Abstract
The comorbidity between alcohol use disorder and post-traumatic stress disorder represents a serious health care burden with few effective treatment options. The current study was designed to evaluate the effect of an alpha 1 receptor antagonist (doxazosin) and a novel anticonvulsant (zonisamide) in a model of alcohol (ethanol) dependence and stress exposure. The main dependent variable was voluntary ethanol intake in mice that experienced chronic intermittent ethanol (CIE) exposure and forced swim stress (FSS) alone, and in combination. Adult male and female C57BL/6J mice had access to a single bottle of 15% (v/v) ethanol for 1-hr in the home cage, 3-hr into the dark phase of the light/dark cycle. Once stable ethanol intake was established (~4 weeks), mice were separated into four groups (CTL, CIE, FSS, CIE + FSS). Mice in the FSS condition received 10-min FSS exposure 4-hr prior to drinking sessions (remaining mice were not disturbed). During baseline and the first two test cycles, all mice received vehicle (saline) injections (IP) 30-min before ethanol access. As previously observed, FSS increased ethanol drinking in dependent (CIE-exposed) mice but not in nondependent control (CTL) mice. In the following test cycles mice were evaluated for ethanol intake after administration of doxazosin, zonisamide or their combination. Results indicated that the three doses of doxazosin evaluated significantly reduced voluntary ethanol intake in all mice. Zonisamide had a more modest effect and may require a more prolonged treatment regime. The combined administration of both compounds was not more effective than each drug alone. This study suggests that doxazosin is reliable at reducing voluntary ethanol intake in mice independently of their history of ethanol dependence and stress exposure.
Keywords: Ethanol dependence, Stress, Mouse, Zonisamide, Doxazosin
Introduction
Alcohol abuse and dependence are serious medical and social problems in the United States and constitute a major public health concern. In the past decade, alcohol use and abuse has significantly increased in the U.S (Dawson, Goldstein, Saha, & Grant, 2015). Likewise, post-traumatic stress disorder (PTSD) is a debilitating neuropsychiatric disorder with an especially high prevalence in Veterans (Wisco, Marx, Miller, et al., 2016). Further aggravating the situation, there is a high comorbidity between PTSD and alcohol use disorder (AUD). Between 28% and 75% of people with PTSD also present with AUD (Petrakis, Simpson 2017). Conversely, individuals with AUD are more likely to suffer PTSD (Grant; Goldstein, Saha, et al., 2015). Further, co-occurrence of PTSD and AUD is associated with more severe clinical symptoms compared to either disorder alone (Blanco et al., 2013), and clinical studies indicate that alcohol abuse exacerbates PTSD symptoms and contributes to the maintenance of PTSD-related problems/symptoms (Jacobsen, Southwick, Koste, 2001; Ouimette, Read, Wade, & Tirone, 2010). Despite the significant health care burden of this clinical issue, there are too few treatments that effectively address the problem of PTSD-AUD comorbidity (Allen, Crawford, & Kudler, 2016; Petrakis, Simpson 2017). Use of animal models is critical for advancing our understanding of underlying mechanisms for these co-occurring disorders, as well as providing platforms for evaluating potential new and novel treatment interventions. The present study was conducted to evaluate the effect of two pharmacological agents on voluntary alcohol intake using a model involving stress exposure and alcohol dependence in mice.
Several studies have implicated a role for adrenergic alpha-1 receptors in alcohol and substance use disorders. For example, the alpha-1 receptor antagonist prozasin, used to treat hypertension, has also been shown to reduce both cocaine self-administration and reinstatement of cocaine seeking behavior (Wee, Mandyam, Lekic, & Koob, 2008; Zang et al., 2005, 2007). Similarly, the administration of prozasin was reported to reduce alcohol drinking in rats selectively bred for high alcohol preference (Froehlich, Hausauer, Federoff, Fischer, & Rasmussen, 2013; Froehlich JC, Hausauer BJ and Rasmussen DD, 2013; Rasmussen, Alexander, Raskind, & Froehlich, 2009). Prazosin also was effective in reducing alcohol responding in dependent rats (Walker, Rasmussen, Raskind, & Koob, 2008), as well as intake in relapse models involving repeated alcohol deprivation periods (Froehlich, Hausauer, Fischer, Wise, & Rasmussen, 2015) and stress-induced reinstatement of alcohol seeking behavior (Le et al., 2011). However, this drug has a relatively short half-life and requires repeated dosing throughout the day in humans. Doxazosin, another noradrenergic alpha-1 adrenoblocker, has emerged as an alternative due to its longer half-life (22-hr vs. 3-4-hr for prozasin). Similar to the effects of prozasin, doxazosin has been shown to block the development and expression of sensitization to chronic cocaine administration in rats (Haile, Hao, O'Malley, Newton, & Kosten, 2012). Doxazosin also reduced alcohol intake in alcohol-preferring (P) rats (O'Neil et al., 2013) and stress-induced reinstatement of alcohol seeking in rats (Funk et al., 2016). Recent clinical evidence indicates that doxazosin can reduce alcohol intake in patients with a positive family history of AUD (Kenna et al., 2016). Further, doxazosin has been used to treat patients with PTSD (De Jong, Wauben, Huijbrechts, Oolders, & Haffmans, 2010). Thus, it is reasonable to suspect that doxazosin will be effective in reducing stress-enhanced alcohol consumption.
Anticonvulsant agents have also been used in clinical and pre-clinical studies to treat alcohol dependence. Old (e.g., valproate, carbamazepine) as well as new (e.g., gabapentin, topiramate) anticonvulsants have been used for detoxification and have shown some efficacy in reducing alcohol intake in clinical trials (Knapp, Ciraulo, Sarid-Segal et al., 2015; Litten, Wilford, Falk, Ryan, & Fertig, 2016; Rubio, Lopez-Munoz, Ferre, et al., 2010). Zonisamide is a relatively new anticonvulsant agent related to topiramate, and it has been shown to reduce alcohol intake in clinical trials. Importantly, zonisamide may have fewer side effects compared to topiramate, especially regarding cognitive function (Buoli, Grassi, Ciappolino, Serati, & Altamura, 2017; Knapp, Ciraulo, Sarid-Segal et al., 2015; Litten et al., 2016). Studies have also shown that zonisamide can reduce alcohol intake in rodents (Knapp et al., 2007). Finally, there is some evidence indicating that drug combinations may be more effective in treating excessive alcohol intake. For example, co-administration of naltrexone and bupropion has been found to reduce binge-like drinking with a greater effect than either drug alone in mice (Navarro, Luhn, Kampov-Polevoy, Garbutt, & Thiele, 2019) and humans (Walter et al., 2020). Additionally, several studies found prazosin in combination with naltrexone to be more effective in reducing drinking than either drug given alone (Froelich, Hausauer, Rasmussen 2013; Rasmussen, Kincaid, Froelich 2015; Verplaetse, Czachowski 2015).
The current study was designed to evaluate the effect of doxazosin and zonisamide, alone and in combination, on voluntary alcohol intake using a model of stress-enhanced alcohol drinking in male and female mice. The model involves repeated cycles of chronic intermittent ethanol (CIE) vapor exposure along with exposure to forced swim stress (FSS) that leads to significantly higher levels of voluntary alcohol intake in alcohol-dependent mice using a limited access procedure (Anderson, Lopez, & Becker, 2016a, 2016b). The main hypotheses tested were that treatment with these compounds would decrease voluntary alcohol consumption, and the effect would be most robust in stressed, alcohol-dependent subjects. It was also hypothesized that the combined administration of these drugs, an alpha-1 receptor antagonist and an anticonvulsant, would be more effective in reducing alcohol drinking in this model.
Material and methods
Subjects
Adult male and female C57BL/6J mice (10 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were individually housed in standard polycarbonate cages with corncob bedding in a temperature- and humidity-controlled vivarium within an AAALAC-accredited facility. Subjects were maintained on a 12-hr modified light/dark cycle with ad libitum access to food and water throughout experimentation. Procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (8th edition, National Research Council, 2011) and under protocols approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
Procedure
All mice were evaluated in the CIE-Stress Drinking model, as illustrated in Fig. 1. Briefly, mice were first trained to drink alcohol (15% v/v) using a limited access (1 h/day) procedure starting 3 h after lights off. Once stable baseline intake was established (~3 weeks), mice were separated into four groups (balanced for baseline level of alcohol intake): control (CTL), FSS alone, CIE alone, and CIE + FSS. Mice in the CIE and CIE + FSS groups received CIE vapor exposure in inhalation chambers (16-hr/day for 4 days). The remaining groups (CTL and FSS) were similarly handled but maintained in control (air) inhalation chambers. Limited access drinking sessions were suspended during inhalation exposure. After a 72-hr abstinence period, test drinking sessions resumed for 5 consecutive days under the same limited access conditions as before. This pattern of weekly CIE (or air) exposure alternating with weekly limited access drinking sessions was repeated for 5 cycles (Test Cycles 1–5) (Fig. 1). Mice in the FSS and CIE + FSS groups experienced brief (10-min) FSS exposure 4-hr prior to each of the test drinking sessions as detailed below. The remaining non-stressed CTL and CIE groups remained in their home cage undisturbed. During the last week of Baseline and during Tests 1 and 2, all mice received injections (IP) of vehicle 30-min prior to the start of the drinking sessions. During Tests 3 and 4, separate groups of mice in each of the four groups received injections (IP) of zonisamide (0, 25, 50, 100 mg/kg) or doxazosin (0, 5, 10, 20 mg/kg) 30-min prior to the drinking sessions. The order of drug tested was counter-balanced across Test 3 and Test 4 for all groups within each dose level (i.e., mice were assigned to the low, medium, or high dose of both drugs and half the mice in each of the groups were tested with zonisamide during Test 3 and doxasozin during Test 4 while the other half received doxazosin during Test 3 and zonisamide during Test 4). During Test 5, mice received injections of vehicle, zonisamide (25 mg/kg) or doxazosin (10 mg/kg), or the combination (25 mg/kg zonisamide + 10 mg/kg doxazosin) 30-min prior to the test drinking sessions.
Fig. 1.
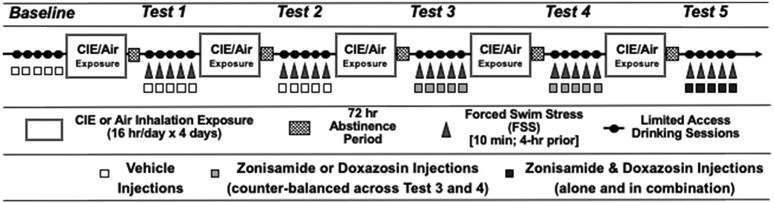
Schedule of cycles of voluntary ethanol intake, CIE or air control exposure, stress experience, and pre-treatment with doxazosin, zonisamide, or their combination during the different phases of the study.
Chronic intermittent ethanol (CIE) exposure
Ethanol vapor was administered in inhalation chambers according to procedures previously described (Anderson et al., 2016a, 2016b; Griffin, Lopez, & Becker, 2009; Griffin, Lopez, Yanke, Middaugh and Becker 2009; Lopez, Anderson & Becker, 2016). Briefly, mice were placed in Plexiglas inhalation chambers (60W x 60L x 36H cm) and exposed to ethanol vapor at levels set to yield stable blood ethanol concentrations (BEC) in the range of 175–225 mg/dl. Separate sets of inhalation chambers were used for males and females to better control vapor concentrations that yielded similar BEC values for males and females. Housing conditions were similar to those in the colony room. Blood samples (40 μl) were obtained from the retro-orbital sinus with heparinized capillary tubes during each CIE cycle of exposure. Blood samples were centrifuged, and plasma was processed in an Analox Instrument analyzer (Lunenburg, MA), with blood ethanol levels expressed as mg/dl.
Forced swim stress
Mice were individually placed in glass cylinders (20-cm diameter x 40-cm tall) filled with water maintained at 23–25 °C for 10 min (Anderson et al., 2016a, 2016b; Lopez, Anderson & Becker, 2016).
Locomotor activity test
A separate group of male mice were injected with either vehicle, or doxazosin 10 mg/kg or 20 mg/kg and placed in an activity monitor for 10 min (Med Associates Inc. Fairfax, VT). Half of the vehicle treated mice (n = 4) were placed in the open field 30 min after injection, and the other half 90 min after injection. Similarly, separate groups of mice that received each dose of doxazosin (7–8/group) were evaluated at 30- or 90-min post-injection. The time to evaluate locomotor activity was selected to match the start and the end of the ethanol drinking session.
Drugs
Doxazosin mesylate was obtained from Tocris and zonisamide was obtained from SunPharma. Doses of these drugs were prepared fresh daily with saline used as the vehicle. The volume of intra-peritoneal (IP) administration was 10 ml/kg.
Design and data analysis
Daily voluntary alcohol intake (g/kg) was recorded for each animal throughout Baseline and during each Test cycle. Daily intake values were averaged over the last five days of Baseline and the five days of each Test cycle. Alcohol intake data during Baseline and during the first two Test cycles were analyzed using a repeated measures ANOVA model with Sex and Group (CTL, FSS, CIE, CIE + FSS) as between subject factors and Phase (Baseline, Test 1, Test 2) as the within subject repeated measure (n = 31–32/group). Data obtained during Test cycles 3 and 4 were analyzed using a repeated measures ANOVA model, with Sex, Group (CTL, FSS, CIE, CIE + FSS), and Treatment (Vehicle, Zon 25, Zon 50, Zon 100, Dox 5, Dox 10, Dox 20) as between subject factors (n = 7–8/group) and Order (Test 3 or Test 4) as a repeated measure. Data from Test 5 was analyzed by ANOVA, with Sex, Group (CTL, FSS, CIE, CIE + FSS), and Treatment (Vehicle, Zon 25, Dox 10, Zon + Dox) as between subject factors (n = 6–12/group). Data obtained in the locomotor activity test were analyzed with one-way ANOVA. Data from vehicle groups was combined and the five groups under analysis were: Vehicle; Dox 10 mg/kg, 30 min; Dox 20 mg/kg, 30 min; Dox 10 mg/kg, 90 min; Dox 20 mg/kg, 90 min. All data analyses were conducted using SAS (ver. 9.4). For post-hoc comparisons, p-values were adjusted for multiple comparisons using False Discovery Rate (FDR.
Results
Early test cycles
Analysis of Baseline, Test 1, and Test 2 levels of intake (g/kg) indicated significant main effects of Group [F (3,264) = 7.85; p < 0.001], Phase [F (2,488) = 62.46; p < 0.001], and significant interactions between Sex and Phase [F (2,488) = 45.16; p < 0.001] and Group and Phase [F (6,507) = 6.58; p < 0.001]. Post-hoc comparisons based on the Group × Phase interaction indicated that alcohol intake for the four experimental groups did not differ during the last week of baseline (confirming that mice exhibited the same level of alcohol intake before they were randomly assigned to one of the four experimental groups). Mice in the CTL condition continued to drink at the same level registered during Baseline. Mice in the FSS, CIE, and CIE + FSS groups evidenced significantly higher levels of alcohol intake during Test 2 compared to their own Baseline (p < 0.001, * in Fig. 2). Further, mice in the CIE + FSS consumed significantly more alcohol than mice in the other three groups during Test 2 (p < 0.001, # in Fig. 2).
Fig. 2.
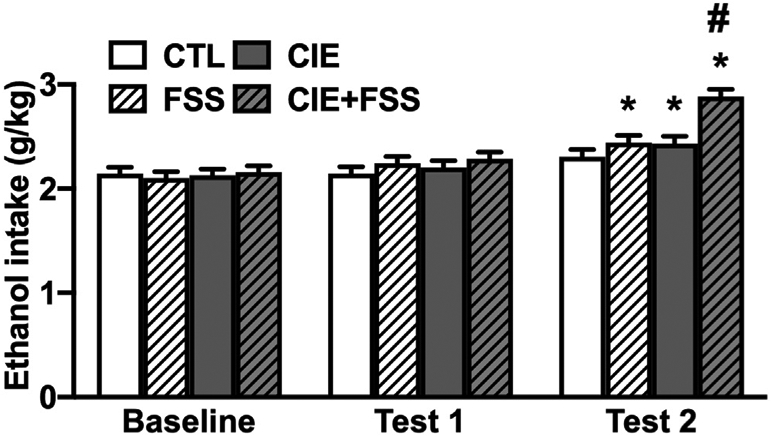
Ethanol intake (g/kg) in mice that experienced CIE or CTL exposure with or without stress before drinking during baseline and the first two test cycles. During this phase of the study all mice received vehicle injections before drinking ethanol. * indicates and increase in intake compared to their own baseline (p < 0.05). # indicates higher level of intake compare to the other groups in this test cycle (p < 0.05). Data are averaged across sex and are depicted as mean ± SEM
Test cycles 3 and 4: evaluation of doxazosin and zonisamide treatments
Alcohol intake values were averaged within Test Cycles 3 and 4 for each group. ANOVA indicated significant main effects of Sex [F (1,138) = 26.93; p < 0.001], Group [F (3,201) = 26.93; p < 0.001], Treatment [F (6,337) = 20.67; p < 0.001], and Order [F (1,224) = 94.26, p < 0.001], as well as a significant Sex × Group interaction [F (3,201) = 2.79; p < 0.05]. The main effect of Sex was due to greater overall alcohol consumption in males compared to females (p < 0.001). The main effect of Order was driven by an overall higher level of alcohol consumption during Test 3 compared to Test 4, but the variable Order did not interact with any other factor in the analysis. Mice in the CIE and the CIE + FSS groups consumed more alcohol than CTL mice (p < 0.001), and the CIE + FSS group showed the highest level of intake during Tests 3 and 4. Paired comparisons based on the Sex × Group interaction indicated that females in the CTL, FSS, and CIE groups consumed less alcohol than males in the same respective groups (ps < 0.05). Finally, post-hoc comparisons based on the main effect of Treatment indicated that all doses of doxazosin (5, 10, 20 mg/kg) and the middle dose of zonisamide (50 mg/kg) produced overall lower levels of alcohol intake compared to control vehicle-injected subjects (p < 0.002, * in Fig. 3). Also, regardless of group assignment, alcohol intake was significantly lower in mice that received doxazosin treatment compared to mice that received zonisamide treatment (p < 0.001). The Group × Treatment interaction did not achieve statistical significance [F (18 337) = 1.01; p > 0.05].
Fig. 3.
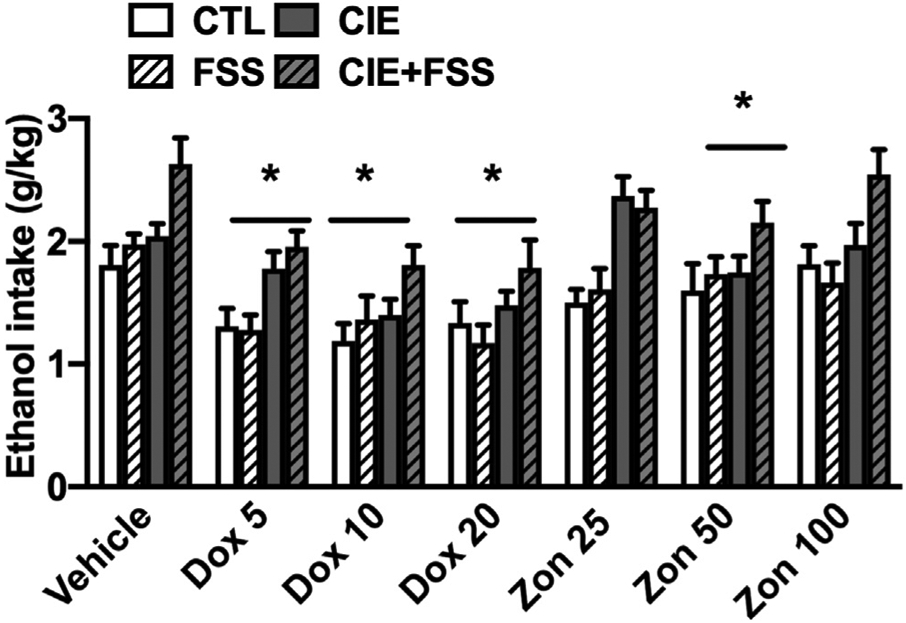
Ethanol intake for CTL, CIE, FSS, and CIE + FSS subjects that received vehicle or different doses of doxazosin or zonisamide before drinking in their home cage. * indicates a significant decrease in intake compared to the vehicle condition (p < 0.05). Data are averaged across sex and are depicted as mean ± SEM
Test cycle 5: evaluation of doxazosin and zonisamide, alone and in combination
ANOVA indicated a main effect of Sex [F (1,142) = 57.01; p < 0.001] due to lower intake in females compared to males, and a significant effect of Group [F (3,206) = 19.99; p < 0.001] due to greater alcohol consumption in the CIE and CIE + FSS groups compared to mice in the CTL and FSS groups (p < 0.05). Also, mice in the CIE + FSS group showed the highest level of intake among all groups. The analysis also indicated a significant main effect of Treatment [F (3,215) = 11.73; p < 0.001]. Post-hoc comparisons indicated that mice that received doxazosin (10 mg/kg), zonisamide (25 mg/kg), and the combination of both drugs consumed less alcohol than vehicle treated mice (p < 0.005, * in Fig. 4). Notably, the reduction in alcohol consumption was no different in the mice that received the combination of doxazosin and zonisamide in comparison to mice that received either drug alone. The Group x Treatment and Group x Treatment × Sex interactions did not achieve statistical significance [F (9,214) = 0.36; p > 0.05, and F (9,214) = 0.98; p > 0.05, respectively].
Fig. 4.
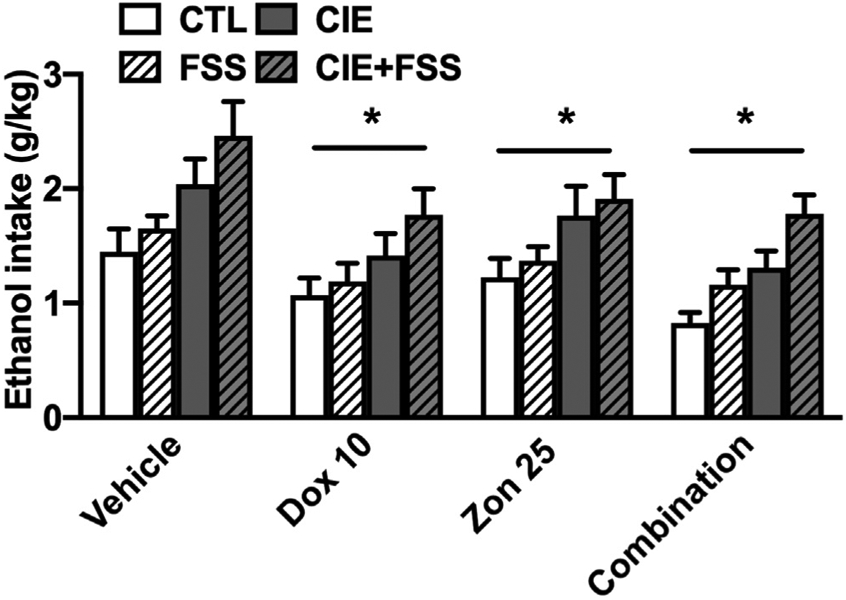
Ethanol intake for CTL, CIE, FSS, and CIE + FSS subjects that received vehicle, doxazosin (10 mg/kg), zonisamide (25 mg/kg) or the combination before drinking in their home cage. * indicates a significant decrease in intake compared to the vehicle condition (p < 0.05). Data are averaged across sex and are depicted as mean ± SEM
Locomotor activity test
Analysis of total ambulatory counts during the 10 min test failed to indicate an effect of doxazosin treatment 30 or 90 min after injections [F (4,34) = 1.79; p = NS) (Table 1). Similar results were obtained in the analysis of time displaying locomotor behavior [F (4,34) = 1.58; p = NS] (data not shown).
Table 1.
Total Ambulatory counts during a 10 min open field test
| Group | Vehicle | Dox 10 mg/kg 30 min |
Dox 20 mg/kg 30 min |
Dox 10 mg/kg 90 min |
Dox 20 mg/kg 90min |
|---|---|---|---|---|---|
| Mean ± | 791.11 | 821.13 | 644.38 | 708.71 | 573.25 |
| SEM | 82.10 | 80.82 | 97.52 | 68.80 | 45.59 |
Discussion
The results of this study show that male and female mice that experienced repeated cycles of chronic intermittent ethanol (CIE) vapor exposure and were exposed to stress showed a significant increase in voluntary ethanol intake compared to mice that received CIE only, stress only, or neither treatment. While this stress-enhanced drinking in CIE-exposed mice has been demonstrated in several studies in male mice (Lopez & Becker, 2005, Anderson et al., 2016a, 2016b, Becker and Lopez, 2004), this is the first report of a similar effect in female mice. As previously noted, female mice typically consume more alcohol than males (both volume and g/kg dosage) (Becker & Koob, 2016; Lopez, Grahame & Becker, 2011, Lopez, Miles, Williams & Becker, 2017). Typically, female mice consume more alcohol than males (both volume and g/kg dosage) (Becker, Koob 2016; Lopez, Grahame & Becker, 2011, Lopez, Miles, Williams & Becker, 2017). However, these studies involved access to alcohol for two, four, or even 24 h periods. In the present study, with access restricted to alcohol for only 1 h, there were negligible differences in alcohol consumption between males and females. Regardless, the selective effect of stress (FSS) increasing drinking in CIE-exposed mice was comparable in males and females.
Across all groups, all doses of doxazosin (5–20 mg/kg) evaluated significantly reduced alcohol intake compared to the vehicle condition. Although this effect appeared to be most robust in the CIE + FSS group, this did not achieve statistical significance. Given that decreased alcohol consumption was evident in all experimental groups, this raises the possibility that the drug effect was due to a general non-specific sedative effect, In one study, a dose of doxazosin (10 mg/kg) that reduced alcohol intake in alcohol-preferring (P) rats also produced a slight reduction in locomotor activity, but this dose did not affect sucrose or food intake (O'Neil et al., 2013). In addition, the reduction in alcohol intake was offset by an increase in water intake, which also argues against a sedative effect of doxazosin (O'Neil et al., 2013). In addition, the data presented in this manuscript also showed that the doses of doxazosin that reduced voluntary ethanol intake do not reduce locomotor activity in an open field in mice. Therefore, it is unlikely doxazosin-induced reduction in alcohol drinking was related to a general sedative effect of the drug.
The anticonvulsant zonisamide had a more modest, dose-related effect on alcohol consumption in the CIE-Stress Drinking model. During the first set of tests, the middle (50 mg/kg) dose of zonisamide reduced alcohol intake in all groups of mice. In subsequent testing, the lower dose of zonisamide (25 mg/kg) was examined again alone and also in combination with doxazosin. In this case, 25 mg/kg zonisamide administered alone significantly reduced alcohol intake across all experimental groups. It is possible that prior history with drug treatment may have influenced results, but further studies will be needed to evaluate this possibility. Unlike doxazosin, the information about the effect of zonisamide administration on alcohol intake in rodents is very limited. The doses used here were selected based on the only study that, to our knowledge, has shown that zonisamide reduced alcohol intake in mice (Knapp et al., 2007).
Contrary to our hypothesis, the combined treatment with doxazosin and zonisamide was no more effective in reducing alcohol consumption than the effect of doxazosin or zonisamide treatment alone. One caveat to consider regarding the study design is that initial evaluation of the drug effects given alone (dose–response effects) was conducted over two test cycles. Mice that first received doxazosin treatment were then evaluated with zonisamide, and vice versa. Further, mice that received the low dose of one drug in Test cycle 3 received the corresponding low dose of the other drug in Test 4. Therefore, although the order of treatment was counter-balanced, all mice had a unique drug history prior to the second evaluation (Test 4). As it is difficult to discern the potential influence of this factor, subsequent studies will need to test the two drugs in independent groups of animals. Another important consideration is that both drugs and their combination were administered at the same time prior to the test drinking sessions. The pretreatment times were also based on previous studies, but it is possible that these may need to be adjusted. Perhaps pretreatment with doxasozin would have a different effect if administered before exposure to stress, or zonisamide may produce a different profile of results if it was administered during early withdrawal (prior to the test drinking sessions). Nevertheless, results presented here suggest that treatment with these drugs offer a promising alternative to effectively reduce voluntary alcohol intake.
Acknowledgments
Supported by the PASA Consortium W81XWH-15- 2–0077, NIAAA grants U24AA020929, U01 AA014095, and P50 AA010761, and VA Medical Research (BX000813).
References
- Allen JP, Crawford EF, & Kudler H (2016). Nature and treatment of comorbid alcohol problems and post traumatic stress disorder among American military personnel and Veterans. Alcohol Res, 38(1), 133–140. PMCID: PMC4872608. [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, & Becker HC (2016a). Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology, 233(11), 2035–2043. PMCID: PMC4864090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, & Becker HC (2016b). Stress-induced enhancement of ethanol intake in C57bl/6J mice with a history of chronic ethanol exposure: Involvement of kappa opioid receptors. Frontiers in Cellular Neuroscience, 10, 45. PMCID: PMC4763044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Koob GF (2016). Sex differences in animal models: Focus on addiction. Pharmacological Reviews, 68(2), 242–263. PMCID: PMC4813426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, & Lopez MF (2004). Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism: Clinical and Experimental Research, 28(12), 1829–1838. [DOI] [PubMed] [Google Scholar]
- Blanco C, Xu Y, Brady K, Perez-Fuentes G, Okuda M, & Wang S (2013). Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: Results from national epidemiological survey on alcohol and related conditions. Drug and Alcohol Dependence, 132(3), 630–638. PMCID: PMC3770804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoli M, Grassi S, Ciappolino V, Serati M, & Altamura AC (2017). The use of zonisamide for the treatment of psychiatric disorders: A systematic review. Clinical Neuropharmacology, 40(2), 85–92. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Saha TD, & Grant BF (2015). Changes in alcohol consumption: United States, 2001-2002 to 2012-2013. Drug and Alcohol Dependence, 148, 56–61. PMCID: PMC4330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong J, Wauben P, Huijbrechts I, Oolders H, & Haffmans J (2010). Doxazosin treatment for posttraumatic stress disorder. Journal of Clinical Psychopharmacology, 30(1), 84–85. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, & Rasmussen DD (2013). Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcoholism: Clinical and Experimental Research, 37(9), 1552–1560. PMCID: PMC3775948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer B, Fischer S, Wise B, & Rasmussen DD (2015). Prazosin reduces alcohol intake in an animal model of alcohol relapse. Alcoholism: Clinical and Experimental Research, 39(8), 1538–1546. PMCID: PMC4515780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, & Rasmussen DD (2013). Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcoholism: Clinical and Experimental Research, 37(10), 1763–1770. PMCID: PMC3795831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Li Z, Loughlin A, & Le AD (2016). Effects of prozasin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacology, 233, 2197–2207. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry, 72(8), 757–766. PMCID: PMC5240584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, & Becker HC (2009). Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice Alcoholism: Clinical and Experimental Research, 33(11), 1893–1900. PMCID: PMC2995298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Yanke AB, Middaugh LD, & Becker HC (2009). Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology, 201(4), 569–580. PMCID: PMC2590623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Hao Y, O'Malley PW, Newton TF, & Kosten TA (2012). The alpha1 antagonist doxazosin alters the behavioral effects of cocaine in rats. Brain Sciences, 2(4), 619–633. PMCID: PMC4061810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, & Kosten TR (2001). Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. American Journal of Psychiatry, 158(8), 1184–1190. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, et al. (2016). Role of the alpha1 blocker doxazosin in alcoholism: A proof-of-concept randomized controlled trial. Addiction Biology, 21(4), 904–914. PMCID: PMC4668239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp CM, Ciraulo DA, Sarid-Segal O, Richardson MA, Devine E, Streeter CC, et al. (2015). Zonisamide, topiramate, and levetiracetam: Efficacy and neuropsychological effects in alcohol use disorders. Journal of Clinical Psychopharmacology, 35(1), 34–42. PMCID: PMC4276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp CM, Mercado M, Markley TL, Crosby S, Ciraulo DA, & Kornetsky C (2007). Zonisamide decreases ethanol intake in rats and mice. Pharmacology Biochemistry and Behavior, 87(1), 65–72. PMCID: PMC2867456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, et al. (2011). Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology, 218(1), 89–99. PMCID: PMC3168954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Wilford BB, Falk DE, Ryan ML, & Fertig JB (2016). Potential medications for the treatment of alcohol use disorder: An evaluation of clinical efficacy and safety. Substance Abuse, 37(2), 286–298. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, & Becker HC (2016). Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol, 51, 17–23. PMCID: PMC4799834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, & Becker HC (2005). Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology, 181(4), 688–696. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, & Becker HC (2011). Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcoholism: Clinical and Experimental Research, 35(5), 953–962. PMCID: PMC3083457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Miles MF, Williams RW, & Becker HC (2017). Variable effects of chronic intermittent ethanol exposure on ethanol drinking in a genetically diverse mouse cohort. Alcohol, 58, 73–82. PMCID: PMC5253308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Luhn KL, Kampov-Polevoy AB, Garbutt JC, & Thiele TE (2019). Bupropion, alone and in combination with naltrexone, blunts binge-like ethanol drinking and intake following chronic intermittent access to ethanol in male C57bl/6J mice. Alcoholism: Clinical and Experimental Research, 43(5), 783–790. PMCID: PMC6502646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil ML, Beckwith LE, Kincaid CL, & Rasmussen DD (2013). The alpha1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) Rats. Alcoholism: Clinical and Experimental Research, 37(2), 202–212. PMCID: PMC3470781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette P, Read JP, Wade M, & Tirone V (2010). Modeling associations between posttraumatic stress symptoms and substance use. Addictive Behaviors, 35(1), 64–67. PMCID: PMC2763948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, & Simpson TL (2017). Posttraumatic stress disorder and alcohol use disorder: A critical review of pharmacologic treatments. Alcoholism: Clinical and Experimental Research, 41(2), 226–237. PMCID: PMC5375032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, & Froehlich JC (2009). The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcoholism: Clinical and Experimental Research, 33(2), 264–272. PMCID: PMC2692839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Kincaid CL, & Froehlich JC (2015). Prazosin + naltrexone decreases alcohol drinking more effectively than does either drug alone in P rats with a protracted history of extensive voluntary alcohol drinking, dependence, and multiple withdrawals. Alcoholism: Clinical and Experimental Research, 39(9), 1832–1841. PMCID: PMC4558320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio G, Lopez-Munoz F, Ferre F, Martinez-Gras I, Ponce G, Pascual JM, et al. (2010). Effects of zonisamide in the treatment of alcohol dependence. Clinical Neuropharmacology, 33(5), 250–253. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, & Czachowski CL (2015). Low-dose prazosin alone and in combination with propranolol or naltrexone: Effects on ethanol and sucrose seeking and self-administration in the P rat. Psychopharmacology, 232(15), 2647–2657. PMCID: PMC4504773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, & Koob GF (2008). alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol, 42(2), 91–97. PMCID: PMC2587143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Navarro M, Thiele TE, Pedersen C, Kampov-Polevoy A, & Garbutt JC (2020). A preliminary, open-label study of naltrexone and bupropion combination therapy for treating binge drinking in human subjects. Alcohol and Alcoholism, 55(1), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, & Koob GF (2008). Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. European Neuropsychopharmacology, 18(4), 303–311. PMCID: PMC2376122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco BE, Marx BP, Miller MW, Wolf EJ, Mota NP, Krystal JH, et al. (2016). Probable posttraumatic stress disorder in the US veteran population according to DSM-5: Results from the national health and resilience in Veterans study. Journal of Clinical Psychiatry, 77(11), 1503–1510. [DOI] [PubMed] [Google Scholar]
- Zhang XY, & Kosten TA (2005). Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biological Psychiatry, 57(10), 1202–1204. [DOI] [PubMed] [Google Scholar]
- Zhang XY, & Kosten TA (2007). Previous exposure to cocaine enhances cocaine self-administration in an alpha 1-adrenergic receptor dependent manner. Neuropsychopharmacology, 32(3), 638–645. [DOI] [PubMed] [Google Scholar]


