Abstract
Older adults are more susceptible to viral and bacterial infection, and experience higher incidence and severity of infectious diseases. Although vaccination is the most logical solution in preventing infectious diseases, primary vaccine responses in individuals aged ≥65 years-old fail to generate complete protection. This is presumably attributed to immunosenescence, a term that describes functional differences associated with the immune system and natural age advancement. Both the innate and adaptive immune systems experience age-related impairments that contribute to insufficient protection following vaccination. This review addresses current knowledge of age-related changes that affect vaccine responsiveness; including the deficits in innate cell functions, dampened humoral and cell-mediated immune responses, current vaccination schedules for older adults, and concludes with potential strategies for improving vaccine efficacy specifically for this age group. Due to an age-related decline in immunity and poor vaccine responses, infectious diseases remain a burden among the aged population.
Keywords: immune, senescence, vaccine, older adults
1. Introduction
Currently, the United Nations reports that there are 700 million individuals over the age of 65 years old globally, and population projections indicate that the population size will double by year 2050 [1]. While growing at an unprecedented rate, the population size of older adults (≥65 years) will exceed the global population of young individuals (≤15 years) as a result. In the United States, older adults constitute 16% of the total population, but account for 27% of medical practitioner visits and 38% of all hospital discharges [2]. The over-representation of older adults at medical facilities reflects an age-associated increase in prevalence and severity of some infectious diseases, specifically dominated by respiratory, gastrointestinal and urinary tract infections [3]. Compared to younger individuals, older adults often experience long-term sequelae and increased morbidity as a result of infection as well [4]. For example, influenza, respiratory syncytial virus (RSV), herpes zoster (HZ) or shingles, pneumococcal disease, and foodborne illnesses disproportionately affect the elderly [5–8]. Due to continuous improvements in life expectancy, age-related diseases are of critical concern and warrant scientific interest to improve the quality of life of the elderly population.
A series of age-associated changes to the immune system, termed immunosenescence, greatly contribute to the increased vulnerability to infections among older adults. In tandem with biological aging, there is a progressive decline in immune function that results in impaired humoral and cellular responses to pathogens [9]. Importantly, primary vaccine responses in older individuals fail to generate complete protection and exhibit reduced efficacy. For example, inactivated influenza vaccines effectively prevent disease in 70-90% of children and younger adults, but efficacy decreases dramatically to 30-50% in older adults [10, 11]. The duration of long term protection is compromised in older individuals as well, as the rate of antibody decline increases with advancing age [12, 13]. Therefore, due to the age-related decline in immunity and vaccine responses, infectious diseases remain a burden among the older adult population. This review will discuss our current knowledge of key features of immunosenescence (summarized in Figure 1), its implications for vaccinating older adults, and vaccination strategies designed to specifically target the aged immune system for improved protection.
Figure 1.
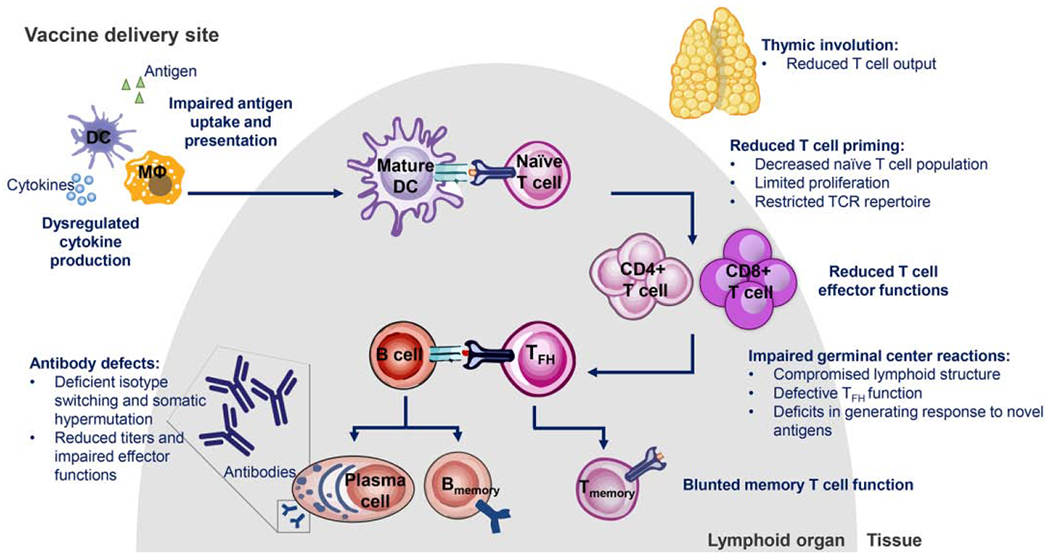
Schematic diagram of immune deficits in older adults. Abbreviations: DC, dendritic cell; MΦ, macrophage; TFH, T follicular helper; TCR, T cell receptor.
2. Deficits in innate immunity in older adults
The innate immune system functions as the first line of defense, in which physical barriers and immune cells nonspecifically respond to pathogens to protect the host. Studies on aged mice and samples from individuals ≥ 65 years old provide evidence of a dysregulated innate immune system as we age. Older adults obtain chronically elevated levels of pro-inflammatory cytokines, termed “inflamm-ageing”, that is specifically characterized by increased basal unstimulated levels of IL-6, TNF-α, and acute phase reactants [14, 15]. This low-grade inflammation is thought to contribute to other age-associated diseases and is a common feature of innate immunosenescence.
Innate cell types, such as dendritic cells (DCs), monocytes, neutrophils and natural killer (NK) cells as discussed herein, appear affected by age and contribute to age-associated immune dysfunction. Functioning as the bridge between innate and adaptive immunity, DCs patrol the body, recognize and phagocytose antigens, then migrate to the draining lymph nodes where they secrete cytokines and present antigen to T cells to launch an immune response. Although the quantity and morphology of circulating DCs appear unaffected by age, DCs from aged hosts exhibit defects at each one of these steps [16]. First, prominent pattern recognition receptors, such as toll-like receptors (TLRs) on DCs demonstrate an age-associated decline. Studies examining the function of TLRs on DCs revealed that DCs from older subjects displayed decreased production of pro-inflammatory cytokines, such as IL-6, IL-12, and TNF-α, when stimulated with TLR specific ligands [17]. This decrease in stimulated cytokine secretion can be attributed to both the reduced surface expression of TLR1, TLR3, and TLR8 and defects in intracellular signaling in older subjects, when compared to young adults [18]. Further, it has been hypothesized that deficient TLR function negatively affects DC activation and could further diminish activation of adaptive immune responses.
Regarding vaccination, one study presents a relationship between reduced TLR-induced cytokine production and influenza antibody response in older adults receiving Fluzone®. Intracellular cytokine staining of DCs from vaccinated young and older adults revealed that older individuals demonstrated deficits in IL-6, IL-12, and TNF-α levels, and are shown to be associated with lower hemagglutination antibody inhibition (HAI) titers and seroprotection rates to Fluzone®. [17]. This highlights the functional significance of age-associated TLR defects in immunized humans.
Secondly, several studies suggest that aged DCs have an impaired capacity to achieve complete T cell priming and activation [18]. For example, CD8+ T cells from HLA-A2+ older volunteers primed with HLA-matched DCs in vitro have been shown to expand and express effector memory (CD45RA− CCR7−) cell markers at lower levels, suggesting less efficient activation, in comparison to samples from middle-aged adults; the same trend holds true for murine CD4+ T cells [19, 20]. This is partially attributed to the age-associated decline in antigen uptake, subsequent presentation and expression of co-stimulatory molecules [16]. DCs from older adults demonstrated decreased uptake of FITC dextran compared to that of younger subjects, representing impaired phagocytic abilities with age. To investigate the impacts of ageing on antigen presentation, an in vivo study of aged mice showed a decrease in peptide-MHC surface expression on DCs from lymph nodes of immunized mice, compared to adults [19]. Further, the expression of the co-stimulatory molecule, CD86, remained comparable among both aged and adult mice, however, upregulation of CD40 was impaired in the old DCs [19]. Since CD4+ T cells rely on continuous antigen presentation and strong expression of co-stimulatory molecules for their proliferation and differentiation, these DC-intrinsic deficits likely augment the age-associated decrease in CD4+ T cell function (discussed further in subsequent sections). Collectively, these defects negatively impact generation of an immune response to novel antigens and contribute to vaccine hyporesponsiveness in older adults.
While other innate cell types in older adults have yet to be extensively studied, it is apparent that the function of monocytes, neutrophils, and NK cells declines with age. Aged human monocytes display impaired phagocytosis, and macrophages from aged mice produce lower levels of nitric oxide, suggesting there may be defects in bacterial killing by older macrophages [21, 22]. Additionally, neutrophils from aged humans demonstrate impaired phagocytosis of opsonized Streptococcus pneumoniae and reduced ability to kill these organisms [23]. Lastly, aged human NK cells exhibit decreased cytokine and chemokine production, as well as diminished cytotoxicity [24]. These age-associated defects in innate cells collectively hinder protective immunity induced by vaccines.
3. Deficits in adaptive immunity in older adults
Age-associated changes to the adaptive immune system are equally relevant to vaccine efficacy, as older adults experience only incomplete protection upon vaccination [25]. While the mechanisms underlying the decline in both cellular and humoral immunity remain unclear, clinical and animal studies provide critical information about age-associated defects. These studies demonstrate that T and B cells share certain altered attributes that are accompanied with advancing age; such as a reduction in naïve cell output, shift in T and B cell phenotype, changes in clonal composition and breadth of diversity, and impaired differentiation potential.
Both B and T lymphocytes progenerate from hematopoietic stem cells (HSC) residing in the bone marrow. However, the regenerative capacity of HSCs decline as we age and [26] recent studies revealed a bias towards myeloid cell lineages with a decrease in lymphoid cells that occurs with aging [27]. These factors compromise production of mature B cells and result in fewer B cells exiting the bone marrow in older adults [28]. Although the number and function of bone marrow-derived T cell precursors remain unaffected with age, the thymus, site of T lymphocyte maturation, shrinks in size and is replaced by adipose tissue by the age of 40-50 [29–31]. The progressive thymic atrophy, termed thymic involution, leads to a decrease in naïve T cell emigration from the thymus, which results in a continuous decline in peripheral naïve T cells throughout life. Specifically, homeostatic proliferation of CD4+ T cells is sufficient to maintain adequate numbers, but a severe age-related decrease in CD8+ T cells has been reported in humans [32]. However, this decrease in naïve T lymphocytes is compensated by an increase in terminally differentiated memory T cells as pathogens are encountered with age [33, 34]. Additionally, peripheral maintenance factors, such as B cell activating factor (BAFF) and IL-7 that promote survival of naïve B and T cells, continuously decrease with age [35, 36]. The progressive decline in naïve cells limits the ability to generate robust de novo immune responses, as in the case of a novel vaccine antigen.
The absolute number of T cells, but not B cells, in circulation remain constant throughout life, but older individuals obtain strikingly different phenotypic changes to their lymphatic pool [37, 38]. As mentioned, due to the accumulation of antigen-experienced lymphocytes and changes to the bone marrow and thymus, memory B and T cells comprise the main populations identified in the periphery; while naïve cells exist at lower frequencies. Of the differentiated B and T cells, a newly discovered B cell subset, termed age-associated B cells, is present in aged mice and will be discussed further in later sections. Additionally, a T cell subset, known as terminally differentiated memory T cells accumulate within the human T cell compartment with age, and share phenotypic features with effector T cells but are described as hypofunctional [39].
Another hallmark of the aging immune system is the reduced proliferation potential of B and T cells. Similarly to other cell types, B and T cells have a finite number of cell divisions, that is associated with telomere length [40]. Since B and T cells undergo extensive clonal expansion throughout the duration of an immune response, several studies illustrate an age-associated progressive shortening of telomeres that occurs as a consequence of activation and differentiation of lymphocytes [41, 42]. Likewise, blunting of telomeres in human HSCs with each cell division is correlated with age [42], potentially presenting a mechanism that limits lymphocyte activation and subsequent expansion.
3.1. T cells
The cellular immune response comprises T helper lymphocytes (Th) and cytolytic T lymphocytes (CTLs), also known as CD4+ and CD8+ T cells, respectively. Upon infection or vaccination, activated CD4+ T cells secrete cytokines to orchestrate and assist surrounding immune cells also involved in the protective response. CTLs traffic to sites of infection to lyse infected cells and secrete pro-inflammatory cytokines. Therefore, many vaccines aim to target CD4+ and CD8+ T cells to provide a protective immune response. However, increasing evidence displays age-associated cell-mediated immunity (CMI) defects that presumably limit vaccine efficacy and increase vulnerability to infections [43]. There are phenotypic alterations of the T cell population with aging, as the aged T cell pool comprises a higher ratio of CD4+ to CD8+ lymphocytes and fewer naïve T cells, that is accompanied by an increase in differentiated memory T cells. Phenotypically, the loss of surface CD28 expression among CD8+ T cells, a principal co-stimulatory molecule, occurs in elderly individuals and appears to be a consistent biomarker of the aging human immune system [44]. Since CD28+ T cells downregulate co-stimulatory molecules after stimulation, CD8+ CD28null T cells represent a population that has previously experienced repeated exposure to antigen. Therefore, CD28null T cells accumulate with age and importantly, are associated with replicative senescence and chronological aging [45, 46]. While the pre-existing T cell pool that previously encountered pathogens earlier in life remain uncompromised, aged naïve T cells demonstrate reduced proliferative capacity after antigen recognition, decreased cytokine secretion, higher activation thresholds, limited receptor repertoire, and insufficient effector functions upon vaccination [47].
Early investigations in aged mice demonstrated defective T cell proliferation and IL-2 production upon mitogenic anti-T cell receptor (TCR) stimulation [48]. Further, Haynes et al. [49] previously reported that in contrast to memory CD4+ T cells generated in young mice, those generated in aged mice produce less IL-2, exhibited decreased expansion, and showed altered differentiation patterns upon antigen stimulation. Indeed, recent studies report that naïve CD4+ T cells from older adults display an age-dependent reduction in TCR signaling that results in decreased clonal expansion and may provide a mechanistic explanation for the dampened levels of T cell activation seen in older individuals [36, 45, 50].
Due to the age-associated alterations in frequencies of naïve and effector T cells, immunosenescence is associated with a decline in TCR repertoire. Healthy adults possess 2-5- fold times greater diversity in TCRs compared to aged individuals [51]. Specifically, human studies reveal that older adults contain a more restricted oligoclonal CD4+ TCR repertoire [52]. In addition, studies using murine models demonstrate that in contrast to young mice primed with influenza A live vaccine, primed aged mice exhibit reduced CD8+ TCR diversity and lack of clonotypes that respond to immunodominant epitopes [53]. However, the functional significance of the reduction in receptor repertoire remains unclear, since the early primed mice were not better protected than aged mice when both groups were challenged with influenza virus. Regardless, due to the age-dependent decline in breadth of TCRs, it would be beneficial to consider the decrease in clonotypic diversity when designing vaccines.
Taken together, both the quantitative and qualitative properties of aged naïve T cells impede the generation of primary immune responses to new antigens. Despite the defects in priming aged T cells, antigen-specific memory T cells that were generated during younger ages are maintained for 40-60 years even in the absence of antigen [54]. Thus, in the context of vaccination, older individuals will have difficulty mounting a robust cellular immune response when encountering unique vaccine antigens for the first time. This concept is supported by the success story of the Shingles vaccine; since both shingles and chickenpox are caused by varicella zoster virus (VZV), VZV-specific immunity targets both diseases. The shingles vaccine relies on a pre-existing T cell population to undergo a recall response, rather than attempting to generate de novo antigen-specific cellular immunity [55]. Since administration of this vaccine is analogous to a booster response, it appears that immunosenescence plays a lesser role in impairing vaccine efficacy in older adults [56]. Collectively, this observation highlights the age-associated decline in naïve T cells and presents advantages to vaccines that prime the immune system earlier in life.
Clinical evidence suggests that increased risk of infection in the elderly is due to a decline in pathogen-specific antibody activity post-vaccination and serves as an indicator of poor vaccine efficacy. However, other studies and investigators argue that the abundance of CD28null T cells serves as a predictor of immune incompetence and may forecast vaccine responsiveness in the elderly [44]. Most studies demonstrate that CD28null T cells are terminally differentiated effectors and have lower proliferation potential, which is supported by their shortened telomeres [57]. CD28 loss in CD8+ T cells is associated with reduced perforin and granzyme activity and thus inadequate killing of infected cells [58]. Likewise, in tandem with lower CD28 expression, CD4+ CD28null T cells fail to display CD40L and unsuccessfully provide B cell help that normally promotes B-cell proliferation and antibody production [59]. The age-related accumulation of CD28null T cells leads to a reduction in high-affinity B cell responses and may be indicative of immune unresponsiveness. Indeed, aged non-responders to the influenza vaccine possess clonally expanded T cells that are dominated by the CD8+ CD28null phenotype, and is associated with a reduction in HAI neutralizing titers [47]. An additional study showed that in ≥65 year-olds, the likelihood of unsuccessful influenza vaccination correlated with higher frequencies of CD28null CD8+ T cells and inability to produce CTL-specific cytokines, while antibody metrics were not indicative of developing subsequent influenza illness [46, 60]. Therefore, these findings present the potential for an additional metric when assessing vaccine responsiveness in the older population.
3.2. B cells
It is well established that humoral immunity plays an indispensable role in providing vaccine-mediated protection, and importantly, this arm of the immune response also experiences an age-associated decline in immune competence. In comparison to younger adult counterparts, vaccines in older adults fail to elicit protective antibody titers and induce lower levels of antibodies with functional activity [61–64]. For example, aged individuals vaccinated with tetanus, diphtheria and two pertussis antigens elicited lower antibody levels than younger adults [61, 65]. Additionally, a booster dose at age 60 still failed to elicit protective levels of antigen-specific antibody levels, further highlighting the decline in humoral immunity with age [61]. Due to great societal importance, many studies focus on influenza vaccines; in which older adults overwhelmingly exhibit lower seroconversion rates and protective HAI titers [66].
Specific mechanisms responsible for B cell intrinsic defects remain to be completely understood. Along with declining B cell numbers, several phenotypic differences among B cell subsets in aged individuals have been reported; an accumulation of CD27+ B cells (marker of B cell memory) have been discussed throughout the literature, as well as a decrease in plasma cell differentiation [12]. Further, there is an increase in frequency of class-switched memory B cells with age in comparison to B cells that have yet to encounter antigen [67, 68]. Surprisingly, in comparison to younger adults immunized with consecutive influenza vaccines, older individuals obtained similar frequencies of memory B cells and plasmablasts. However, vaccine-specific serum titers and Blimp-1 expression levels (plasma cell master regulator) appeared drastically lower in the aged group, indicative of a defect in the ability of memory B cells to differentiate into plasma cells, which actively produce antibodies [12]. In addition, spectratype analysis revealed that individuals aged 86+ years obtained much lower B cell receptor (BCR) diversity, in comparison to younger adults [69]. Therefore, similarly to the T cell compartment, the contraction of receptor repertoire accompanied by an increase in class-switched B cell frequency most likely reflects the cumulative exposure to antigens throughout life. Furthermore, the reduced pool of naïve B cells available in older individuals restricts the number of possible clones able to respond to novel antigens. Indeed, aged individuals vaccinated against influenza obtain hypermutated IgG but produce fewer B cell lineages in comparison to adults, indicating that a germinal center reaction occurred but resulted in fewer responding clones [70]. Importantly, vaccine-induced antibodies failed to elicit optimal effector functions that are normally induced in younger adults. Specifically, reduced protective HAI titers following trivalent influenza vaccination and lower opsonization activity in the case of the 23-valent pneumococcal polysaccharide vaccine (PPV23) are observed in older individuals [66, 71]. Therefore, even when sufficient antibody titers are generated, the protective capacity of the vaccine-induced antibodies may be significantly reduced in the aged population.
There are age-associated cellular mechanisms that compromise the generation of isotype-switched high-affinity antibodies in B cells. Human studies reveal that activation-induced cytidine deaminase (AID), an enzyme responsible for facilitating class switch recombination (CSR) and somatic hypermutation (SHM) during germinal center reactions, is expressed at lower levels in adults of advanced age [68]. Indeed, aged mice demonstrate AID-associated impaired CSR and decreased effector antibody isotypes [72], and it has been shown that a decline in AID mRNA in human B cells correlates with decreased antibody affinity maturation following influenza vaccination [73].
Interestingly, a subset of mature B cells was identified in aged mice, termed age-associated B cells (ABCs), and are described as responsive to innate stimuli but resistant to BCR and CD40 stimulation [74, 75]. The presence of this senescent subset remains to be confirmed in humans, however, B cells with a similar profile have been identified during autoimmune diseases and viral infections in people [74, 76]. The functional consequences of ABCs in humans and the distinction from exhausted B cells requires further investigation, but these cells likely contribute to humoral immunosenescence and warrant attention during vaccine development.
3.3. Lymphoid tissue and germinal center reactions
In addition to intrinsic B cell defects, insufficient germinal center reactions may be attributed to diminished CD4+ T cell cognate functions and reduced antigen presentation capacity of follicular dendritic cells, as both have been shown to contribute to weakened humoral responses [77, 78]. T follicular helper cells (TFH, CXCR5+ CD4+) are a critical subset of T cells residing in B cell follicles of lymphoid tissue that provide antigen-experienced B cell signals during germinal center reactions to facilitate their proliferation, SHM, isotype switching, and subsequent generation of memory B cells and plasma cells. As with other cell types, TFH’s perturbations correlate with age and likely negatively affect humoral responses [79, 80]. Indeed, studies show that older adults fail to produce increased numbers of activated circulating TFH cells, characterized by lack of ICOS expression, following influenza vaccination, while younger individuals elicit significantly heightened levels of this subset. Since ICOS+-TFH correlates with HAI titers in the young, the defects in vaccine-induced TFH activation may underly reduced influenza-specific antibody responses and presumably functions as an immune biomarker in the aged population [80]. Further, using a murine adoptive transfer model, it has been reported that transfer of aged CD4+ T cells to nucleoprotein (NP)-immunized 2-4 month old mice led to a decrease in NP-specific IgG titers, that can be attributed to reduced expansion and differentiation of B cells within observed germinal centers. Within this model, it appears the migration of CD4+ T cells are unaffected, but expression of CD40L is reduced and presumably impairs the cognate helper functions of CD4+ T cells as well [77].
Likewise, recent studies reveal age-associated alterations to lymphoid structures that negatively impact both B and T cells and results in decreased vaccine efficacy [81]. Specifically, reduced lymph node size and fibrosis of lymphoid tissue has been reported and thought to compromise adaptive immunity [82–84]. Furthermore, factors promoting B cell survival, such as BAFF and APRIL, are detected at lower levels in serum and correlate with decreased B cell survival in older adults [85]. Since these stromal microenvironments maintain lymphocytes throughout life, it has been proposed that restoring the lymphoid structure may provide a therapeutic strategy for rejuvenating the aging immune system to improve vaccine responses [81].
4. Current vaccine status for older adults
Given these age-associated immune shortcomings, vaccine-induced protection remains challenging in the elderly. Older adults are disproportionately affected by several viral and bacterial infections and experience higher case fatality rates to these infectious diseases. Prominent infectious agents of clinical concern include influenza virus, RSV, herpes zoster virus, Streptococcus pneumoniae, Klebsiella pneumoniae, Pseudomonas aeruginosa, Clostridium difficile and Staphylococcus aureus. Since vaccination serves as a viable and efficient strategy to establish protective immunity for some of these diseases, the Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC), recommends that adults aged 65+ years obtain immunization against HZ virus, Streptococcus pneumoniae, and influenza virus (Table 1). The subsequent portion describes the vaccines that are currently licensed for older adults.
Table 1.
Vaccines licensed for use in older adults
| Infectious agent | Vaccine | Vaccine formulation |
|---|---|---|
| Influenza virus | Fluzone® High-Dose Quadrivalent Inactivated influenza vaccine (IV) FLUAD® IV with adjuvant |
60 μg HAa antigen of each recommended influenza strain 15 μg HA antigen of each recommended influenza strain + MF59® adjuvant |
| Streptococcus pneumoniae | Pneumovax® 23-valent polysaccharide vaccine (PPV23) Prevnar® 13-valent conjugated vaccine PCV13 |
25 μg polysaccharide of each serotype 2.2 μg polysaccharide of each serotype conjugated to CRM197 + 0.125 mg aluminum phosphate |
| Varicella zoster virus | Zostavax® Zoster live vaccine Shingrix® Recombinant zoster vaccine |
20,000 Plaque forming Units (PFU) Oka strain 50 μg VZVb glycoprotein E (gE) + AS01B adjuvant |
HA, hemagglutinin
VZV, Varicella Zoster Virus
4.1. Influenza
A placebo-controlled trial conducted by Govaert et al. in 1994 demonstrated that administration of influenza vaccine to older individuals reduced the risk of obtaining clinically and serologically confirmed influenza by about 50% [86]. In addition, of the currently licensed vaccines for older adults, the protective efficacy of both live attenuated influenza vaccine (LAIV) and trivalent inactivated influenza virus vaccine has shown to be reduced in older adults [87], and is potentially attributed to a reduction in antibody titers and dampened effector function upon vaccination [62, 71]. Taken together, the disparity in humoral responses with age is consistent with defective B cell function among older individuals.
However, recently licensed adjustments to the influenza vaccine, such as increased dosage and addition of an adjuvant, have improved immunogenicity in older adults. First, Fluzone® High-Dose (containing 4X the antigen dose) has demonstrated significant improvements in influenza-specific humoral responses in comparison to the standard dose; generating higher anti-HA titers and seroconversion rates [88]. Secondly, Fluad®-MF59®-Adjuvanted quadrivalent Influenza, containing four-component inactivated influenza vaccine plus adjuvant, is a recently approved vaccine formulation used specifically in older adults that has demonstrated improved HAI titers, greater protection against influenza and reduced the need for hospitalization [89].
4.2. Shingles
Another prominent disease affecting aged individuals, HZ or shingles, manifests as a result of reactivation of latent varicella zoster virus (VZV). In the 1970s, Takahashi and colleagues developed an attenuated varicella virus, termed the Oka strain, to protect against pediatric chickenpox caused by VZV [90]. However, despite previous vaccination, an age-associated decline in VZV-specific CMI responses allow for reactivation of virus and results in HZ disease [91]. More recently, the Oka strain was granted licensure in the US for individuals aged >50 to prevent shingles. This vaccine, known as Zostavax®, comprises 14 times more virus than the vaccine strain used to target chickenpox in children and provides older adults protection against HZ [92]. However, Zostavax® vaccine efficacy for HZ declines by 7 years following vaccination (61.1% versus 37.7%), and efficacy has been shown to decrease with age of the vaccinee; vaccine efficacy for adults aged 50-59 years-old is 70% and drops to 37% in adults aged ≥70 years [88, 93]. Due to these shortcomings, a recently approved ASO1B-adjuvanted subunit HZ vaccine, Shingrix® (RZV), is preferred for vaccinating older adults against HZ complications. RZV comprises a mixture of HZ glycoprotein E (gE) antigen and AS01B adjuvant, and demonstrates high efficacy among all age groups ranging from 50 to ≥70 years old, with protection maintained at least 9 years following vaccination [89]. Therefore, Shingrix®, or the combination of subunit antigen and adjuvant, potentially exemplifies a general strategy for vaccines in circumventing immunosenescence in older adults.
5. Strategies to overcome age-related limitations
Development of successful vaccines will provide tremendous improvements to the health and quality of life of older adults. To design the best possible preventative strategies, we must specifically target well understood pathways that deleteriously affect immune aging, with the goal of ameliorating their effects. Previously reported approaches include the use of adjuvants, increased antigen dose, and additional vaccine booster doses.
5.1. Adjuvants
By definition, adjuvants enhance immunogenicity of vaccine antigens and importantly, can reduce non-responsiveness in target populations. Therefore, research efforts aim to develop vaccine formulations that contain a protective antigen and an adjuvant with immune stimulatory properties that circumvent the limitations of immunosenescence. Approved adjuvants currently in use for the older population include Novartis’ MF59® and GSK’s Adjuvant System (AS), approved for influenza vaccine and recombinant HZ gE antigen respectively [94–96]. A large-scale study spanning three influenza seasons provides estimates that Fluad®-MF59®-Adjuvanted influenza vaccine further prevents influenza- and pneumonia-related hospitalizations by 25%, compared to nonadjuvanted vaccine [89]. These adjuvants encompass similar compositions, as they all comprise squalene in an oil-water emulsion. While the exact mechanisms of action remain unknown, it has been hypothesized that these adjuvants induce a local pro-inflammatory environment that results in increased innate immune cell recruitment and antigen-presenting cell (APC) activation that subsequently amplifies T and B cell expansion to increase immune competence.
5.2. Increased dosage
As mentioned, high-dose vaccines have proven to be effective clinically in the case of influenza and HZ. Immunization with increased dosage is believed to generate greater numbers of antigen-presenting follicular DCs that ultimately results in increased B cell stimulation. The 7- and 9-valent pneumococcal conjugate vaccines (PCV7 and PCV9) were assessed for dose-dependent immune responses in subjects aged 70 years or older [97]. Despite demonstrating improved immunogenicity in individuals that received the high-dose vaccine, PCV7 and PCV9 as well as other vaccines containing an increased dosage specifically for the older population have yet to reach the market. However, these findings provide insight into the biology of immune aging, in which older adults may require more antigen to reach activation thresholds of immune cells and ultimately mount an immune response.
5.3. Multiple doses
Another approach to improving vaccine responsiveness in aged individuals involves regular booster vaccinations every 10-20 years. Healthy subjects of the ages 59-91 that received a booster of multivalent vaccine containing antigens from tetanus, diphtheria, pertussis, and polio produced better humoral responses than the younger individuals receiving a single dose [61]. Likewise, administration of an additional booster dose of Zostavax® spaced 10 years apart given to individuals over 70 years of age showed great success in improving antibody titers and representative CMI cytokines [98]. Therefore, routine vaccine booster programs may be sufficient in inducing humoral immunity to provide protection.
5.4. Other approaches
Other novel vaccination approaches in early developmental stages include the use of viral vectors, exploring intradermal routes, signaling pathway inhibitors, and genetically engineering live vaccines with targeted alterations to enhance immunogenicity. Since CD8+ T cell responses to some viruses appear unperturbed in aged individuals, vector-based vaccines are currently being explored as a solution to generating protective adaptive immunity. For example, recent studies investigating the use of Vaccinia virus Ankara (MVA) expressing influenza antigens demonstrated that older adults obtain detectable levels of influenza-specific CD8+ polyfunctional T cells [99]. In addition, administration of Fluzone® via the intradermal route confers a superior immune response in comparison to the currently licensed intramuscular vaccination [100]. It has been postulated that intradermal administration results in accelerated immune cell recruitment that subsequently leads to generation of a more potent immune response. Since increasing evidence indicates that the cellular aging process can be manipulated with signal transduction modifiers, recent studies investigated the use of a rapamycin analog (mTOR inhibitor to block cell cycle) treatment 6 weeks prior to influenza vaccination. Rapamycin-treated older individuals produced higher antibody titers and exhibited a lower percentage of T cells expressing inhibitory molecules. Due to the enhanced vaccine response and sufficient safety profile, mTOR inhibitors demonstrate substantial promise to be used in conjunction with vaccines to ameliorate age-related immunosenescence [101]. Finally, a novel approach involves creating genetically engineered live attenuated vaccines in which genes that contribute to immune suppression are removed, with the ultimate goal of eliminating immune barricades to enhance immunogenicity [102].
6. Conclusion
It is evident that there are distinct differences between the immune systems of older and younger adults, and the implications on vaccine responses have yet to be completely understood. However, it has been established that some of these age-associated immune changes result in reduced vaccine efficacy and an increased susceptibility to infections. Recent interventions to vaccines for older adults, such as increased dosage and addition of adjuvants, have demonstrated great promise for protecting this population. However, a complete mechanistic understanding of immunosenescence will further our knowledge of immune aging and instruct rational vaccine design for the aged population, with the overall goal of improving the quality of life of older adults.
Acknowledgments
This work was funded by NIH/NIAID U19 AI142725 (PI: Myron M. Levine; Project 2 leader: Sharon M. Tennant).
All authors attest they meet the ICMJE criteria for authorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].United Nations. World Population Prospects 2019. 2019. [Google Scholar]
- [2].CDC/NCHS National Hospital Discharge Survey. Number, percent distribution, rate, days of care with average length of stay, and standard error of discharges from short-stay hospitals, by sex and age: United States, 2010. 2010. [Google Scholar]
- [3].Yoshikawa TT, Norman DC. Geriatric Infectious Diseases: Current Concepts on Diagnosis and Management. Journal of the American Geriatrics Society. 2017;65:631–41. [DOI] [PubMed] [Google Scholar]
- [4].Albright JWA JF. Aging, Immunity, and Infection. In: Press H, editor. Totowa, NJ: 2003. [Google Scholar]
- [5].Scallan E, Crim SM, Runkle A, Henao OL, Mahon BE, Hoekstra RM, et al. Bacterial Enteric Infections Among Older Adults in the United States: Foodborne Diseases Active Surveillance Network, 1996–2012. Foodborne Pathog Dis. 2015;12:492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wroe PC, Finkelstein JA, Ray GT, Linder JA, Johnson KM, Rifas-Shiman S, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis. 2012;205:1589–92. [DOI] [PubMed] [Google Scholar]
- [7].Gordon A, Reingold A. The Burden of Influenza: a Complex Problem. Curr Epidemiol Rep. 2018;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pera A, Campos C, Lopez N, Hassouneh F, Alonso C, Tarazona R, et al. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas. 2015;82:50–5. [DOI] [PubMed] [Google Scholar]
- [10].Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–74. [DOI] [PubMed] [Google Scholar]
- [11].Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. [DOI] [PubMed] [Google Scholar]
- [12].Frasca D, Diaz A, Romero M, Blomberg BB. The generation of memory B cells is maintained, but the antibody response is not, in the elderly after repeated influenza immunizations. Vaccine. 2016;34:2834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–60. [DOI] [PubMed] [Google Scholar]
- [14].Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–8. [DOI] [PubMed] [Google Scholar]
- [15].Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nature reviews Endocrinology. 2018;14:576–90. [DOI] [PubMed] [Google Scholar]
- [16].Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase signaling pathway. Journal of immunology (Baltimore, Md : 1950). 2007;178:6912–22. [DOI] [PubMed] [Google Scholar]
- [17].Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. Journal of immunology (Baltimore, Md : 1950). 2010;184:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Agrawal A, Agrawal S, Gupta S. Role of dendritic cells in inflammation and loss of tolerance in the elderly. Front Immunol. 2017;8:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pereira LF, de Souza AP, Borges TJ, Bonorino C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells. Mech Ageing Dev. 2011;132:187–94. [DOI] [PubMed] [Google Scholar]
- [20].Briceno O, Lissina A, Wanke K, Afonso G, von Braun A, Ragon K, et al. Reduced naive CD8(+) T-cell priming efficacy in elderly adults. Aging Cell. 2016;15:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–75. [DOI] [PubMed] [Google Scholar]
- [22].Kissin E, Tomasi M, McCartney-Francis N, Gibbs CL, Smith PD. Age-related decline in murine macrophage production of nitric oxide. J Infect Dis. 1997;175:1004–7. [DOI] [PubMed] [Google Scholar]
- [23].Simell B, Vuorela A, Ekstrom N, Palmu A, Reunanen A, Meri S, et al. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine. 2011;29:1929–34. [DOI] [PubMed] [Google Scholar]
- [24].Le Garff-Tavernier M, Beziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–35. [DOI] [PubMed] [Google Scholar]
- [25].Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lansdorp PM, Dragowska W, Thomas TE, Little MT, Mayani H. Age-related decline in proliferative potential of purified stem cell candidates. Blood Cells. 1994;20:376–80; discussion 80-1. [PubMed] [Google Scholar]
- [27].Haas S, Trumpp A, Milsom MD. Causes and Consequences of Hematopoietic Stem Cell Heterogeneity. Cell stem cell. 2018;22:627–38. [DOI] [PubMed] [Google Scholar]
- [28].Cancro MP. B cells and aging: gauging the interplay of generative, selective, and homeostatic events. Immunol Rev. 2005;205:48–59. [DOI] [PubMed] [Google Scholar]
- [29].Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–75. [DOI] [PubMed] [Google Scholar]
- [30].George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today. 1996;17:267–72. [DOI] [PubMed] [Google Scholar]
- [31].Eaton SM, Maue AC, Swain SL, Haynes L. Bone marrow precursor cells from aged mice generate CD4 T cells that function well in primary and memory responses. Journal of immunology (Baltimore, Md : 1950). 2008;181:4825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nasi M, Troiano L, Lugli E, Pinti M, Ferraresi R, Monterastelli E, et al. Thymic output and functionality of the IL-7/IL-7 receptor system in centenarians: implications for the neolymphogenesis at the limit of human life. Aging Cell. 2006;5:167–75. [DOI] [PubMed] [Google Scholar]
- [34].den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–97. [DOI] [PubMed] [Google Scholar]
- [35].Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Seminars in immunology. 2012;24:342–9. [DOI] [PubMed] [Google Scholar]
- [36].Becklund BR, Purton JF, Ramsey C, Favre S, Vogt TK, Martin CE, et al. The aged lymphoid tissue environment fails to support naive T cell homeostasis. Sci Rep. 2016;6:30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. Journal of immunology (Baltimore, Md : 1950). 2005;175:3262–7. [DOI] [PubMed] [Google Scholar]
- [38].Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. Journal of immunology (Baltimore, Md : 1950). 2008;180:2741–6. [DOI] [PubMed] [Google Scholar]
- [39].Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699–706. [DOI] [PubMed] [Google Scholar]
- [41].Bodnar AG, Kim NW, Effros RB, Chiu CP. Mechanism of telomerase induction during T cell activation. Exp Cell Res. 1996;228:58–64. [DOI] [PubMed] [Google Scholar]
- [42].Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, et al. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. Journal of immunology (Baltimore, Md : 1950). 1995;155:3711–5. [PubMed] [Google Scholar]
- [43].Nikolich-Zugich J Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. Journal of immunology (Baltimore, Md : 1950). 2014;193:2622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–69. [DOI] [PubMed] [Google Scholar]
- [45].Vallejo AN, Brandes JC, Weyand CM, Goronzy JJ. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. Journal of immunology (Baltimore, Md : 1950). 1999;162:6572–9. [PubMed] [Google Scholar]
- [46].Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. Journal of immunology (Baltimore, Md : 1950). 2002;168:5893–9. [DOI] [PubMed] [Google Scholar]
- [48].Effros RB, Walford RL. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983;81:298–305. [DOI] [PubMed] [Google Scholar]
- [49].Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100:15053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. Journal of immunology (Baltimore, Md : 1950). 2014;192:2689–98. [DOI] [PubMed] [Google Scholar]
- [52].Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Mowes B, et al. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35:1987–94. [DOI] [PubMed] [Google Scholar]
- [53].Valkenburg SA, Venturi V, Dang TH, Bird NL, Doherty PC, Turner SJ, et al. Early priming minimizes the age-related immune compromise of CD8(+) T cell diversity and function. PLoS Pathog. 2012;8:e1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Crotty S, Ahmed R. Immunological memory in humans. Seminars in immunology. 2004;16:197–203. [DOI] [PubMed] [Google Scholar]
- [55].Weksler ME, Pawelec G, Franceschi C. Immune therapy for age-related diseases. Trends Immunol. 2009;30:344–50. [DOI] [PubMed] [Google Scholar]
- [56].Weinberg A, Kroehl ME, Johnson MJ, Hammes A, Reinhold D, Lang N, et al. Comparative immune responses to licensed herpes zoster vaccines. J Infect Dis. 2018;218:S81–s7. [DOI] [PubMed] [Google Scholar]
- [57].Scheuring UJ, Sabzevari H, Theofilopoulos AN. Proliferative arrest and cell cycle regulation in CD8(+)CD28(−) versus CD8(+)CD28(+) T cells. Hum Immunol. 2002;63:1000–9. [DOI] [PubMed] [Google Scholar]
- [58].Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4+ CD28− T cells in the aging immune system. Mech Ageing Dev. 1998;102:131–47. [DOI] [PubMed] [Google Scholar]
- [60].McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. Journal of immunology (Baltimore, Md : 1950). 2006;176:6333–9. [DOI] [PubMed] [Google Scholar]
- [61].Kaml M, Weiskirchner I, Keller M, Luft T, Hoster E, Hasford J, et al. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine. 2006;24:6808–11. [DOI] [PubMed] [Google Scholar]
- [62].Kolibab K, Smithson SL, Shriner AK, Khuder S, Romero-Steiner S, Carlone GM, et al. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I. Antibody concentrations, avidity and functional activity. Immun Ageing. 2005;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94. [DOI] [PubMed] [Google Scholar]
- [64].Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Weinberger B, Schirmer M, Matteucci Gothe R, Siebert U, Fuchs D, Grubeck-Loebenstein B. Recall responses to tetanus and diphtheria vaccination are frequently insufficient in elderly persons. PLoS One. 2013;8:e82967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. [DOI] [PubMed] [Google Scholar]
- [67].Paganelli R, Quinti I, Fagiolo U, Cossarizza A, Ortolani C, Guerra E, et al. Changes in circulating B cells and immunoglobulin classes and subclasses in a healthy aged population. Clinical and experimental immunology. 1992;90:351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. Journal of immunology (Baltimore, Md : 1950). 2008;180:5283–90. [DOI] [PubMed] [Google Scholar]
- [69].Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jiang N, He J, Weinstein JA, Penland L, Sasaki S, He XS, et al. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5:171ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Schenkein JG, Park S, Nahm MH. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine. 2008;26:5521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. Journal of immunology (Baltimore, Md : 1950). 2004;172:2155–62. [DOI] [PubMed] [Google Scholar]
- [73].Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog. 2012;8:e1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49:725–39. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Aydar Y, Balogh P, Tew JG, Szakal AK. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. Journal of immunology (Baltimore, Md : 1950). 2003;171:5975–87. [DOI] [PubMed] [Google Scholar]
- [79].Sage PT, Tan CL, Freeman GJ, Haigis M, Sharpe AH. Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep. 2015;12:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Herati RS, Reuter MA, Dolfi DV, Mansfield KD, Aung H, Badwan OZ, et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. Journal of immunology (Baltimore, Md : 1950). 2014;193:3528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Thompson HL, Smithey MJ, Surh CD, Nikolich-Zugich J. Functional and homeostatic impact of age-related changes in lymph node stroma. Front Immunol. 2017;8:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kityo C, Makamdop KN, Rothenberger M, Chipman JG, Hoskuldsson T, Beilman GJ, et al. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J Clin Invest. 2018;128:2763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Richner JM, Gmyrek GB, Govero J, Tu Y, van der Windt GJ, Metcalf TU, et al. Age-dependent cell trafficking defects in draining lymph nodes impair adaptive immunity and control of west nile virus infection. PLoS Pathog. 2015;11:e1005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci Rep. 2016;6:25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Jin R, Kaneko H, Suzuki H, Arai T, Teramoto T, Fukao T, et al. Age-related changes in BAFF and APRIL profiles and upregulation of BAFF and APRIL expression in patients with primary antibody deficiency. Int J Mol Med. 2008;21:233–8. [PubMed] [Google Scholar]
- [86].Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. Jama. 1994;272:1661–5. [PubMed] [Google Scholar]
- [87].Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. The Journal of infection. 2017;75:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Robertson CA, DiazGranados CA, Decker MD, Chit A, Mercer M, Greenberg DP. Fluzone(R) High-Dose Influenza Vaccine. Expert Rev Vaccines. 2016;15:1495–505. [DOI] [PubMed] [Google Scholar]
- [89].Tsai TF. Fluad(R)-MF59(R)-Adjuvanted influenza vaccine in older adults. Infect Chemother. 2013;45:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Takahashi M, Okuno Y, Otsuka T, Osame J, Takamizawa A. Development of a live attenuated varicella vaccine. Biken J. 1975;18:25–33. [PubMed] [Google Scholar]
- [91].Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc. 2009;109:S13–7. [PubMed] [Google Scholar]
- [92].Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. [DOI] [PubMed] [Google Scholar]
- [93].Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lecrenier N, Beukelaers P, Colindres R, Curran D, De Kesel C, De Saegher JP, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vaccines. 2018;17:619–34. [DOI] [PubMed] [Google Scholar]
- [95].Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS, et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis. 2011;11:91–101. [DOI] [PubMed] [Google Scholar]
- [96].Villa M, Black S, Groth N, Rothman KJ, Apolone G, Weiss NS, et al. Safety of MF59-adjuvanted influenza vaccination in the elderly: results of a comparative study of MF59-adjuvanted vaccine versus nonadjuvanted influenza vaccine in northern Italy. Am J Epidemiol. 2013;178:1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lode H, Schmoele-Thoma B, Gruber W, Ahlers N, Fernsten P, Baker S, et al. Dose-ranging study of a single injection of pneumococcal conjugate vaccine (1 x, 2 x, or 4 x) in healthy subjects aged 70 years or older. Vaccine. 2011;29:4940–6. [DOI] [PubMed] [Google Scholar]
- [98].Levin MJ, Schmader KE, Pang L, Williams-Diaz A, Zerbe G, Canniff J, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis. 2016;213:14–22. [DOI] [PubMed] [Google Scholar]
- [99].Antrobus RD, Lillie PJ, Berthoud TK, Spencer AJ, McLaren JE, Ladell K, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS One. 2012;7:e48322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tsang P, Gorse GJ, Strout CB, Sperling M, Greenberg DP, Ozol-Godfrey A, et al. Immunogenicity and safety of Fluzone((R)) intradermal and high-dose influenza vaccines in older adults >/=65 years of age: a randomized, controlled, phase II trial. Vaccine. 2014;32:2507–17. [DOI] [PubMed] [Google Scholar]
- [101].Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. [DOI] [PubMed] [Google Scholar]
- [102].Li Y, Wang S, Xin W, Scarpellini G, Shi Z, Gunn B, et al. A sopB deletion mutation enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Infect Immun. 2008;76:5238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


