Abstract
Background:
Black women have greater than a three-fold risk of pregnancy-associated death compared to White women; cardiomyopathy is a leading cause of maternal mortality.
Objectives:
This study examined racial disparities in health outcomes among women with peripartum cardiomyopathy.
Study Design:
Retrospective cohort of women with peripartum cardiomyopathy per the National Heart, Lung, and Blood Institute definition from January 2000–November 2017 from a single referral center. Selected health outcomes among Black and White women were compared; primary outcome was ejection fraction at diagnosis. Secondary outcomes included cardiovascular outcomes, markers of maternal morbidity, resource utilization, and subsequent pregnancy outcomes.
Results:
Ninety-five women met inclusion criteria: 48% Black, 52% White. Nearly all peripartum cardiomyopathy diagnoses were postpartum (95.4% Black, 93% White, p=0.11). Ejection fraction at diagnosis was not different between Black and White women (26.8% ± 12.5 vs 28.7% ± 9.9, p=0.41). Though non-significant, fewer Black women had myocardial recovery to EF ≥ 55% (35% vs 53%, p = 0.07); however, 11 (24%) of Black women vs 1 (2%) White woman had an ejection fraction ≤ 35% at 6 – 12 months postpartum (p < 0.01). More Black women underwent implantable cardioverter defibrillator placement: n = 15 (33%) vs n = 7 (14%), p=0.03. Eight women (8.4%) died in the study period, not different by race (p = 0.48). Black women had higher rates of healthcare utilization. In the subsequent pregnancy, Black women had a lower initial ejection fraction (40% vs 55%, p = 0.007) and were less likely to recover postpartum (37.5% vs 55%, p = 0.02).
Conclusions:
Black and White women have similar mean ejection fraction at diagnosis of peripartum cardiomyopathy, but Black women have more severe left ventricular systolic dysfunction leading to worse outcomes, increased resource use, and lower ejection fraction entering the subsequent pregnancy.
Keywords: maternal morbidity, maternal mortality, peripartum cardiomyopathy, racial disparities
Introduction
Black women in the United States are more than three times more likely to die from pregnancy-related complications compared to White women. Recent US maternal mortality data from 13 participating states reported 1,252 Black and 1,385 White women died during pregnancy or up to 1 year postpartum from 2011 – 2015, equating to a pregnancy-related mortality ratio (defined as number of pregnancy-related deaths per 100,000 live births) of 42.8 and 13.0 for Black and White women, respectively.[1] Furthermore, this Centers for Disease Control and Prevention (CDC) data described cardiomyopathy as the leading cause of maternal mortality postpartum, accounting for 24.4% of maternal deaths from 7 – 42 days postpartum and 45% of deaths from 43 – 365 days postpartum.[1]
Other data sources have suggested racial disparities in maternal mortality among women with cardiomyopathy. CDC data from 1991 – 1997 investigating pregnancy-related mortality from cardiomyopathy identified 171 deaths from peripartum cardiomyopathy and demonstrated that Black women had a greater than six-fold increased risk of death compared to White women.[2] A 2002 – 2003 cohort from North Carolina including 78 women found that Black women had nearly a fourfold increased risk of and fatality from cardiomyopathy compared to White women.[3] A study reporting outcomes of women from the University of Southern California and Louisiana State University including 52 Black women with peripartum cardiomyopathy showed an 11.5% versus 4.8% mortality rate among Black versus White women, respectively (p = 0.09).[4] The Investigations of Pregnancy-Associated Cardiomyopathy (IPAC) Study similarly found that Black women had more left ventricular dysfunction at presentation and at 6 and 12 months postpartum. [5]
An association between peripartum cardiomyopathy and hypertensive disorders of pregnancy has been reported.[6] Although the majority of peripartum cardiomyopathy occurs in normotensive women,[7] the prevalence of hypertensive disorders of pregnancy in women with peripartum cardiomyopathy is increased.[8] It is well documented that Black women experience significant disparities in health outcomes.[9, 10, 11, 12, 13, 14, 15] Our objective was to further investigate racial disparities in health outcomes among a cohort of women with peripartum cardiomyopathy (PPCM) at a single tertiary care center in Alabama, hypothesizing that disproportionate morbidity and mortality among Black women with peripartum cardiomyopathy may originate from a lower ejection fraction (EF) at presentation compared to White women.
Materials and Methods
The Institutional Review Board (IRB) at the University of Alabama at Birmingham approved research activities (IRB-170302005) prior to study initiation. A waiver of informed consent was granted; STROBE guidelines were followed.[16] The database query was performed through the Center for Clinical Translational Science (CCTS) Informatics for Integrating Biology and the Bedside (i2b2) program as previously described.[17, 18] We searched our electronic medical record database for patients with ICD 9 and 10 codes for peripartum cardiomyopathy and the following search parameters: age ≥18 years old, female, patient in the Heart Failure Clinic at the University of Alabama at Birmingham (UAB) Hospital, patient on the Heart Failure Hospital Service at UAB Hospital or an obstetric patient with a consultation from the heart failure service from January 2000 – November 2017. The electronic medical records were then reviewed to determine study eligibility. Patients were included if they were White or Black per self-reported race fields in the electronic medical record, managed by UAB cardiology, and had PPCM defined per the National Heart, Lung, and Blood Institute (NHLBI) defined as development of heart failure one month prior to delivery or within five months after delivery in the absence of an identifiable cause or evidence of heart disease prior to last month of pregnancy with concomitant left ventricular systolic dysfunction on echocardiogram.[19]
Data were manually chart-abstracted by one author (EK) and ten percent of the charts were independently validated by a second author (ZW). Demographic and delivery characteristics were obtained including age at diagnosis, marital status, payor source, smoking, parity, hypertensive disorders of pregnancy, gestational age at delivery, mode of delivery, and delivery location. The primary outcome was left ventricular ejection fraction at time of diagnosis as documented on the echocardiogram report signed by the attending cardiologist. Secondary outcomes included any of the following after receiving the NHLBI-classified diagnosis of PPCM: EF < 35% at diagnosis, recovery of EF to ≥ 55% within twelve months postpartum; number of months from diagnosis to recovery for those who recovered; persistent left ventricular systolic dysfunction at 6 – 12 months postpartum stratified as ejection fraction ≤ 45%, ≤ 40%, ≤35%, ≤30%, and ≤25%; extracorporeal membrane oxygenation (ECMO); hemodialysis; continuous renal replacement therapy (CRRT); mechanical ventilation; left-ventricular assist device (LVAD); implantable cardioverter defibrillator (ICD) placement; heart transplantation; death; and duration from diagnosis to death among women who died. Additionally, medication use was abstracted from the chart as a categorical variable; medications of interest included angiotensin-converting enzyme inhibitors, aldosterone receptor antagonists, angiotensin receptor blockers, beta blockers, bromocriptine, calcium channel blockers, digoxin, diuretics, and nitrates. Resource utilization within our hospital system was determined and defined by the number of emergency room visits, inpatient admissions, and intensive care unit (ICU) stays after the hospital admission associated with the PPCM diagnosis. Charts were reviewed for subsequent pregnancies; if documented, the EF in the first and second trimesters were recorded, as well as gestational age at delivery, mode of delivery, and EF postpartum.
Demographic and delivery characteristics between Black and White women were compared using Chi-square or Fisher’s exact test for categorical variables and Student’s t-test or Wilcoxon rank-sum test for continuous variables, as appropriate. All continuously measured outcomes including the primary outcome of EF at diagnosis were compared between Black and White patients with the two-sample t-test or the Wilcoxon rank-sum test where applicable. Missing values were tracked and analyses performed using those with the available information on relevant variables specific for the comparison.
A convenience sample from the i2b2 bioinformatics search was utilized. Given 59,552 births in the state of Alabama in 2003[20] with a similar total of 58,941 in 2017[21] and assuming a similar birth rate during the interim study period, assuming at least one-half of patients with PPCM were referred to the University of Alabama at Birmingham, one of two tertiary referral centers in the state, and a conservative PPCM incidence of 1 in 4,000 live births,[22, 23] we estimated that an approximate sample of 130 women would yield at least 50 Black and 50 White women. This sample size provides 80% power to detect a difference of 0.56 standard deviations in EF at a 0.05 level of significance, and over 95% power to detect a full standard deviation difference in mean EF between groups.
Secondary outcomes (e.g., any recovery to EF > 55%) were compared using the chi-square test of association. Multivariable logistic regression models were used to model odds of these outcomes while adjusting for clinically relevant characteristics. P values less than 0.05 were deemed statistically significant. SAS 9.4 (Cary, North Carolina) was used to analyze data.
Results
The database query identified 140 women with ICD 9 and 10 codes for PPCM; after applying study eligibility criteria 95 women were included in the study – 46 (48%) were Black and 49 (52%) were White (Figure 1). Age was similar between groups, but White women were more likely to be married (66.7% vs 41.3%, p = 0.01) and have private insurance (79.6% vs 34.8%, p< 0.0001) compared to Black women. Mode of delivery was similar between groups, but Black women were delivered approximately 2 weeks before White women: 36.5 ± 3.8 vs 38.5 ± 2.0 weeks gestation (p = 0.02) (Table 1). Nearly all diagnoses were postpartum (vs antepartum): 95.4% Black and 93% White women, p=0.11.
Figure 1. Flow diagram of included patients.
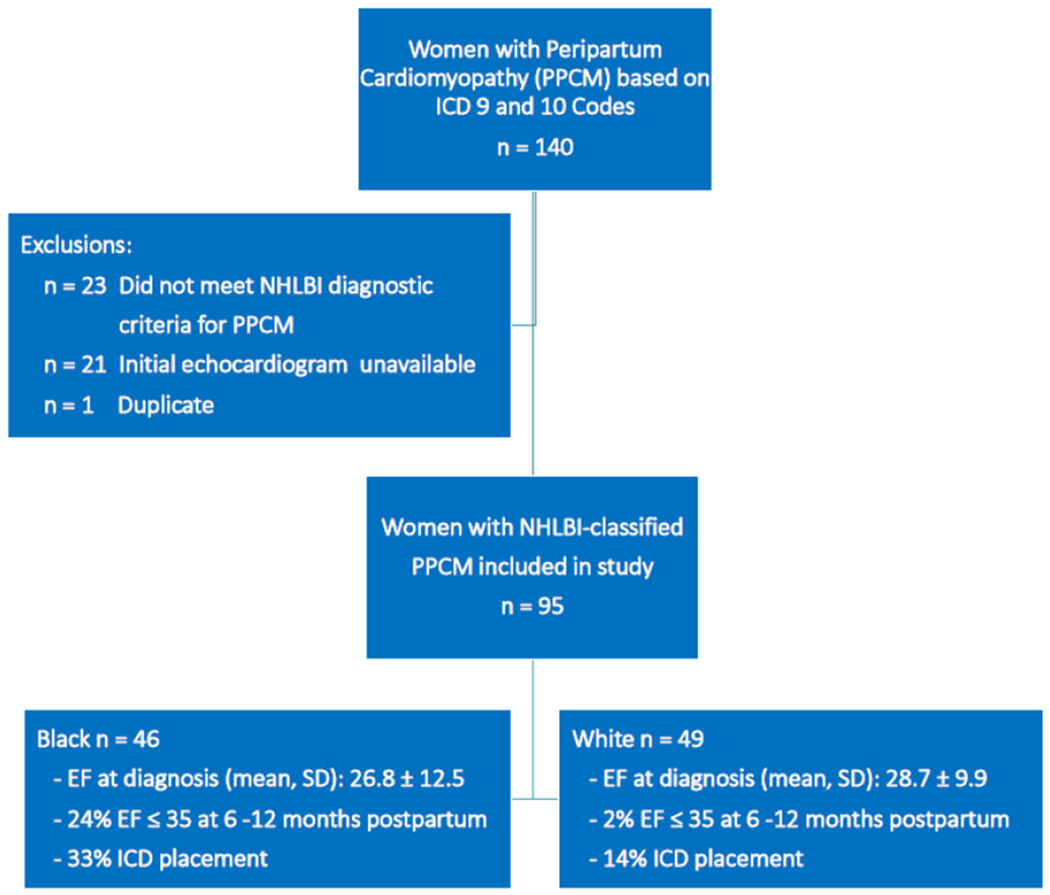
Flow diagram depicting women included in the study after application of inclusion and exclusion criteria. Key study findings include: 1) Black and white women had similar mean EF at diagnosis: 26.8 ± 12.5 vs 28.7 ± 9.9, p=0.41, respectively; 2) 24% of Black women and 2% of White women had persistent left systolic dysfunction ≤ 35 % at 6-12 months postpartum (p = 0.0006), and 3) 33% of Black women had ICD placement compared to 14% of White women (p = 0.03).
PPCM = peripartum cardiomyopathy; NHLBI = National Heart, Lung and Blood Institute; EF = ejection fraction; ICD = implantable cardioverter defibrillator
Table 1:
Demographic and Delivery Characteristics of Women with Peripartum Cardiomyopathy
| Black n = 46 |
White n = 49 |
p value | |
|---|---|---|---|
| Age at PPCM diagnosis (years), mean ± SD* | 29.1 ± 6.6 | 28.6 ± 6.1 | 0.69 |
| Age ≥ 35 years, n (%) | 13 (28.3%) | 7 (14.3%) | 0.10 |
| Married, n (%) * | 19 (41.3%) | 32 (66.7%) | 0.01 |
| Payor source, n (%) | <0.0001 | ||
| Public | 30 (65.2%) | 10 (20.4%) | |
| Private | 16 (34.8%) | 39 (79.6%) | |
| Smoking, n (%) * | 11 (23.9%) | 17 (35.4%) | 0.22 |
| Delivered at UAB † | 9 (20.0%) | 4 (8.5%) | 0.11 |
| Gestational age at delivery (weeks), mean (SD) ‡ | 36.5 ±3.8 | 38.5 ±2.0 | 0.02 |
| Mode of delivery § | 0.98 | ||
| Vaginal | 14 (36.8%) | 15 (36.6%) | |
| Cesarean | 24 (63.2%) | 26 (63.4%) |
value missing for 1 White patient
3 missing: 1 Black and 2 White
30 missing: 11 Black, 19 White
16 missing: 8 Black and 8 White
PPCM = Peripartum Cardiomyopathy; UAB = University of Alabama at Birmingham
The primary outcome, EF at diagnosis, was not significantly different between Black and White women (26.8 ± 12.5 vs 28.7 ± 9.9, p=0.41) (Table 2); 76.1% of Black women versus 69.4% of White women had an EF of < 35% at diagnosis (p = 0.46). Thirty-five percent of Black women compared to 53.1% of White women (p=0.07) recovered to an EF of ≥ 55% within 12 months of PPCM diagnosis. Months from diagnosis to recovery were similar between groups with a median duration of 6 months, p = 0.28. Black women more commonly had persistent left ventricular systolic dysfunction at 6–12 months postpartum compared to white women when stratified at EF ≤ 40% (12 vs 1, p = 0.0002), EF ≤ 35% (11 vs 1, p = 0.0006), and EF ≤ 30% (4 vs 0, p = 0.04). ECMO and CRRT were not utilized for any patient, and there were similar numbers of Black and White women who had hemodialysis, mechanical ventilation, and LVAD use. ICD placement was significantly higher among Black women: 32.6% vs 14.3%, p = 0.03. Two cardiac transplants occurred in the cohort, both in black women (p = 0.23), 4 and 13 years after initial PPCM diagnosis. Five Black and 3 White women died during the study period (p=0.48). Duration from diagnosis to death was 8.8 (0.5 – 14.3) vs 16.5 (0.3 – 24.4) years for Black and White women, respectively (p = 0.57).
Table 2:
Outcomes of Women with NHLBI – Classified Peripartum Cardiomyopathy from 2000–2017
| Black n=46 |
White n=49 |
p value | |
|---|---|---|---|
| Primary Outcome | |||
| EF at diagnosis | 26.8 ± 12.5 | 28.7 ±9.9 | 0.41 |
| Secondary Outcomes | |||
| EF < 35% at diagnosis | 35 (76.1%) | 34 (69.4%) | 0.46 |
| Clinical Outcomes | |||
| Recovered EF to ≥ 55% within 12 months of diagnosis | 16 (34.8%) | 26 (53.1%) | 0.07 |
| If recovered, months from diagnosis to recovery, median (Q1-Q3) | 6 (1-9) | 6 (6-12) | 0.28 |
| Persistent LV dysfunction defined as: † | |||
| EF ≤ 45 at 6 -12 months postpartum | 13 (28%) | 7 (14%) | 0.05 |
| EF ≤ 40 at 6 -12 months postpartum | 12 (26%) | 1 (2%) | 0.0002 |
| EF ≤35 at 6-12 months postpartum | 11 (24%) | 1 (2%) | 0.0006 |
| EF ≤30 at 6-12 months postpartum | 4 (0%) | 0 (0%) | 0.04 |
| EF ≤ 25 at 6 -12 months postpartum | 2 (4%) | 0 (0%) | 0.21 |
| Hemodialysis | 2 (4.4%) | 1 (2.0%) | 0.61 |
| Mechanical Ventilation | 6(13.0%) | 9(18.4%) | 0.48 |
| LVAD | 5 (10.9%) | 1 (2.0%) | 0.10 |
| ICD Placement | 15 (32.6%) | 7 (14.3%) | 0.03 |
| Heart Transplantation | 2 (4.4%) | 0 (0%) | 0.23 |
| Death | 5 (10.9%) | 3 (6.1%) | 0.48 |
| Duration from diagnosis to death (years), median (min, max) * | 8.8 (0.5–14.3) | 16.5 (0.3-24.4) | 0.57 |
| Medication Use | |||
| Angiotensin-converting enzyme inhibitors | 36 (78.3%) | 36 (73.5%) | 0.59 |
| Aldosterone receptor antagonists | 23 (50.0%) | 13 (27.1%) | 0.02 |
| Angiotensin receptor blockers | 5 (10.9) | 10 (20.4%) | 0.20 |
| Beta blocker | 45 (97.8%) | 44 (89.8%) | 0.21 |
| Bromocriptine | 0 (0%) | 0 (0%) | -- |
| Calcium channel blocker | 5 (10.9%) | 3 (6.1%) | 0.48 |
| Digoxin | 23 (50.0%) | 13 (26.5%) | 0.02 |
| Diuretic | 42 (91.3%) | 32 (65.3%) | 0.002 |
| Nitrates | 9 (19.6%) | 2(4.1%) | 0.02 |
| Resource Utilization | |||
| Number of ER Visits | 0(0-21) | 0 (0-4) | 0.006 |
| Number of Inpatient Admissions | 1 (0-10) | 0 (0-4) | 0.01 |
| Number of ICU Stays | 0 (0-5) | 0(0-1) | 0.005 |
Restricted to 8 deaths in cohort;
missing 19 Black, 18 White
EF = ejection fraction; ER = emergency room; ICU = intensive care unit; LV = left ventricular; LVAD = left ventricular assist device
Details of the eight women who died are as follows. Seven (88%) had an EF < 35% at diagnosis. Two of the eight deaths occurred ≤ 6 months of diagnosis. Neither patient underwent LVAD or ICD placement or cardiac transplantation and they never experienced an EF recovery to ≥ 55%. The other six deaths occurred ≥ 1 year after diagnosis, with a median time to death of 12.8 years (minimum 3.4 years – maximum 24.4 years). Only one of the six women experienced an EF recovery ≥ 55%, and it was noted six months after diagnosis. Among the six women whose deaths were delayed, three underwent LVAD placement, five underwent ICD placement, and one underwent cardiac transplantation.
Medication use is also presented in Table 2. Angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, beta blockers, and calcium channel blockers were similarly used between the groups. Aldosterone receptor antagonists, digoxin, diuretics and nitrates were more commonly used among Black women. No patient in either group was medically managed with bromocriptine.
Regarding resource utilization, Black women with PPCM were more likely to have emergency room visits (p < 0.01), inpatient admissions (p = 0.01), and ICU admissions (p = 0.005) than White women (Table 2).
There were 31 documented subsequent pregnancies (15 Black women and 16 White women). White women had a higher EF during the first trimester (40% vs 55%, p = 0.007) and postpartum (37.5% vs 55%, p = 0.02) (Table 3). There was no difference in gestational age at time of delivery between Black and White women (31.8 ± 10.2 vs 30.54 ± 13.1 weeks, p=0.77), nor mode of delivery in the subsequent pregnancies (60% vs 63.6% vaginal births, respectively, p = 1.00).
Table 3:
First Subsequent Pregnancy Documented after an NHLBI – Classified Peripartum Cardiomyopathy Diagnosis
| N=15 | N=16 | p value | |
|---|---|---|---|
| EF First Trimester * | 40 (30-55) | 55 (55-55) | 0.007 |
| EF Second Trimester † | 45 (35-50) | 55 (55-55) | 0.08 |
| EF Postpartum ‡ | 37.5 (30-40) | 55 (55-55) | 0.02 |
| Gestation age at delivery (weeks) § | 31.8 ± 10.2 | 30.54 ± 13.1 | 0.77 |
| Mode of delivery if delivery ≥ 20 weeks | 1.00 | ||
| Vaginal | 6 (60%) | 7 (63.6%) | |
| Cesarean | 4 (40%) | 4 (36.4%) |
Missing 8 Black, 3 White
Missing 10 Black, 12 White
Missing 9 Black, 7 White
Missing 3 Black, 1 White
EF = Ejection Fraction
Ten women had documented second subsequent pregnancies (4 Black women and 6 White women). Due to small numbers descriptive data are presented. Despite a normal mean ejection fraction at pregnancy entry in both groups, Black women had a mean postpartum EF of 42.5% whereas White women had a mean postpartum EF of 50%. On average, both groups delivered at term.
Discussion
We found that the EF at PPCM diagnosis was not significantly different between Black and White women. However, our findings suggest that Black women have more severe persistent left ventricular systolic dysfunction compared to White women leading to worse outcomes including need for ICD placement, increased resource utilization, and poorer cardiac function entering a subsequent pregnancy.
Our primary outcome that mean EF at diagnosis was not significantly different between Black and White women did not support our hypothesis. Considering reports of healthcare disparities among Black women in the Deep South[9], we expected to also find disparities in health outcomes among Black women defined by worse cardiac function at PPCM presentation. This disparity was identified in a prospectively enrolled multicenter cohort from North American tertiary centers including thirty Black and 70 White (or other race) women; the Investigations of Pregnancy Associated Cardiomyopathy (IPAC) investigators found that Black women had a mean EF of 31% at entry to care compared to 36% for the remaining cohort (p = 0.009).[5] It is not clear why our finding regarding race differs from the multicenter cohort but differences in the source population including differences in referral patterns to the reporting centers may be responsible. Better definition of a source population may resolve this question.
This study was not appropriately powered for most secondary outcomes, but our finding of persistent LV dysfunction at 6 – 12 months postpartum in Black women is collaborated by IPAC investigators who found that race was a significant predictor of myocardial recovery.[5] In their study Black women had lower EF at study entry, 6 months and 12 months postpartum. Higher resource utilization among Black women in our cohort may reflect an urban population with convenient access to our center and our inability to capture resource utilization by patients living in rural areas who present to their local hospital for care. Geospatial mapping may be helpful to better clarify access to care disparities.
It is important to address EF in the first trimester of the subsequent pregnancy. Black women in our cohort had a reduced EF compared to White women; surprisingly, all White women in our study had a normal EF in subsequent pregnancies. Prior studies have shown that EF is a strong predictor of poor outcomes in subsequent pregnancies.[24] It is possible that the burden of known disparities in PPCM-associated morbidity and mortality may be from pregnancies following a PPCM diagnosis.
Our study had several strengths. First, we had a large cohort of 95 women from the largest tertiary care referral center in Alabama, even distribution between races, and confirmation of NHLBI-classified PPCM based on individual chart review. Our nearly equal number of Black and White women with PPCM is uncommon in the literature and provided a unique opportunity to study the impact of race on PPCM. This nearly equal distribution of PPCM cases between Black and White women identifies a disparity in the frequency of PPCM. Our center is the main tertiary care center in Alabama; this cohort reflects most cases of PPCM in the state. Birth data from 2017[21] reported White women had 34,377 births while Black women had 17,963 births for an approximate birth ratio of 2:1. Therefore, the frequency of PPCM among Black women in Alabama is approximately twice as high as white women, consistent with the literature.[23, 25]
This study is not without limitations. Since many of these patients delivered elsewhere and were subsequently transferred to our center to the cardiology / heart failure service, obstetric variables such as parity and hypertensive disorders of pregnancy are lacking. These are important variables that could confound our results. Follow-up is also limited as some women either had loss of follow up or followed up with a local cardiologist; differential follow up by race may impact our findings. The next limitation is generalizability. We did not have any documented Asian or Hispanic women in our cohort, consistent with our source population.[21] Therefore, we are only able to comment on Black/White racial disparities. The generalizability of our results are also limited by the high smoking rate and its negative implications on cardiovascular health.[26, 27] Twenty-four percent of Black women and 35% of White women in this cohort smoked, nearly 2–3 times the national rate of 13.7%.[28] Next, we fell short of our anticipated sample size based on our baseline assumptions; it is unlikely that five additional women would change the primary outcome, but we were not powered for the primary outcome.
These data reveal disparities in health outcomes between Black and White women with peripartum cardiomyopathy; more research is needed to understand why and to identify action items that can be implemented at a systems level to reduce known disparities in PPCM-related maternal morbidity and mortality. Critical gaps of knowledge remain. Were women of both races counseled equally about subsequent pregnancies, contraception, and follow-up care? Did women of both races have equal access to follow-up care? Was the payor source a barrier to healthcare access? Were there differences in family and social support systems? These important questions need to be answered as we strategize optimal ways to reduce disparities in health outcomes among women with PPCM.
In conclusion, these data suggest that Black and White women in Alabama who presented with or were referred for PPCM in our center had comparable EF at PPCM diagnosis, but that Black women have more severe persistent left ventricular systolic dysfunction and lower EF at entry in the subsequent pregnancy. Further studies are needed to investigate these differences and to further address racial disparities in PPCM to alleviate disparities in maternal morbidity and mortality.
Table 4:
Second Subsequent Pregnancy Documented after an NHLBI – Classified Peripartum Cardiomyopathy Diagnosis
| Black N=4 | White N=6 | |
|---|---|---|
| EF First Trimester * | 55 (55 - 55) | 55 (45 - 55) |
| EF Second Trimester † | 55 (55 - 55) | 45 (45 - 45) |
| EF Postpartum ‡ | 42.5 (30-55) | 50 (45 - 55) |
| Gestation age at delivery (weeks) § | 38.3 (35.6-39.8) | 39 (38.4-39.2) |
| Mode of delivery if delivery ≥ 20 weeks§§ | ||
| Vaginal | 3 (75%) | 1 (25%) |
| Cesarean | 1 (25%) | 3 (75%) |
Data reported as median (q1 - q3) or n (%)
Missing 3 Black, 2 White
Missing 2 Black, 5 White
Missing 2 Black, 3 White
Missing 2 White
Missing, 2 White
EF = Ejection Fraction
Acknowledgments
“Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR003096. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
Accepted for presentation at the 40th Annual Pregnancy Meeting of the Society for Maternal-Fetal Medicine, Grapevine, Texas, February 3 – 8, 2020.
Declaration of Interest Statement
The authors report no conflict of interest.
References:
- 1.Petersen EE, Davis NL, Goodman D, et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morbidity and mortality weekly report. 2019. May 10;68(18):423–429. doi: 10.15585/mmwr.mm6818e1.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead SJ, Berg CJ, Chang J. Pregnancy-related mortality due to cardiomyopathy: United States, 1991–1997. Obstetrics and gynecology. 2003. Dec;102(6):1326–31. doi: 10.1016/j.obstetgynecol.2003.08.009.; eng. [DOI] [PubMed] [Google Scholar]
- 3.Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstetrics and gynecology. 2012. Nov;120(5):1013–9. doi: http://10.1097/AOG.0b013e31826e46a110.1097/aog.0b013e31826e46a1.; eng. [DOI] [PubMed] [Google Scholar]
- 4.Goland S, Modi K, Hatamizadeh P, et al. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. Journal of cardiac failure. 2013. Apr;19(4):214–8. doi: 10.1016/j.cardfail.2013.03.004.; eng. [DOI] [PubMed] [Google Scholar]
- 5.McNamara DM, Elkayam U, Alharethi R, et al. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). Journal of the American College of Cardiology. 2015. Aug 25;66(8):905–14. doi: 10.1016/j.jacc.2015.06.1309.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rana KF, Saeed A, Shamim SA, et al. The Association between Hypertensive Disorders of Pregnancy and Peripartum Cardiomyopathy. Cureus. 2019. Oct 8;11(10):e5867. doi: 10.7759/cureus.5867.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens I, Basit S, Lykke JA, et al. Hypertensive disorders of pregnancy and peripartum cardiomyopathy: A nationwide cohort study. PloS one. 2019;14(2):e0211857. doi: 10.1371/journal.pone.0211857.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bello N, Rendon ISH, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2013. Oct 29;62(18):1715–1723. doi: 10.1016/j.jacc.2013.08.717.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prather C, Fuller TR, Jeffries WLt, et al. Racism, African American Women, and Their Sexual and Reproductive Health: A Review of Historical and Contemporary Evidence and Implications for Health Equity. Health equity. 2018;2(1):249–259. doi: 10.1089/heq.2017.0045.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prather C, Fuller TR, Marshall KJ, et al. The Impact of Racism on the Sexual and Reproductive Health of African American Women. Journal of women’s health (2002). 2016. Jul;25(7):664–71. doi: 10.1089/jwh.2015.5637.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holdt Somer SJ, Sinkey RG, Bryant AS. Epidemiology of racial/ethnic disparities in severe maternal morbidity and mortality. Seminars in perinatology. 2017. Aug;41(5):258–265. doi: 10.1053/j.semperi.2017.04.001.; eng. [DOI] [PubMed] [Google Scholar]
- 12.Ross J, Braswell KV, Madeira da Silva L, et al. Unraveling the etiology of ovarian cancer racial disparity in the deep south: Is it nature or nurture? Gynecologic oncology. 2017. May;145(2):329–333. doi: 10.1016/j.ygyno.2017.02.025.; eng. [DOI] [PubMed] [Google Scholar]
- 13.Khariton Y, Nassif ME, Thomas L, et al. Health Status Disparities by Sex, Race/Ethnicity, and Socioeconomic Status in Outpatients With Heart Failure. JACC Heart failure. 2018. Jun;6(6):465–473. doi: 10.1016/j.jchf.2018.02.002.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant AS, Worjoloh A, Caughey AB, et al. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. American journal of obstetrics and gynecology. 2010. Apr;202(4):335–43. doi: 10.1016/j.ajog.2009.10.864.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grobman WA, Bailit JL, Rice MM, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstetrics and gynecology. 2015. Jun;125(6):1460–7. doi: 10.1097/aog.0000000000000735.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of clinical epidemiology. 2008. Apr;61(4):344–9. doi: 10.1016/j.jclinepi.2007.11.008.; eng. [DOI] [PubMed] [Google Scholar]
- 17.Murphy SN, Weber G, Mendis M, et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). Journal of the American Medical Informatics Association : JAMIA. 2010. Mar-Apr;17(2):124–30. doi: 10.1136/jamia.2009.000893.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visweswaran S, Becich MJ, D’Itri VS, et al. Accrual to Clinical Trials (ACT): A Clinical and Translational Science Award Consortium Network. JAMIA open. 2018. Oct;1(2):147–152. doi: 10.1093/jamiaopen/ooy033.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. Jama. 2000. Mar 1;283(9):1183–8.; eng. [DOI] [PubMed] [Google Scholar]
- 20.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2003. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2005. Sep 8;54(2):1–116.; eng. [PubMed] [Google Scholar]
- 21.Martin JA, Hamilton BE, Osterman MJK, et al. Births: Final Data for 2017. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2018. Nov;67(8):1–50.; eng. [PubMed] [Google Scholar]
- 22.Arany Z, Elkayam U. Peripartum Cardiomyopathy. Circulation. 2016. Apr 5;133(14):1397–409. doi: 10.1161/circulationaha.115.020491.; eng. [DOI] [PubMed] [Google Scholar]
- 23.Kolte D, Khera S, Aronow WS, et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. Journal of the American Heart Association. 2014. Jun 4;3(3):e001056. doi: 10.1161/jaha.114.001056.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yameogo NV, Samadoulougou AK, Kagambega LJ, et al. Maternal and fetal prognosis of subsequent pregnancy in black African women with peripartum cardiomyopathy. BMC cardiovascular disorders. 2018. Jun 18;18(1):119. doi: 10.1186/s12872-018-0856-7.. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isogai T, Kamiya CA. Worldwide Incidence of Peripartum Cardiomyopathy and Overall Maternal Mortality. International heart journal. 2019. May 30;60(3):503–511. doi: 10.1536/ihj.18-729.; eng. [DOI] [PubMed] [Google Scholar]
- 26.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2014. Mar;34(3):509–15. doi: 10.1161/atvbaha.113.300156.; eng. [DOI] [PubMed] [Google Scholar]
- 27.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American College of Cardiology. 2004. May 19;43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047.; eng. [DOI] [PubMed] [Google Scholar]
- 28.Creamer MR, Wang TW, Babb S, et al. Tobacco Product Use and Cessation Indicators Among Adults – United States, 2018. Morbidity and Mortality Weekly Report 2019, 68(45);1013–1019 [accessed 2019 Nov 14]. [DOI] [PMC free article] [PubMed] [Google Scholar]


