Abstract
Rationale
Patients with coronavirus disease-19-related acute respiratory distress syndrome (C-ARDS) could have a specific physiological phenotype as compared with those affected by ARDS from other causes (NC-ARDS).
Objectives
To describe the effect of positive end-expiratory pressure (PEEP) on respiratory mechanics in C-ARDS patients in supine and prone position, and as compared to NC-ARDS. The primary endpoint was the best PEEP defined as the smallest sum of hyperdistension and collapse.
Methods
Seventeen patients with moderate-to-severe C-ARDS were monitored by electrical impedance tomography (EIT) and evaluated during PEEP titration in supine (n = 17) and prone (n = 14) position and compared with 13 NC-ARDS patients investigated by EIT in our department before the COVID-19 pandemic.
Results
As compared with NC-ARDS, C-ARDS exhibited a higher median best PEEP (defined using EIT as the smallest sum of hyperdistension and collapse, 12 [9, 12] vs. 9 [6, 9] cmH2O, p < 0.01), more collapse at low PEEP, and less hyperdistension at high PEEP. The median value of the best PEEP was similar in C-ARDS in supine and prone position: 12 [9, 12] vs. 12 [10, 15] cmH2O, p = 0.59. The response to PEEP was also similar in C-ARDS patients with higher vs. lower respiratory system compliance.
Conclusion
An intermediate PEEP level seems appropriate in half of our C-ARDS patients. There is no solid evidence that compliance at low PEEP could predict the response to PEEP.
Keywords: ARDS, PEEP, Mechanical ventilation, COVID-19, Electrical impedance tomography
Introduction
Respiratory failure is the main cause of admitting patients with COVID-19 to intensive care unit (ICU). Contrary to the classical picture of acute respiratory distress syndrome (ARDS), studies have reported many COVID-19 patients presenting with severe hypoxemia despite normal respiratory system compliance (1, 2). Two phenotypes have been suggested (3). L phenotype combined low lung weight, low elastance, and low recruitability. Hypoxemia in these patients was possibly related to impaired pulmonary perfusion, hence the theoretically limited effect of high positive end-expiratory pressure (PEEP) levels. H phenotype may combine high lung weight, high elastance, and high recruitability, which fits typical ARDS picture where standard management including relatively high PEEP could be applied. Lung physiological phenotyping of COVID-19-related ARDS (C-ARDS) is still debated, especially when compared to non-COVID-19 ARDS (NC-ARDS). Hypoxemia is a hallmark of ARDS, and positive end-expiratory pressure (PEEP) and prone position are two common tools used for its management.
The objective of this study was to describe the physiological effects of PEEP on respiratory mechanics in supine and prone position in patients who required invasive ventilation for C-ARDS and to compare it to NC-ARDS.
Material and methods
Patients
Patients admitted to the medical intensive care unit of Henri Mondor University Hospital for ARDS between February 27, 2019, and April 4, 2020, were included. ARDS was defined according to the Berlin definition (4). C-ARDS was confirmed by positive nasopharyngeal polymerase chain reaction for SARS-CoV-2. Patients were excluded in case of a contraindication to impedance tomography (pacemaker, implantable defibrillator, skin lesion).
Monitoring
Patients were investigated by EIT (Enlight 1800, Timpel, Sao Paulo, Brazil). For such, a belt containing 32 electrodes was placed around the patient’s chest at the fifth or sixth intercostal space. EIT data were generated upon passing small alternate electrical current through that belt. Regional variations in impedance (∆Z) during ventilation map the tidal volume distribution in the lung and estimate regional compliance as follows. The fraction of Vt in each pixel is: V(pix) = Vt × ∆Z(pix)/∆Zglobal, and the compliance of a pixel is V(pix) divided by the global driving pressure. The PEEP titration tool helps map lung hyperdistension (regions associated with increase in local compliance when PEEP decreases) and lung collapse (regions associated with decrease in local compliance when PEEP decreases). The PEEP titration tool was used to determine the best PEEP which is defined by the best compromise between pulmonary hyperdistension and collapse.
It should be noted that compliance of the respiratory system measured by EIT may slightly differ from static compliance measured by the standard method, because plateau pressure and total PEEP are estimated on the airway pressure curve, without end-expiratory and end-inspiratory pauses.
Protocol
Investigations were performed on deeply sedated and paralyzed patients. The tidal volume was set at 6 mL/kg of ideal body weight, the respiratory rate was adjusted to maintain normal PaCO2, and the insufflation flow was set at 60 L/min. PEEP titration was performed using EIT tool, starting from a PEEP at 18 cmH2O (if the plateau pressure remained below 35 cmH2O) with a decrease of 3 cmH2O every two minutes, until reaching 6 cmH2O. No recruitment maneuver was performed before the PEEP trial. The titration began 5–10 min after setting PEEP at 18 cmH2O, after stabilization of the end-expiratory impedance.
In C-ARDS patients, we also performed measurement of the airway opening pressure (by insufflation at minimum flow to avoid resistive pressure) (5) and of the recruitment-to-inflation ratio (in order to determine the recruitment potential) (6). If the patient was turned prone within 24 h, the PEEP titration was repeated and then compared with the data measured in the supine position. The duration of prone positioning was 18 h. Readings of arterial blood gases (ABG) prior to exploration in supine, and last ABG at the end of proning, were collected.
If echocardiography (echo) was performed within 48 h before or after the explorations, the presence of acute cor pulmonale on standard echo or the presence of a patent foramen ovale after contrast injection was recorded. Similarly, if chest computed tomography (CT) scan was performed within 48 h before or after the explorations, the results were collected, including the presence or absence of posterior pulmonary consolidation on CT scan or pulmonary embolism on CT angiography. All CT scans were reviewed by the attending radiologist.
In all cases, at the end of the investigations, PEEP was set at the best level as evidenced by the PEEP titration tool and the respiratory mechanics.
Statistics
Quantitative data are expressed as median [first, third quartiles]. Curves of respiratory system compliance, hyperdistention, and collapse at different PEEP levels were assessed by computing areas under the curves (AUCs), as suggested by Matthews et al. (7). Briefly, the AUC was calculated by adding the areas under the graph between each pair of consecutive observations. For the measurements Y15 at PEEP15 and Y12 at PEEP12 for example, the area between those two PEEP was the product of the PEEP difference by the average of the two measurements:
We then compared AUCs between groups using Wilcoxon–Mann–Whitney test. Effects of prone positioning on continuous variables were studied using Wilcoxon paired test. Comparisons between C-ARDS and NC-ARDS patients relied on Mann–Whitney test for continuous variables. After determining median respiratory system compliance, patients with higher compliance values (i.e., > median) were compared with those with lower values. Owing to the exploratory nature of the study, no sample size calculation was needed.
Ethical issues
This is an ancillary report of an ongoing prospective monocentric observational study on EIT in patients with ARDS (CPP-66/17). Written informed consent was waived due to the observational nature of the study.
Results
Patient characteristics and outcomes
A total of 135 ARDS patients were admitted during the study period. Among them, 105 could not be included because of a contraindication to impedance tomography [including pacemaker or implantable defibrillator (n = 4), skin lesion (n = 4)], or lack of availability of material or personnel (n = 97). Thus, the present study comprises 30 patients investigated by EIT with PEEP titration, including 17 with C-ARDS and 13 with NC-ARDS [bacterial pneumonia (n = 5), tuberculosis (n = 1), pneumocystis (n = 1), aspiration pneumonia (n = 3), interstitial lung disease (n = 1), and extra-pulmonary sepsis (n = 2)]. Patients were explored a median of 1 [1, 2] days after intubation. The characteristics and outcomes of included patients are summarized in Table 1. C-ARDS and NC-ARDS patients had similar characteristics and outcomes, except for significantly lower SAPS 2 at admission, and more cor pulmonale on echocardiography in the former group.
Table 1.
Characteristics of 30 patients with acute respiratory distress syndrome induced or not by coronavirus disease-19
| C-ARDS (n = 17) | NC-ARDS (n = 13) | p value | |
|---|---|---|---|
| Patients' characteristics | |||
| Age (years) | 54 [50, 67] | 69 [53, 71] | 0.15 |
| Male, n (%) | 16/17 (94%) | 11/13 (85%) | 0.56 |
| Weight (kg) | 90 [80, 106] | 80 [66, 90] | 0.07 |
| Body mass index, kg/m2 | 30.2 [27.8, 33.2] | 28.7 [24.8, 31.1] | 0.16 |
| History of COPD, n (%) | 1/17 (6%) | 1/13 (8%) | > 0.99 |
| History of chronic heart failure, n (%) | 3/17 (18%) | 4/13 (31%) | 0.67 |
| History of chronic kidney failure, n (%) | 3/17 (18%) | 3/13 (23%) | > 0.99 |
| Immunosuppression, n (%) | 1/17 (6%) | 2/13 (15%) | 0.56 |
| Time from first symptoms to intubation (days) | 9 [6.5, 10] | NA | |
| SAPS 2 | 34 [27, 38] | 62 [42, 83] | 0.01 |
| PaO2/FiO2 at intubation (mmHg) | 98 [90, 144] | 135 [80, 182] | 0.64 |
| CT scan and echocardiography | |||
| Presence of lung consolidation on CT scan, n (%) | 2/11 (18%) | 4/5 (80%) | 0.04 |
| Pulmonary embolism on CT angiography, n (%) | 3/8 (38%) | 1/5 (20%) | > 0.99 |
| Acute cor pulmonale on echocardiography, n (%) | 8/17 (47%) | 1/13 (8%) | 0.04 |
| Patent foramen ovale, n (%) | 1/17 (6%) | 0 | > 0.99 |
| Outcomes | |||
| Need for vasopressor, n (%) | 11/17 (65%) | 11/13 (85%) | 0.41 |
| ECMO upon ICU stay, n (%) | 2/17 (12%) | 1/13 (8%) | > 0.99 |
| Tracheotomy during ICU stay, n (%) | 3/17 (18%) | 0 | 0.24 |
| Duration of mechanical ventilation (days) | 13 [9, 29] | 13 [8, 22] | 0.53 |
| Duration of ICU stay (days) | 18 [12, 30] | 17 [11, 22] | 0.75 |
| Death in ICU, n (%) | 4/17 (24%) | 6 /13 (46%) | 0.26 |
Continuous variables are expressed as median [interquartile range]
C-ARDS coronavirus disease-19-related acute respiratory distress syndrome, NC-ARDS noncoronavirus disease-19-related acute respiratory distress syndrome, BMI body mass index, COPD chronic obstructive pulmonary disease, SAPS simplified acute physiology score, CT computed tomography, ECMO extracorporeal membrane oxygenation, ICU intensive care unit, NA not available
Respiratory mechanics and PEEP titration
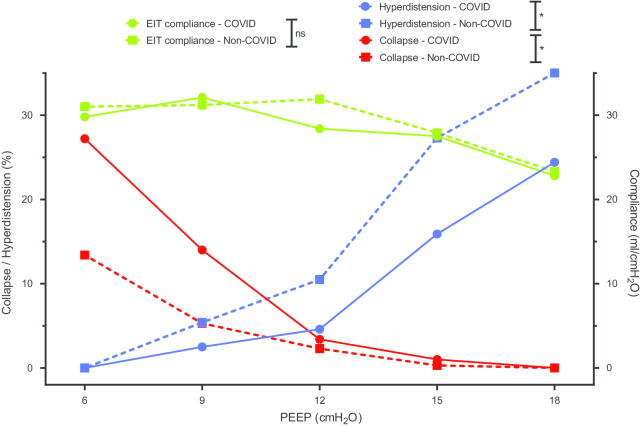
C-ARDS and NC-ARDS patients were similar in terms of hypoxemia, but with a trend to have higher body weights and respiratory system compliance in the former group (Tables 1, 2). The best PEEP (defined using EIT as the smallest sum of hyperdistension and collapse) ranged from 6 to 18 cmH2O, with a higher value in C-ARDS than NC-ARDS (Table 2, Fig. 1). C-ARDS patients had more derecruitment at lower PEEP and less hyperdistension at higher PEEP as compared to NC-ARDS patients (Table 2; Fig. 2).
Table 2.
Comparison of respiratory mechanics and PEEP titration in supine position, for patients with acute respiratory distress syndrome induced or not by coronavirus disease-19
| C-ARDS (n = 17) | NC-ARDS (n = 13) | p value | |
|---|---|---|---|
| Respiratory mechanics | |||
| Respiratory rate (breath/min) | 30 [28, 35] | 31 [29, 31] | 0.74 |
| Tidal volume (ml/kg of PBW) | 6.1 [5.9, 6.3] | 6.2 [6.0, 6.3] | 0.58 |
| CRS at low PEEP (mL/cmH2O) | 40 [32, 47] | 35 [26, 38] | 0.07 |
| Airway opening pressure (cmH2O) | 2 [0, 4] | NA | |
| Recruitment-to-inflation ratio | 0.46 [0.33, 0.52] | NA | |
| PaO2/FiO2 before EIT explorations (mmHg) | 133 [96, 180] | 120 [110, 137] | 0.40 |
| EIT-PEEP titration | |||
| Best PEEPa (cmH2O) | 12 [9, 12] | 9 [6, 9] | < 0.01 |
| Hyperdistension at PEEP 18 cmH2O, % | 24 [17, 30] | 35 [25, 41] | 0.02 |
| AUC for hyperdistension | 111 [68, 136] | 193 [119, 226] | 0.03 |
| Collapse at PEEP 6 cmH2O, % | 27 [20, 35] | 13 [7, 19] | < 0.01 |
| AUC for collapse | 94 [59, 141] | 45 [31, 61] | < 0.01 |
aDefined using EIT as the smallest sum of hyperdistension and collapse. Continuous variables are expressed as median [interquartile range]
C-ARDS coronavirus disease-19-related acute respiratory distress syndrome, NC-ARDS noncoronavirus disease-19-related acute respiratory distress syndrome, PBW predicted body weight, CRS respiratory system compliance, PEEP positive end-expiratory pressure, EIT electrical impedance tomography, AUC area under the curve, NA not available
Fig. 1.
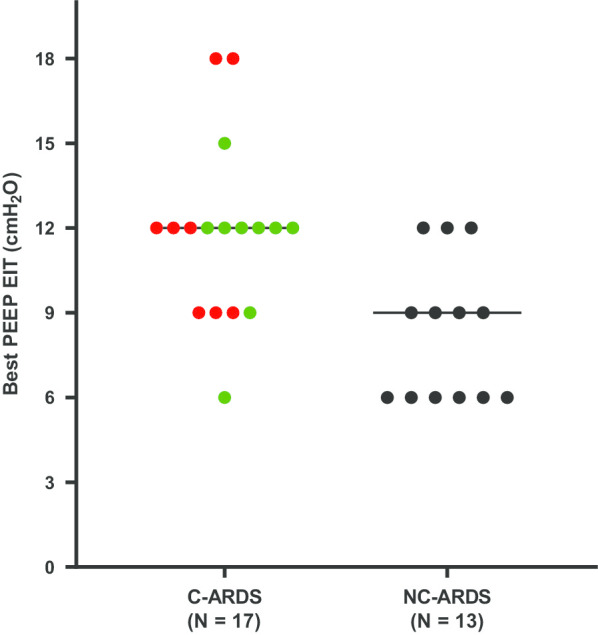
Best positive end-expiratory pressure as defined by electric impedance tomography in patients with acute respiratory distress syndrome related to coronavirus disease-19 (a) or not (b), with lower (red circles) versus higher (green circles) respiratory system compliance
Fig. 2.
Positive end-expiratory pressure titration in patients with acute respiratory distress syndrome associated or not with coronavirus disease-19. * denotes a p value < 0.05 for Wilcoxon–Mann–Whitney test comparing area under the curve.
Effect of respiratory system compliance and proning in C-ARDS patients
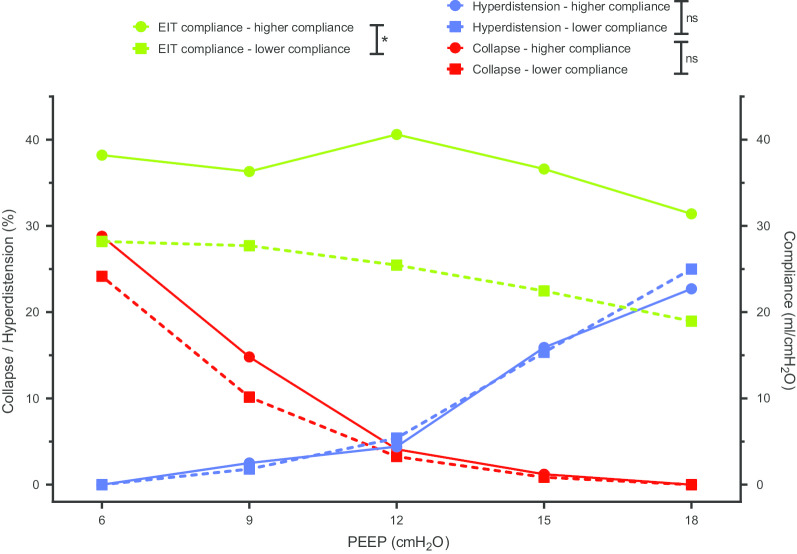
We also compared two C-ARDS patient subgroups based on the compliance of the respiratory system at low PEEP (Table 3, Fig. 3). Body mass index, time from first symptoms to exploration, recruitment-to-inflation ratio, and PaO2/FiO2 were similar in C-ARDS patients with lower versus higher respiratory system compliance. The response to PEEP was similar in the two subgroups in terms of collapse, hyperdistension, or best PEEP (Table 3, Fig. 3). There was no correlation between recruitment-to-inflation ratio and best PEEP (rho = − 0.37, p = 0.14).
Table 3.
Comparison of patients with coronavirus disease-19-related acute respiratory distress syndrome associated with lower versus higher respiratory system compliance
| Compliance ≥ 40 mL/cmH2O (n = 9) | Compliance < 40 mL/cmH2O (n = 8) | p value | |
|---|---|---|---|
| Body mass index (kg/m2) | 29.8 [27.9, 33.1] | 30.3 [24.4, 33.4] | 0.67 |
| Time from first symptoms to exploration (days) | 9 [6.5, 12] | 10 [9, 11] | 0.63 |
| PaO2/FiO2 before EIT (mmHg) | 138 [89, 182] | 130 [118, 150] | 0.96 |
| Respiratory system compliance (mL/cmH2O) | 47 [46, 52] | 31.5 [29, 37] | < 0.01 |
| Recruitment-to-inflation ratio | 0.48 [0.41, 0.62] | 0.4 [0.31, 0.49] | 0.25 |
| Increase in PaO2/FiO2 after proning (mmHg) | 52.5 [38, 91] | 52 [12, 68] | 0.53 |
| Duration of proning (h) | 18.5 [18, 21] | 18 [17.5, 20.5] | 0.87 |
| EIT-PEEP titration | |||
| Best PEEPa (cmH2O) | 12 [12] | 12 [9, 13.5] | 0.92 |
| AUC for compliance | 444 [345, 513] | 293 [260, 316] | < 0.01 |
| Hyperdistension at PEEP 18 (%) | 23 [17, 31] | 25 [18, 27] | 0.70 |
| AUC for hyperdistension | 111 [68, 141] | 108 [78, 131] | 0.81 |
| Collapse at PEEP 6 (%) | 29 [21, 39] | 24 [19 – 31] | 0.61 |
| AUC for collapse | 104 [59, 141] | 78 [66, 115] | 0.74 |
aDefined using EIT as the smallest sum of hyperdistension and collapse. Continuous variables are expressed as median [interquartile range]
PEEP positive end-expiratory pressure, EIT electrical impedance tomography, AUC area under the curve
Fig. 3.
Positive end-expiratory pressure titration in patients with coronavirus disease-19-related acute respiratory distress syndrome, with lower versus higher respiratory system compliance. * denotes a p value < 0.05 for Wilcoxon–Mann–Whitney test comparing area under the curve
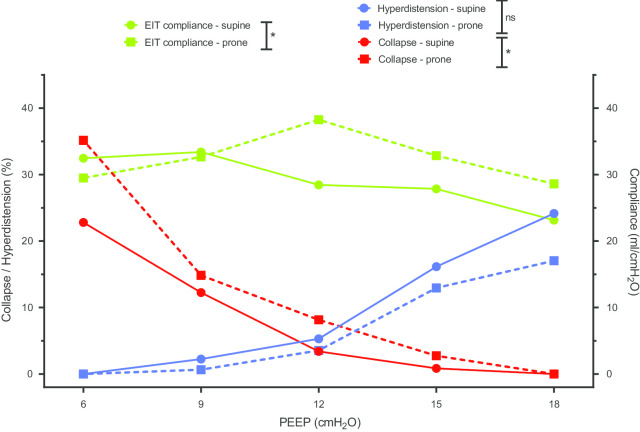
In C-ARDS patients, proning was resulted in higher values of EIT-measured respiratory system compliance and more collapse at lower PEEP as compared to supine position, while hyperdistension and best PEEP did not significantly change (Table 4, Fig. 4). The median delay between prone positioning and PEEP titration was 4 [2, 11] h, depending on the availability of EIT device and investigators.
Table 4.
Comparison of PEEP titration in supine versus prone position, for patients with coronavirus disease-19-related acute respiratory distress syndrome
| C-ARDS in supine position (n = 14) | C-ARDS in prone position (n = 14) | p value | |
|---|---|---|---|
| PaO2/FiO2 (mmHg) | 107 [93, 136] | 179 [127, 190] | < 0.01 |
| EIT-PEEP titration | |||
| Best PEEPa (cmH2O) | 12 [9, 12] | 12 [10, 15] | 0.59 |
| AUC for compliance | 344 [316, 439] | 455 [332, 494] | 0.04 |
| Hyperdistension at PEEP 18 cm H2O (%) | 24 [17, 30] | 17 [15, 41] | 0.63 |
| AUC for hyperdistension | 113 [68, 134] | 80 [40, 194] | 0.82 |
| Collapse at PEEP 6 cm H2O (%) | 23 [19, 37] | 35 [23, 42] | 0.07 |
| AUC for collapse | 86 [58, 139] | 173 [135, 261] | < 0.01 |
aDefined using EIT as the smallest sum of hyperdistension and collapse. Continuous variables are expressed as median [interquartile range]
PEEP positive end-expiratory pressure, EIT electrical impedance tomography, AUC area under the curve in supine and prone position (see Fig. 4)
Fig. 4.
Positive end-expiratory pressure titration in patients with coronavirus disease-19-related acute respiratory distress syndrome, in supine versus prone position. * denotes a p value < 0.05 for Wilcoxon–Mann–Whitney test comparing area under the curve
Discussion
The main findings of our report are as follows: (1) the median value of the best PEEP defined using EIT as the smallest sum of hyperdistension and collapse was 12 cmH2O in C-ARDS patients in prone and supine position; (2) baseline respiratory system compliance was not predictive of the response to PEEP; and (3) compared with NC-ARDS, C-ARDS exhibited more collapse at low PEEP and less hyperdistension at high PEEP.
PEEP level setting
In our study, an intermediate PEEP level of around 12 cmH2O seemed appropriate in more than half of C-ARDS patients. A lower PEEP level was associated with significant alveolar collapse. Another study reporting PEEP titrations by EIT in 15 C-ARDS patients showed almost 50% collapse at low PEEP (8). In our work, this collapse was worse than that observed in NC-ARDS patients. This finding could be explained by a higher prevalence of obesity or overweight in COVID-19 patients (9); our results showed a trend toward higher body weight in the C-ARDS group. On the other hand, this collapse does not seem to be completely explained by higher airway opening pressures in C-ARDS patients as only two out of 17 patients had an airway opening pressure greater than 5 cmH2O.
A higher PEEP level was associated with hyperdistension, and only three patients had an ideal PEEP level (defined by the EIT PEEP titration tool) greater than 12 cmH2O. These findings are aligned with those from another study in C-ARDS (10), which showed a decrease in compliance and an increase in dead volume at higher PEEP, indicating hyperdistension and absence of recruitment (11). The benefit of higher PEEP should also be weighed against hemodynamic tolerance since the incidence of pulmonary thrombosis is high in C-ARDS patients (12,13) and half of our patients exhibited cor pulmonale. Overall, our results are consistent with recent data suggesting a variable potential for recruitment in C-ARDS patients (14–16).
Prone position
In the prone position, the best PEEP was not significantly different from the supine position. Previous work in NC-ARDS found consistent results. Cornejo and al (17), using CT scan, showed that the percentage of recruitment was similar in both positions (36% in supine et 32% in prone) when PEEP increased from 5 to 15 cmH2O; Aguirre-Bermeo and al (18), using the nitrogen washout/washin technique, showed that PEEP-induced lung volume recruitment did not significantly change in prone versus supine position. The higher collapse at lower PEEP in prone versus supine may be explained by the local increase in ventral chest wall elastance in the former position. However, the benefit of proning in this situation may be explained by the persistence of a predominantly dorsal perfusion in prone (19).
Only six NC-ARDS patients were turned prone within 48 h of exploration in supine position, and these patients did not have a PEEP titration in prone position. Therefore, we were unable to compare PEEP titrations in prone between C-ARDS and NC-ARDS.
Respiratory system compliance
Some authors have described a significant proportion of C-ARDS patients ventilated with normal compliance, with a median value around 50 mL/cmH2O (2). They distinguished two profiles: low recruiters characterized by low elastance, low lung weight, and a priori little benefit from higher PEEP and prone positioning; and high recruiters characterized by high elastance, high lung weight, and possible response to higher PEEP and prone positioning. However, since then, several studies have shown a significant and early alteration of compliance in C-ARDS (16,20,21). Furthermore, in large cohort studies, compliance of C-ARDS patients was either slightly higher (22) or similar (23) to compliance of NC-ARDS patients.
Prior to COVID-19 pandemic, many studies sought to determine factors predicting the response to PEEP or proning. In NC-ARDS, neither compliance at low PEEP nor CT-scan radiological pattern was predictive of recruitment at high PEEP (11) or improvement in oxygenation in the prone position (24). In the present study, we found a comparable response to PEEP in patients with higher versus those with lower respiratory system compliance. Similarly, after prone positioning, the increase in PaO2/FiO2 ratio was comparable in both subgroups. Their numbers (eight to nine patients per subgroups) were certainly low, and the CT-scan profile was not taken into account. However, there is no evidence that the sole measurement of the respiratory system compliance can predict the response to PEEP or proning in C-ARDS patients. Similarly, the recruitment-to-inflation ratio did not correlate with the best PEEP. Therefore, different levels of PEEP should probably be tested regardless of baseline compliance in order to adjust ventilator settings to individual needs.
Strengths and limitations
The strengths of our study rely on the comprehensive physiological assessment and the comparison with NC-ARDS. To our knowledge, this is the first study to compare the PEEP response of C-ARDS and NC-ARDS in supine and prone position, using EIT. The main limitation of our work is the sample size. Patients’ enrollment was greatly impacted by the heavy workload upon COVID pandemic and the lack of time to set up monitoring and conduct investigations. Second, it was not possible to perform chest CT scan in all patients at the time of explorations. As a result, we were neither able to correlate the CT-scan results with physiological findings nor to evaluate the effect of PEEP according to the radiological phenotype. Third, we were unable to compare PEEP titrations in prone between C-ARDS and NC-ARDS.
Conclusion
Our study characterized the lung physiology of C-ARDS with the following findings. An intermediate PEEP seemed appropriate to minimize collapse and hyperdistension in more than half of our C-ARDS patients in supine or prone position. Compliance at low PEEP alone could not predict the response to PEEP.
Supplementary Information
Additional file 1. Median and interquartile values of hyperdistension, collapse and EIT-compliance for each level of PEEP during PEEP titration in: S1) Patients with NC-ARDS versus C-ARDS; S2) C-ARDS patients with lower versus higher respiratory system compliance; S3) C-ARDS patients in supine versus prone position.
Acknowledgements
Not applicable.
Abbreviations
- C-ARDS
Coronavirus disease-19-related acute respiratory distress syndrome
- NC-ARDS
Noncoronavirus disease-19-related acute respiratory distress syndrome
- PEEP
Positive end-expiratory pressure
- EIT
Electrical impedance tomography
- ICU
Intensive care unit
- ABG
Arterial blood gases
- CT
Computed tomography
- AUC
Area under the curve
Authors’ contributions
FP was involved in study design, data collection, analysis, and interpretation, and script writing. ST was involved in study design, data collection, analysis, and interpretation, and script writing. TM was involved in data collection, analysis, and interpretation. GA was involved in data analysis and interpretation. MV was involved in data analysis. AFH was involved in data collection. KR was involved in data interpretation. NDP was involved in data interpretation. MA was involved in data analysis and interpretation. GC was involved in study design, data analysis and interpretation. AMD was involved in study design, data analysis and interpretation, and script writing. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This is an ancillary report of an ongoing prospective monocentric observational study on EIT in patients with ARDS (CPP-66/17). The collection and analysis of data provided by electrical impedance tomography had received the approval of the CPP Ile de France VI, on November 8, 2017. Written informed consent was waived due to the observational nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
François Perier and Samuel Tuffet have contributed equally to this work
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-020-03414-3.
References
- 1.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a «typical» acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care Lond Engl. 2020;24(1):154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1–4. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Del Sorbo L, Grieco DL, Shklar O, Junhasavasdikul D, Telias I, et al. Airway closure in acute respiratory distress syndrome: an underestimated and misinterpreted phenomenon. Am J Respir Crit Care Med. 2018;197(1):132–136. doi: 10.1164/rccm.201702-0388LE. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome: a clinical trial. Am J Respir Crit Care Med. 2020;201(2):178–187. doi: 10.1164/rccm.201902-0334OC. [DOI] [PubMed] [Google Scholar]
- 7.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Zee P, Somhorst P, Endeman H, Gommers D. Electrical impedance tomography for positive end-expiratory pressure titration in COVID-19 related ARDS. Am J Respir Crit Care Med. 2020;202:280–284. doi: 10.1164/rccm.202003-0816LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roesthuis L, van den Berg M, van der Hoeven H. Advanced respiratory monitoring in COVID-19 patients: use less PEEP! Crit Care Lond Engl. 2020;24(1):230. doi: 10.1186/s13054-020-02953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 12.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beloncle FM, Pavlovsky B, Desprez C, Fage N, Olivier P-Y, Asfar P, et al. Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care. 2020;10(1):55. doi: 10.1186/s13613-020-00675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med. 2020;48:1129–1134. doi: 10.1097/CCM.0000000000004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haudebourg A-F, Perier F, Tuffet S, de Prost N, Razazi K, Mekontso Dessap A, et al. Respiratory mechanics of COVID-19 vs. non-COVID-19 associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202:287–290. doi: 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornejo RA, Díaz JC, Tobar EA, Bruhn AR, Ramos CA, González RA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188(4):440–448. doi: 10.1164/rccm.201207-1279OC. [DOI] [PubMed] [Google Scholar]
- 18.Aguirre-Bermeo H, Turella M, Bitondo M, Grandjean J, Italiano S, Festa O, et al. Lung volumes and lung volume recruitment in ARDS: a comparison between supine and prone position. Ann Intensive Care. 2018;8(1):25. doi: 10.1186/s13613-018-0371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perier F, Tuffet S, Maraffi T, Alcala G, Victor M, Haudebourg A-F, et al. Effect of PEEP and proning on ventilation and perfusion in COVID-19 ARDS. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202008-3058LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet Lond Engl. 2020 doi: 10.1101/2020.04.15.20067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the Seattle Region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papazian L, Paladini M-H, Bregeon F, Thirion X, Durieux O, Gainnier M, et al. Can the tomographic aspect characteristics of patients presenting with acute respiratory distress syndrome predict improvement in oxygenation-related response to the prone position? Anesthesiology. 2002;97(3):599–607. doi: 10.1097/00000542-200209000-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Median and interquartile values of hyperdistension, collapse and EIT-compliance for each level of PEEP during PEEP titration in: S1) Patients with NC-ARDS versus C-ARDS; S2) C-ARDS patients with lower versus higher respiratory system compliance; S3) C-ARDS patients in supine versus prone position.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.