the INSTIs by exploiting the exquisite selectivity of magnesium ions for specific coordination geometry and transesterification chemistry.
Figure 2.
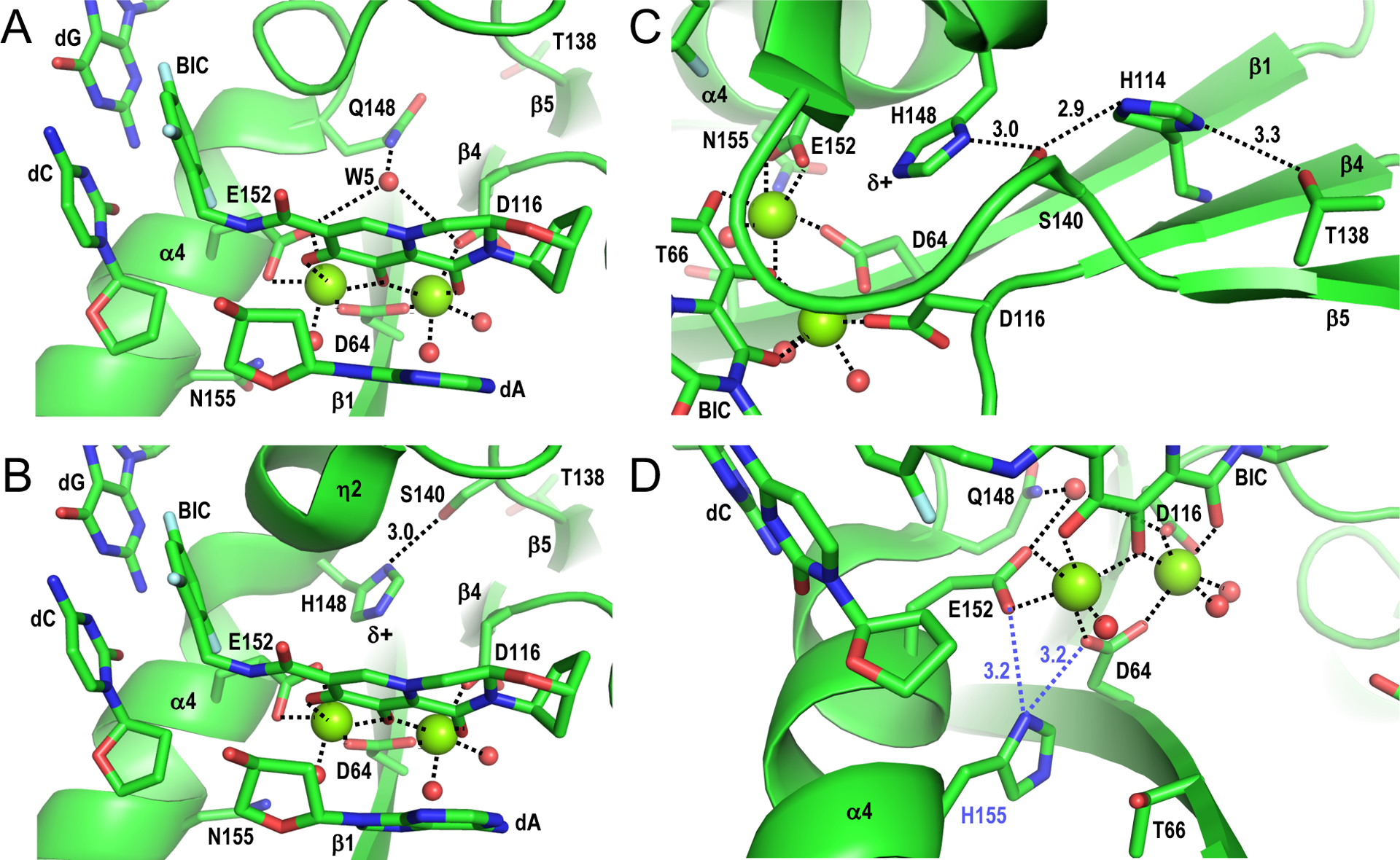
Structural basis of INSTI drug resistance. (A) Structure of SIVrcm intasome in complex with BIC (PDB code 6RWM) oriented to highlight magnesium ion interactions at the IN active site (dashed lines). Red spheres, water molecules; other labeling is the same as in Figure 1C. (B) Structure of the SIVrcm Q148H/G140S intasome bound to BIC (PDB code 6RWO; active site resolution 2.8 Å), oriented as in panel A. The distance between Ser140 Oγ and His148 Nδ1 is given in Å. (C) Same as in panel B, oriented to highlight the hydrogen bonding network involving IN residues Thr138, His114, Ser140, and His148. (D) Hypothetical interaction between mutant His155 side chain with SIVrcm IN active site. Asn155 within the SIVrcm intasome-BIC complex (PDB code 6RWM) was altered to His using the Wizard option of PyMOL [40], selecting the rotamer that most closely mimicked the rotameric configuration of Asn155. In this configuration, His155 (blue label) could potentially disrupt metal ion cluster stabilization by interacting with the carboxylate sidechain of active site residue Asp64 or Glu152 (dashed blue lines). Panel A-D structures were generated using PyMOL [40].
Further analyses of the IN mutant-BIC complex yielded insights into long-range effects of IN substitutions associated with INSTI resistance. As one example, E138T augments the resistance of IN mutant viruses with preexisting Q148H/G140S changes [35, 36]. Fortuitously, position 138 in SIVrcm IN is occupied by Thr (Fig. 1B). Accordingly, substituting Glu for Thr138 in Q148H/G140S SIVrcm conferred levels of BIC and DTG sensitivities similar to those observed for Q148H/G140S HIV-1 [26]. The SIVrcm cryo-EM structure revealed that Thr138 participates in a chain of hydrogen bond interactions extending to His148 Nε2, exacerbating the effect of the primary INSTI resistance mutations [26] (Fig. 2C). L/I74M and T97A mutations are also known to enhance the resistance of Q148H/G140S mutant virus [32]. SIVrcm IN residues Ile74 and Thr97 are in close proximity to Phe121, which makes direct van der Waals contacts with active site carboxylate residue Asp116 [26]. Molecular dynamics confirmed that Ile74 and Thr97 amino acid substitutions affect the conformation of the Phe121 side chain, which is expected to convey the effect onto the metal ion cluster. And indeed, I74M/T97A significantly enhanced SIVrcm Q148H/G140S INSTI resistance [26]. Based on these analyses, we concluded that destabilization of the metal ion chelating cluster at the IN active site underlies the general mechanism of INSTI resistance. Speculatively, this reasoning may extend to some other primary INSTI resistance changes, such as N155H [6, 7, 37]. Asn155 positions nearby DDE residues Asp64 and Glu152 (Fig. 1B, C; Fig. 2A–C), and the mutant His residue can be modeled to within 3.2 Å of these sidechains (Fig. 2D).
Prospects for improved INSTI designs
As initially gleaned from PFV intasome crystal structures, INSTIs mimic critical aspects of the strand transfer reaction. Superposing intasome structures overlays the RAL heteroatoms with the 3′-oxygen of the cleaved vDNA end and the scissile phosphodiester bond in tDNA, revealing the INSTI as a true substrate mimic [5]. Mimicry of strand transfer substrates has since expanded to include more extensive aspects of the tDNA. Substrate envelope is defined as the volume encompassed by an enzyme active site with bound substrate. If inhibitors can be made to maximally fit the substrate envelope while minimally exceeding its limit, selection of drug resistance could potentially be minimized. Initially espoused for design of HIV-1 PR inhibitors [38], the concept has since been leveraged to advance INSTI development [12, 14, 15]. The recent cryo-EM structures revealed that naphthyridine-based INSTIs 4c, 4d, and 4f adopted conformations with the HIV-1 intasome that differed in part from respective interactions initially seen in PFV intasome crystal structures, presumably due to the extent of amino acid differences between the two enzymes [16]. Extensive overlap between the 6-hexanol group of advanced INSTI 4d with tDNA in the HIV-1 intasome may explain the beneficial resistance profile of this compound versus other second-generation INSTIs in vitro [16] (Fig. 3).
Figure 3.
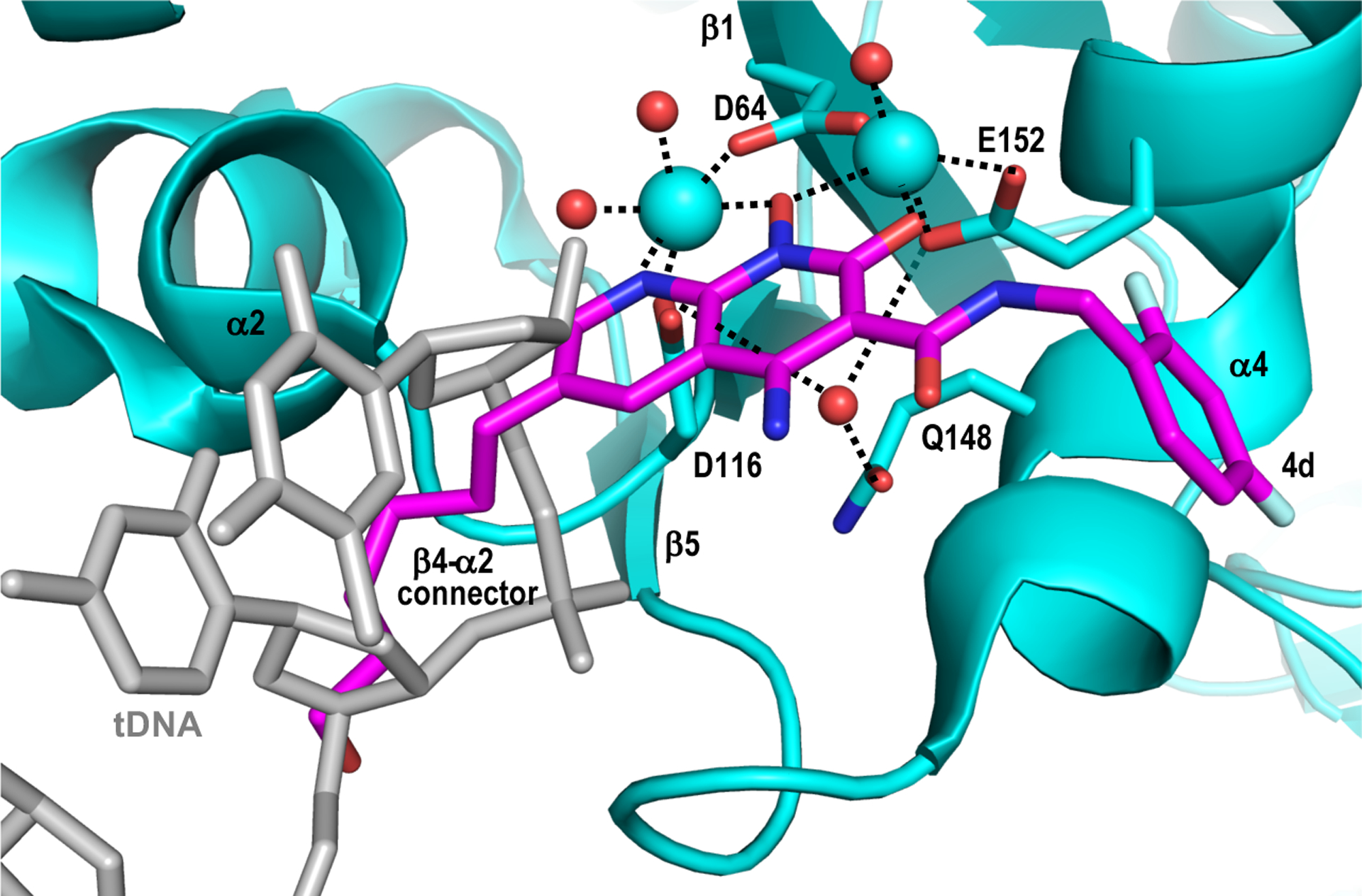
INSTI obstruction of tDNA positioning for strand transfer. The structures of the HIV-1 strand transfer complex (PDB code 5U1C, gray) [22] and 4d-bound intasome (PDB code 6PUY, cyan with magenta INSTI) were superimposed via Cα positions of active site residues Asp64, Asp116, and Glu152 using PyMOL [40]. Note the extensive overlap between the hexanol substituent of compound 4d with tDNA. Other labeling as in Figure 1B and 1C.
The comparatively high resolutions of the cryo-EM structures (Fig. 1) revealed the positions of several solvent molecules in the vicinity of the IN active sites [16, 26] (Fig. 2 and 3). Compounds with improved drug resistance profiles may be envisaged via extending second-generation chemical scaffolds to fill the space occupied by solvent and potentially increase the strength of INSTI-IN active site interactions.
Conclusions
We should fully expect the recent structures of the HIV-1 and SIVrcm intasomes to aid ongoing INSTI development programs. One of the more striking conclusions from this work is the dual edged sword played by the magnesium ion cofactors at the IN active site. Providing an optimal landing platform for the drugs, the INSTI-metal interaction at the same time affords numerous opportunities for IN to engender drug resistance. HIV-1 takes advantage of various points of IN-INSTI contact, some direct but most indirect [26, 39], to detune the stability of the metal chelating cluster, and in doing so lessen the drug’s grip on the active site. As long as sufficient levels of IN 3′-processing and strand transfer activities survive the detuning process, the virus wins out and replication in the presence of the drug can proceed. Engineering additional points of INSTI-IN contacts, while at the same time maximizing substrate envelope utilization, may yield new compounds of superior clinical efficacy.
Acknowledgements
This work was supported by US National Institutes of Health grant R01AI070042.
Abbreviations
- ART
antiretroviral therapy
- BIC
bictegravir
- cryo-EM
cryogenic electron microscopy
- EVG
elvitegravir
- IN
integrase
- INSTI
integrase strand transfer inhibitor
- PDB
Protein Data Bank
- PFV
prototype foamy virus
- PR
protease
- RAL
raltegravir
- SIVrcm
red-capped mangabey simian immunodeficiency virus
- tDNA
target DNA
- vDNA
viral DNA
Footnotes
Conflict of interest
ANE has received fees from ViiV Healthcare Co. over the past 12 months. PC has no potential conflicts of interest to declare.
References
- 1.Coffin J & Swanstrom R (2013) HIV Pathogenesis: Dynamics and Genetics of Viral Populations and Infected Cells. Cold Spring Harb Perspect Med 3, a012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossetti B, Montagnani F & De Luca A (2018) Current and emerging two-drug approaches for HIV-1 therapy in ART-naïve and ART-experienced, virologically suppressed patients. Expert Opin Pharmacother 19, 713–738. [DOI] [PubMed] [Google Scholar]
- 3.Lesbats P, Engelman AN & Cherepanov P (2016) Retroviral DNA Integration. Chem Rev 116, 12730–12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelman AN (2019) Multifaceted HIV integrase functionalities and therapeutic strategies for their inhibition. J Biol Chem 294, 15137–15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hare S, Maertens GN & Cherepanov P (2012) 3′-Processing and strand transfer catalysed by retroviral integrase in crystallo. EMBO J 31, 3020–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper DA, Steigbigel RT, Gatell JM, Rockstroh JK, Katlama C, Yeni P, Lazzarin A, Clotet B, Kumar PN, Eron JE, Schechter M, Markowitz M, Loutfy MR, Lennox JL, Zhao J, Chen J, Ryan DM, Rhodes RR, Killar JA, Gilde LR, Strohmaier KM, Meibohm AR, Miller MD, Hazuda DJ, Nessly ML, DiNubile MJ, Isaacs RD, Teppler H, Nguyen B-Y & the BENCHMRK Study Teams (2008) Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med 359, 355–365. [DOI] [PubMed] [Google Scholar]
- 7.McColl DJ & Chen X (2010) Strand transfer inhibitors of HIV-1 integrase: Bringing IN a new era of antiretroviral therapy. Antiviral Res 85, 101–118. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A & Fujiwara T (2011) In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 55, 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsiang M, Jones GS, Goldsmith J, Mulato A, Hansen D, Kan E, Tsai L, Bam RA, Stepan G, Stray KM, Niedziela-Majka A, Yant SR, Yu H, Kukolj G, Cihlar T, Lazerwith SE, White KL & Jin H (2016) Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother 60, 7086–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddell J,IV (2018) 2018 IAS-USA Recommendations for the Use of Antiretroviral Therapy for HIV: Building on Decades of Progress. JAMA 320, 347–349. [DOI] [PubMed] [Google Scholar]
- 11.Raheem IT, Walji AM, Klein D, Sanders JM, Powell DA, Abeywickrema P, Barbe G, Bennet A, Clas SD, Dubost D, Embrey M, Grobler J, Hafey MJ, Hartingh TJ, Hazuda DJ, Miller MD, Moore KP, Pajkovic N, Patel S, Rada V, Rearden P, Schreier JD, Sisko J, Steele TG, Truchon J-F, Wai J, Xu M & Coleman PJ (2015) Discovery of 2-pyridinone aminals: A prodrug strategy to advance a second generation of HIV-1 integrase strand transfer inhibitors. J Med Chem 58, 8154–8165. [DOI] [PubMed] [Google Scholar]
- 12.Zhao XZ, Smith SJ, Maskell DP, Metifiot M, Pye VE, Fesen K, Marchand C, Pommier Y, Cherepanov P, Hughes SH & Burke TR Jr. (2016) HIV-1 integrase strand transfer inhibitors with reduced susceptibility to drug resistant mutant integrases. ACS Chem Biol 11, 1074–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreier JD, Embrey MW, Raheem IT, Barbe G, Campeau L-C, Dubost D, McCabe Dunn J, Grobler J, Hartingh TJ, Hazuda DJ, Klein D, Miller MD, Moore KP, Nguyen N, Pajkovic N, Powell DA, Rada V, Sanders JM, Sisko J, Steele TG, Wai J, Walji A, Xu M & Coleman PJ (2017) Discovery and optimization of 2-pyridinone aminal integrase strand transfer inhibitors for the treatment of HIV. Bioorg Med Chem Lett 27, 2038–2046. [DOI] [PubMed] [Google Scholar]
- 14.Zhao XZ, Smith SJ, Maskell DP, Métifiot M, Pye VE, Fesen K, Marchand C, Pommier Y, Cherepanov P, Hughes SH & Burke TR (2017) Structure-guided optimization of HIV integrase strand transfer inhibitors. J Med Chem 60, 7315–7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SJ, Zhao XZ, Burke TR & Hughes SH (2018) HIV-1 integrase inhibitors that are broadly effective against drug-resistant mutants. Antimicrob Agents Chemother 62, e01035–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passos DO, Li M, Jóźwik IK, Zhao XZ, Santos-Martins D, Yang R, Smith SJ, Jeon Y, Forli S, Hughes SH, Burke TR, Craigie R & Lyumkis D (2020) Structural basis for strand-transfer inhibitor binding to HIV intasomes. Science 367, 810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espeseth AS, Felock P, Wolfe A, Witmer M, Grobler J, Anthony N, Egbertson M, Melamed JY, Young S, Hamill T, Cole JL & Hazuda DJ (2000) HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc Natl Acad Sci USA 97, 11244–11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hare S, Gupta SS, Valkov E, Engelman A & Cherepanov P (2010) Retroviral intasome assembly and inhibition of DNA strand transfer. Nature 464, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Z, Shi K, Banerjee S, Pandey KK, Bera S, Grandgenett DP & Aihara H (2016) Crystal structure of the Rous sarcoma virus intasome. Nature 530, 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballandras-Colas A, Brown M, Cook NJ, Dewdney TG, Demeler B, Cherepanov P, Lyumkis D & Engelman AN (2016) Cryo-EM reveals a novel octameric integrase structure for betaretroviral intasome function. Nature 530, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballandras-Colas A, Maskell DP, Serrao E, Locke J, Swuec P, Jónsson SR, Kotecha A, Cook NJ, Pye VE, Taylor IA, Andrésdóttir V, Engelman AN, Costa A & Cherepanov P (2017) A supramolecular assembly mediates lentiviral DNA integration. Science 355, 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passos DO, Li M, Yang R, Rebensburg SV, Ghirlando R, Jeon Y, Shkriabai N, Kvaratskhelia M, Craigie R & Lyumkis D (2017) Cryo-EM structures and atomic model of the HIV-1 strand transfer complex intasome. Science 355, 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valkov E, Gupta SS, Hare S, Helander A, Roversi P, McClure M & Cherepanov P (2009) Functional and structural characterization of the integrase from the prototype foamy virus. Nucleic Acids Res 37, 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh Y, Matreyek KA & Engelman A (2011) Differential sensitivities of retroviruses to integrase strand transfer inhibitors. J Virol 85, 3677–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grobler JA, Stillmock K, Hu B, Witmer M, Felock P, Espeseth AS, Wolfe A, Egbertson M, Bourgeois M, Melamed J, Wai JS, Young S, Vacca J & Hazuda DJ (2002) Diketo acid inhibitor mechanism and HIV-1 integrase: Implications for metal binding in the active site of phosphotransferase enzymes. Proc Natl Acad Sci USA 99, 6661–6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook NJ, Li W, Berta D, Badaoui M, Ballandras-Colas A, Nans A, Kotecha A, Rosta E, Engelman AN & Cherepanov P (2020) Structural basis of second-generation HIV integrase inhibitor action and viral resistance. Science 367, 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp PM, Shaw GM & Hahn BH (2005) Simian immunodeficiency vrus infection of chimpanzees. J Virol 79, 3891–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hare S, Vos AM, Clayton RF, Thuring JW, Cummings MD & Cherepanov P (2010) Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc Natl Acad Sci USA 107, 20057–20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delelis O, Malet I, Na L, Tchertanov L, Calvez V, Marcelin A-G, Subra F, Deprez E & Mouscadet J-F (2009) The G140S mutation in HIV integrases from raltegravir-resistant patients rescues catalytic defect due to the resistance Q148H mutation. Nucleic Acids Res 37, 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassounah SA, Alikhani A, Oliveira M, Bharaj S, Ibanescu R-I, Osman N, Xu H-T, Brenner BG, Mesplède T & Wainberg MA (2017) Antiviral activity of bictegravir and cabotegravir against integrase inhibitor-resistant SIVmac239 and HIV-1. Antimicrob Agents Chemother 61, e01695–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SJ, Zhao XZ, Burke TR & Hughes SH (2018) Efficacies of cabotegravir and bictegravir against drug-resistant HIV-1 integrase mutants. Retrovirology 15, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang WW, Cheung PK, Oliveira N, Robbins MA, Harrigan PR & Shahid A (2018) Accumulation of multiple mutations in vivo confers cross-resistance to new and existing integrase inhibitors. J Infect Dis 218, 1773–1776. [DOI] [PubMed] [Google Scholar]
- 33.Margot NA, Ram RR, White KL, Abram ME & Callebaut C (2019) Antiviral activity of HIV-1 integrase strand-transfer inhibitors against mutants with integrase resistance-associated mutations and their frequency in treatment-naïve individuals. J Med Virol 91, 2188–2194. [DOI] [PubMed] [Google Scholar]
- 34.Engelman A, Liu Y, Chen H, Farzan M & Dyda F (1997) Structure-based mutagenesis of the catalytic domain of human immunodeficiency virus type 1 integrase. J Virol 71, 3507–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafer RW (2006) Rationale and uses of a public HIV drug-resistance database. J Infect Dis 194, S51–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naeger LK, Harrington PT,K & Deming D (2016) Effect of dolutegravir functional monotherapy on HIV-1 virological response in integrase strand transfer inhibitor resistant patients. Antivir Ther 21, 481–488. [DOI] [PubMed] [Google Scholar]
- 37.Malet I, Ambrosio FA, Subra F, Herrmann B, Leh H, Bouger M-C, Artese A, Katlama C, Talarico C, Romeo I, Alcaro S, Costa G, Deprez E, Calvez V, Marcelin A-G & Delelis O (2018) Pathway involving the N155H mutation in HIV-1 integrase leads to dolutegravir resistance. J Antimicrob Chemother 73, 1158–1166. [DOI] [PubMed] [Google Scholar]
- 38.King NM, Prabu-Jeyabalan M, Nalivaika EA & Schiffer CA (2004) Combating susceptibility to drug resistance: Lessons from HIV-1 protease. Chem Biol 11, 1333–1338. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan L, Li X, Naraharisetty HL, Hare S, Cherepanov P & Engelman A (2010) Structure-based modeling of the functional HIV-1 intasome and its inhibition. Proc Natl Acad Sci USA 107, 15010–15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLano WL (2002) Pymol: An open-source molecular graphics tool. CCP4 Newsletter Protein Crystallograph 40, 82–92. [Google Scholar]


