Abstract
Background
There is emerging evidence for enhanced blood coagulation in coronavirus 2019 (COVID-19) patients, with thromboembolic complications contributing to morbidity and mortality. The mechanisms underlying this prothrombotic state remain enigmatic. Further data to guide anticoagulation strategies are urgently required.
Methods
We used viscoelastic rotational thromboelastometry (ROTEM) in a single-center cohort of 40 critically ill COVID-19 patients.
Results
Clear signs of a hypercoagulable state due to severe hypofibrinolysis were found. Maximum lysis, especially following stimulation of the extrinsic coagulation system, was inversely associated with an enhanced risk of thromboembolic complications. Combining values for maximum lysis with D-dimer concentrations revealed high sensitivity and specificity of thromboembolic risk prediction.
Conclusions
The study identifies a reduction in fibrinolysis as an important mechanism in COVID-19-associated coagulopathy. The combination of ROTEM and D-dimer concentrations may prove valuable in identifying patients requiring higher intensity anticoagulation.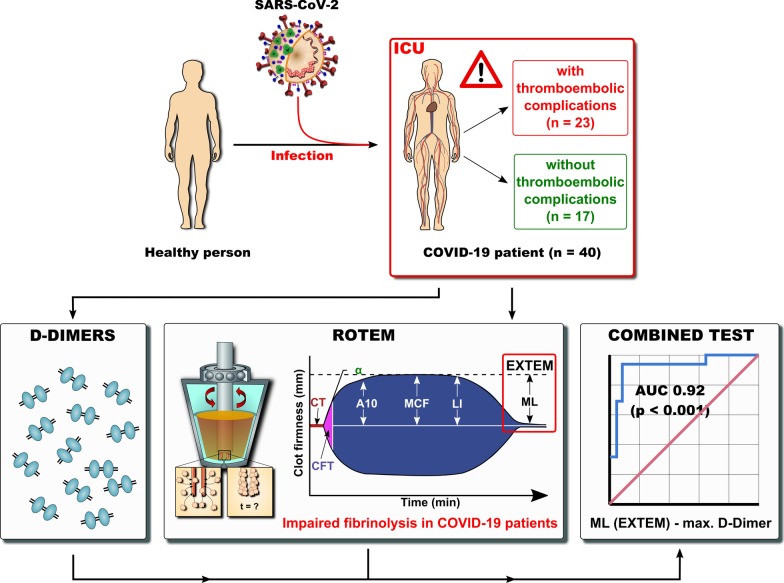
Keywords: COVID-19, Coagulopathy, Hypofibrinolysis, ROTEM, D-dimers
Background
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) has led to a global pandemic posing a major threat to humans [1]. More than 500 000 deaths related to COVID-19 have been so far reported [2].
SARS-CoV-2 primarily affects the respiratory system with a widely heterogeneous clinical presentation, ranging from none or minimal symptoms to significant hypoxia with viral pneumonia, potentially leading to severe acute respiratory distress syndrome (ARDS) and cytokine storm [3]. ARDS with related lung injury is considered one of the main causes of death in COVID-19 patients [4].
However, there is emerging evidence that involvement of other pathomechanisms contributes to morbidity and mortality. Both clinical and autopsy studies have revealed a high incidence of venous and arterial thromboembolic events, including pulmonary embolism, even in patients receiving therapeutic anticoagulation [5–7]. These findings have led to recommendations for higher anticoagulation targets; however, it remains unclear which patients are at increased risk and require anticoagulation [8]. While fibrinogen and D-dimer levels are frequently elevated, neither parameter reliably identifies patients at an increased risk of thromboembolic complications [8]. Although different markers of hypercoagulation have been reported among COVID-19 patients [6, 9], the exact mechanisms underlying the prothrombotic state in these patients remain unclear so far [10, 11]. In particular, it has not been clarified to which extent increased procoagulation and/or impaired fibrinolysis is involved.
In addition to conventional laboratory parameters, rotational thromboelastometry (ROTEM) provides evidence for net coagulation capacity and insight into clot formation time, clot firmness and fibrinolysis in the critically ill patients [12]. Here we report ROTEM data in 40 consecutive, severely ill COVID-19 patients treated in two tertiary intensive care units (ICUs) and assessed the association with thromboembolic complications.
Methods
Coagulation tests
After admission to our ICUs, blood samples were drawn and viscoelastic tests were performed once with citrated blood using a ROTEM sigma point-of-care device (Tem International, Munich, Germany) [13]. In each patient, intrinsically (contact activation, INTEM) and extrinsically (tissue factor activation, EXTEM) activated test assays were performed to analyze the clot dynamics in both coagulation pathways. Furthermore, FIBTEM and HEPTEM were performed. In the FIBTEM, platelets are inactivated with cytochalasin D to enable isolated evaluation of fibrinogen in clot firmness. The heparin effect was determined by comparing the clotting time of the INTEM with the clotting time of the HEPTEM, where heparinase is added.
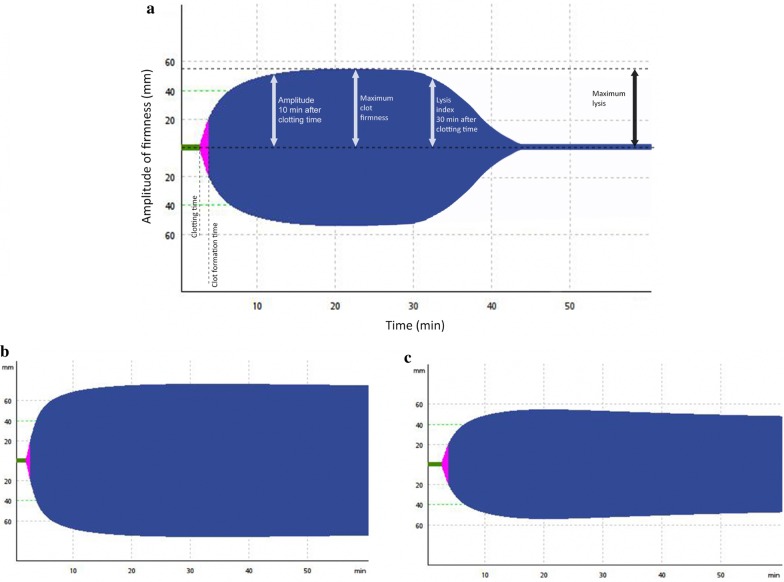
The following ROTEM variables were analyzed: clotting time defined as the time until initiation of clotting; clot formation time (seconds until a clot strength reaches 20 mm), reflecting the kinetics of clot formation; maximum clot firmness (MCF) defined as the maximum amplitude of clot firmness; maximum lysis (ML; %) defined as the difference between MCF and the lowest clot amplitude after MCF, reflecting fibrinolytic activity (Fig. 1).
Fig. 1.
a All measured values in ROTEM analysis, including clotting time (CT [s]), clot formation time (CFT [s]), maximum clot firmness (MCF [mm]) and maximum lysis (ML [%(range)]). b A reduction of fibrinolysis in a COVID-19 patient with a thromboembolic event; the clot amplitude remains unchanged until the end. c A physiological fibrinolysis pattern in a healthy person, reflected by the subtle decrease of the MCF during the measurement
Additional routine laboratory tests performed according to standardized protocols comprised hemoglobin concentration, white blood cell count, platelet count, prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (aPTT) and inflammatory parameters (see Table 2). The levels of tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1) and plasminogen were determined using commercial ELISA Kits (t-PA Antigen ELISA Kit, PAI-1 Antigen ELISA Kit, Glu-Plasminogen, TECHNOZYM®/Technoclone).
Table 2.
Laboratory parameters of total cohort and subcohorts with and without thromboembolic events
| Cohort (N = 40) | Thromboembolic event | ||||||
|---|---|---|---|---|---|---|---|
| Yes (N = 23) | No (N = 17) | ||||||
| Median | [IQR] | Median | [IQR] | Median | [IQR] | p value | |
| Laboratory variables (normal values) | |||||||
| Haemoglobin (12·5–17·2 g/dL) | 10.1 | [8.5–11.2] | 9.70 | [8.3–10.8] | 10.4 | [9.3–11.9] | ns |
| White blood cells (3·5–10·5/nl) | 10.13 | [7.5–13.7] | 10.63 | [7.4–16] | 9.58 | [6.6–12.1] | ns |
| Platelet count (150–370/nl) | 193.5 | [131.3–316.3] | 181 | [116–306] | 209 | [178–325.5] | ns |
| Prothrombin time (70–130%) | 74.5 | [62.8–86] | 79 | [61–83] | 71 | [63.5–87.5] | ns |
| INR (0·9–1·25) | 1.2 | [1.1–1.4] | 1.18 | [1.1–1.4] | 1.26 | [1.1–1.4] | ns |
| PTT (26–40 s) | 45.65 | [39.4–56.1] | 51.10 | [40.8–57.4] | 41.1 | [38.7–54.2] | ns |
| Fibrinogen (1·6–4 g/l) | 6.67 | [4.7–7.7] | 6.72 | [5.0–7.8] | 6.1 | [4.6–7.9] | ns |
| D-dimers (< 0·5 mg/l) | 3.95 | [2.6–5.9] | 4.84 | [3.5–7.2] | 3.06 | [2.3–3.9] | 0.003 |
| max. D-dimers (< 0·5 mg/l) | 8.25 | [3.6–16.2] | 11.57 | [8.2–18.4] | 3.98 | [2.6–6.4] | < 0.001 |
| Procalcitonin (0·5 µg/l) | 0.57 | [0.2–2.5] | 0.81 | [0.4–4.7] | 0.24 | [0.2–1.3] | ns |
| CRP (< 0·5 mg/l) | 123.8 | [84.3–216.5] | 130 | [86–273.7] | 111 | [79.3–185] | ns |
| max. CRP (< 0·5 mg/l) | 312.9 | [208.3–343.9] | 341.4 | [261.1–370.7] | 261.05 | [175.3–312.9] | 0.002 |
| IL-6 (< 7 ng/l) | 103 | [35·6–230] | 88 | [27.7–340] | 153 | [53.7–206.5] | ns |
| max. IL-6 (< 7 ng/l) | 558.6 | [178.8–1792.3] | 550 | [174–2475] | 567.2 | [186.5–1196.5] | ns |
| Ferritin (30–400 µg/l) | 1636 | [1067.8–4028.5] | 1663 | [1218.5–4655] | 1567 | [720–3662] | ns |
| max. Ferritin (30–400 µg/l) | 2523.2 | [1536.7–6635.1] | 2781.5 | [1854.7–7996.2] | 2028.4 | [922.9–4893.4] | ns |
| tPA (2–8 µg/l) | 1 | [0.9–5.5] | 1 | [0.9–3.6] | 2 | [0.9–9.9] | ns |
| PAI-1 (7–43 ng/ml) | 36 | [17–70] | 31 | [12–61] | 42.50 | [25.3–87] | ns |
| tPA/PAI-1 | 0.053 | [0.02–0.18] | 0.05 | [0.02–0.14] | 11 | [0.03–0.24] | ns |
| Antithrombin III (80–120%) | 79 | [58.5–96.5] | 75.5 | [56.8–84] | 94 | [66.5–110] | ns |
| Factor VIII (50–150%) | 258 | [190.5–319.5] | 260 | [219.5–355] | 222 | [149.5–289.5] | ns |
| Plasminogen (80–120%) | 88 | [72.8–114] | 82 | [72.8–109.8] | 101 | [70.8–129.8] | ns |
| ROTEM variables | |||||||
| FIBTEM CT (s) | 88.5 | [78–97.8] | 89 | [78–102] | 88 | [75.5–96] | ns |
| FIBTEM CFT (s) | 68 | [51–104] | 64.5 | [54–95.8] | 71 | [47–165] | ns |
| FIBTEM MCF (mm) | 34.5 | [27.3–39.5] | 35 | [27–38] | 34 | [27–40] | ns |
| EXTEM CT (s) | 86 | [69.5–99.8] | 84 | [69–96] | 86 | [70.5–107.5] | ns |
| EXTEM CFT (s) | 46.5 | [40–60.5] | 47 | [40–61] | 45 | [40.5–56.5] | ns |
| EXTEM MCF (mm) | 75 | [70.3–78] | 75 | [69–78] | 76 | [72.5–78.5] | ns |
| INTEM CT (s) | 208 | [181.3–227.5] | 215 | [197–251] | 189 | [171.5–212] | 0.005 |
| INTEM CFT (s) | 50.5 | [39.5–61.8] | 56 | [39–63] | 45 | [39.5–60.5] | ns |
| INTEM MCF (mm) | 74 | [69–77] | 74 | [65–77] | 73 | [69.5–78] | ns |
| HEPTEM CT (s) | 188.5 | [170.5–208.3] | 193 | [173–209] | 173 | [159–206] | ns |
| HEPTEM CFT (s) | 41 | [35.5–56.5] | 40 | [34–60] | 42 | [37–51] | ns |
| HEPTEM MCF (mm) | 73 | [67.5–75.3] | 73 | [66–76] | 71 | [71–75] | ns |
| ML, EXTEM (%) | 3 | [1.3–5.8] | 3 | [0–5] | 5 | [3.5–8] | 0.001 |
| ML, INTEM (%) | 3 | [1–6] | 2 | [0–3] | 6 | [2.5–6] | 0.001 |
Unless values are designated as maximum values during the ICU stay, these parameters were determined on the day, when ROTEM analysis was performed, after admission to our ICUs
CT clotting time, CFT clot formation time, MCF maximum clot firmness, ML maximum lysis
To combine the parameters maximum D-dimers (mg/l) and ML (%), the difference (maximum D-dimers—ML EXTEM) was calculated and analyzed.
Anticoagulation therapy
In our Intensive care units, all patients included in this trial were treated with either low molecular weight heparin or in the case of ECMO therapy with argatroban. We aimed for a PTT of 50–55 s (normal 26–40 s), and in patients with thromboembolic events we aimed for a PTT of 60–80 s.
Ultrasound
We performed ultrasound examinations in all patients (GE Vivid S70 ultrasound machine with a 9L-D probe) to screen for venous thrombosis, focusing on the jugular, subclavian, brachial, femoral and popliteal veins upon admission to our ICU and subsequently at least once weekly.
Ethics
The study was approved by the ethics committees of Charité – Universitätsmedizin Berlin (EA4/115/20).
Statistics
Statistical analyses were performed using IBM® SPSS® Statistics version 26 (New York, USA). The descriptives are provided as median with limits of the interquartile range (IQR) for continuous variables or as absolute and relative frequencies for categorical variables.
Continuous data were primarily right skewed. Therefore, the Mann–Whitney U test was used to compare differences between patient groups in continuous variables, while Chi-square test was used for categorical data. A two-sided significance level of 0.05 was applied without adjustment for multiple comparison. All p values constitute exploratory data analyses and do not allow for confirmatory generalization of results. To evaluate the strength of different ROTEM variables to distinguish between patients with and without thromboembolic events, receiver operating characteristic (ROC) analysis was carried out including area under the curve measures (AUC) with 95% confidence intervals (CI). Sensitivity, specificity and accuracy (percentage of correctly classified patients) are reported.
Results
Characteristics of the cohort
Forty consecutive patients with COVID-19 confirmed by polymerase chain reaction in throat swabs were admitted to two ICUs within our department between March 25th and May 11th. All patients received viscoelastic testing using the ROTEM system and were included in the analysis, which was censored on May 11th.
Table 1 shows baseline characteristics of the study cohort. As most patients were referred from community hospitals within a regional network, patients were mostly severely ill with a median sequential organ failure assessment (SOFA) score of 9 and a mean acute physiology and chronic health evaluation (APACHE) II of 28 points. Mechanical ventilation via either endotracheal tube or tracheostomy was administered to 78% of patients, whereas extracorporeal membrane oxygenation was required for 25% and kidney replacement therapy for 53% of patients. Evidence for macrothromboembolic events was found in 23 of 40 patients (58%). In five patients, we identified thromboembolic events upon admission to our ICUs (N = 3 prediagnosed pulmonary emboli, N = 2 deep venous thrombosis). Nineteen patients developed thromboembolic complications during the ICU stay, comprising deep vein thrombosis (N = 14), pulmonary embolism (N = 4), ischemic stroke (N = 3), complete thrombosis of the ECMO-circuit requiring emergency circuit-change (N = 1) and a clotted ECMO cannula (N = 1).
Table 1.
Patient characteristics of total cohort and subcohorts with and without thromboembolic events
| Cohort (n = 40) | Thromboembolic events (N = 23) | No thromboembolic events (N = 17) | p value | ||||
|---|---|---|---|---|---|---|---|
| Age (years, (median, [IQR])) | 67 | [57.3–76.6] | 66 | [56–76] | 68 | [62–77.5] | ns |
| Gender, male (n, %) | 35 | 87.5% | 20 | 87% | 15 | 88% | ns |
| BMI, kg/m2 (median, [IQR]) | 28.1 | [24.8–32.8] | 27.8 | [24.2–33] | 28.7 | [25.7–32.3] | ns |
| Duration of ICU stay, days (median, [IQR]) | 39.5 | [24–54.25] | 42 | [28–58] | 25 | [8.5–47.5] | 0.05 |
| Death during ICU stay (n, %) | 11 | 27.5% | 9 | 39.1% | 2 | 11.8% | 0.58 |
| Intubation (n, %) | 31 | 77.5% | 20 | 87% | 11 | 65% | ns |
| ECMO (n, %) | 10 | 25% | 9 | 39.1% | 1 | 6% | ns |
| CRRT (n, %) | 21 | 52.5% | 16 | 69.6% | 5 | 29.4% | 0.013 |
| SOFA score (median, [IQR]) | 9 | [6.3–11.8] | 10 | [6–11] | 8 | [4.5–11] | ns |
| SIC score (median, [IQR]) | 3 | [2–4] | 3 | [2–4] | 3 | [2–4] | ns |
| APACHE score (median, [IQR]) | 28 | [22–33] | 29 | [23–34] | 26 | [19–31.8] | ns |
| Preexisting conditions | |||||||
| Coronary artery disease (n, %) | 9 | 22.5% | 6 | 26% | 3 | 18% | ns |
| Hypertension (n, %) | 25 | 62.5% | 14 | 61% | 11 | 65% | ns |
| Diabetes mellitus/insulin resistance (n, %) | 13 | 32.5% | 10 | 43% | 3 | 18% | ns |
| Chronic kidney disease (n, %) | 7 | 17.5% | 6 | 26% | 1 | 6% | ns |
| Chronic dialysis (n, %) | 1 | 2.5% | 1 | 4% | 0 | 0% | ns |
| Lung disease (n, %) | 7 | 17.5% | 6 | 26% | 1 | 6% | ns |
ECMO Extracorporeal membrane oxygenation, SOFA sequential organ failure assessment, CRRT continuous renal replacement therapy, SIC sepsis-induced coagulopathy, APACHE acute physiology and chronic health evaluation
Laboratory parameters
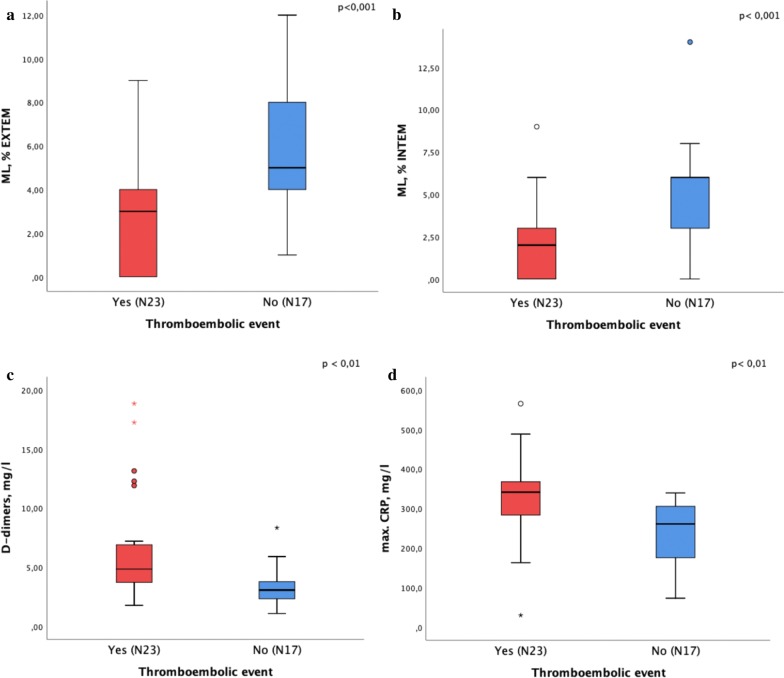
Table 2 shows laboratory parameters for the study cohort and in patients with and without thromboembolic events. Hematological parameters were similar in both patient groups. Patients with thromboembolic events had a significantly higher maximum C-reactive protein (CRP) value, with a median value of 341 mg/l [IQR 261.1–370.7] versus 261.1 mg/l [IQR 175.3–312.9], respectively (p = 0.002). Other markers of inflammation such as procalcitonin (PCT), ferritin and interleukin-6 did not differ significantly between groups.
Analyses of the coagulation parameters revealed no significant differences between the groups with the exception of a prolonged PTT in the group with thromboembolic events. Patients had significantly elevated levels of fibrinogen without significant differences between groups.
Moreover, the median initial D-dimer levels were 4.84 mg/l [IQR 3.5–7.2] in the group with thromboembolic complications in comparison with 3.06 mg/l [IQR 2.3–3.9] in the group without thromboembolic complications (p = 0.003).
ROTEM parameters
Substantial abnormalities in the ROTEM analysis were found in the overall cohort. Maximum clot firmness in INTEM, EXTEM, FIBTEM and HEPTEM was markedly elevated in the entire cohort compared to reference values with median values of 74 mm [IQR 69–77], 75 mm [IQR 70.3–78], 34.5 mm [IQR 27.3–39.5] and 73 mm [IQR 67.5–75.3], respectively. Of note, there was no significant difference in these parameters between the subgroups with and without thromboembolic complications. However, the median clotting time detected in INTEM was significantly longer in the group of patients with thromboembolic complications: 215 s [IQR 197–251] versus 189 s [IQR 171.5–212]; p = 0.005. Clotting times in FIBTEM, EXTEM and HEPTEM showed no significant differences between groups.
Figure 2 depicts ML in INTEM and EXTEM. Under both conditions, ML was reduced and significantly lower in the group with thromboembolic complications (INTEM median 2% [IQR 0–3.0] versus 6% [IQR 2.5–6]; p = 0.001; EXTEM median 3% [IQR 0–5] versus 5% [IQR 3.5–8], p = 0.001), indicating substantially impaired fibrinolysis in both groups. This was observed to be more pronounced in patients with thromboembolic complications.
Fig. 2.
a Maximum lysis (ML) in EXTEM, b maximum lysis (ML) in INTEM, c D-dimers on the day of ROTEM and d max. CRP in COVID-19 patients with and without thromboembolic complications
ROC analysis to distinguish patients with and without thromboembolic complications
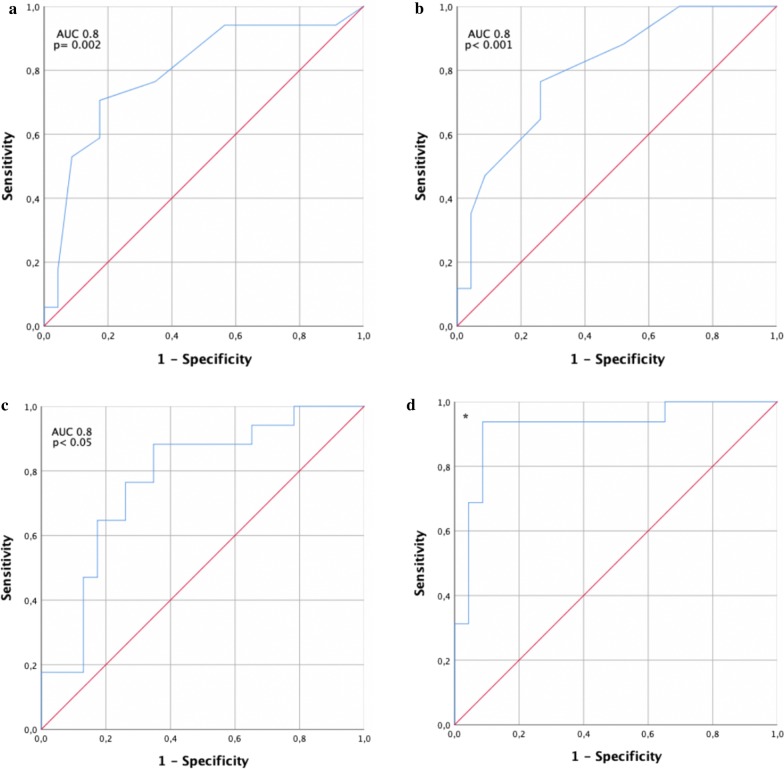
Based on the above findings, we evaluated the potential of different ROTEM variables to distinguish between patients with and without thromboembolic events using ROC analysis (Fig. 3). Maximum lysis in EXTEM resulted in an area under the curve (AUC) of 0.8 [95% CI 0.7–0.9] for thromboembolic events (p = 0.001), while the ML in INTEM resulted in an AUC of 0.79 [95% CI 0.6–0.9] (p = 0.002). D-dimers showed an AUC of 0.78 [95% CI 0.6–0.9], and maximum D-dimers had an AUC of 0.82 [95% CI 0.7–1.0]. Combined analysis showed that the difference in D-dimers and ML EXTEM resulted in an AUC of 0.92 [95% CI 0.8–1].
Fig. 3.
ROC analysis of a maximum lysis (ML) in EXTEM, b D-dimer and c ML INTEM d difference of ML in EXTEM and max. D-dimer for prediction of thromboembolic events in our cohort [*AUC of 0·92 (p < 0.001)]
Discussion
This study provides evidence that hypofibrinolysis is an important contributor to the hypercoagulable state in COVID-19 patients. Maximum lysis assessed in ROTEM analysis, especially in the EXTEM analysis, was reduced more profoundly in patients with thromboembolic events. Based on these observations, we propose that ROTEM analysis is useful for patient stratification according to their prothrombotic risk. In particular, combined consideration of ROTEM maximum lysis and D-dimers may identify patients that benefit from therapeutic anticoagulation.
In this small cohort of severely ill COVID-19 patients, we observed thromboembolic complications in more than 50% of patients. Analysis of routine coagulation parameters should be interpreted with caution, as many of the patients were treated with therapeutic anticoagulation. However, in accordance with previous studies, fibrinogen and factor VIII were elevated in our cohort and D-dimers were significantly elevated in the subgroup with thromboembolic complications [14]. Other conventional markers of the coagulation system showed no significant differences between the two groups.
In contrast to individual parameters, viscoelastic methods, such as thromboelastography and ROTEM, permit functional evaluations by recording most components of the coagulation process in vitro in the presence of cellular blood components. This provides insight into the different coagulation phases, including the initiation, formation and stabilization of a clot, and finally, clot lysis. The influence of the endothelium as an important co-factor of coagulation, however, is not directly reflected in ROTEM assessment. In several studies, hypercoagulable conditions were identified using ROTEM in disease states with an increased risk of thromboembolic events [15, 16]. Moreover, viscoelastic systems, such as ROTEM and thromboelastography, were successfully established to detect hypo- or hyperfibrinolysis in patients with traumatic injury or severe septic shock [17, 18].
Panigada et al. used thromboelastography in 20 patients with COVID-19 in addition to plasmatic tests of coagulation [19]. Similar to our study, they also found increased levels of fibrinogen and factor VIII, and almost normal routine coagulation tests. Thromboelastography data showed elevated clot firmness as reflected by maximal amplitude and reduced fibrinolysis measured as reduced clot lysis at 30 min (Lys 30), consistent with our observations. Spiezia and colleagues and Pavoni and co-workers also recently showed severe hypercoagulopathy in critically-ill COVID-19 patients using ROTEM [20, 21]. They found a significantly higher maximal clot firmness in INTEM, EXTEM and FIBTEM, and shorter INTEM clot formation time in comparison with a healthy control group. However, they observed no differences between COVID-19 patients with and without thrombosis [20]. In a cohort of 19 patients, Ibañez et al. noted markedly reduced fibrinolysis in COVID-19 patients; however, no distinction with respect to the presence of thromboembolic events was made [23].
While our findings confirm these results, we noted not only a markedly reduced fibrinolysis in the whole cohort but a significantly reduced ML in the group with thromboembolic complications. The clot lysis parameter ML provides information on the fibrinolytic activity, with low values providing evidence for hypofibrinolysis. In the current study, we found the ML in both EXTEM and INTEM to be markedly below normal values. Furthermore, the ML under both conditions was even lower in the group with thromboembolic complications. Therefore, we conclude that a severely impaired fibrinolysis plays an important role in the hypercoagulable state and thromboembolic risk in COVID-19 patients [23].
It is, however, somewhat surprising that highly elevated levels of D-dimers were found in a state of hypofibrinolysis. As a hypothesis, it has been suggested that intra-alveolar fibrin deposition accounts for local activation of fibrinolysis in ARDS.
The mechanisms leading to hypofibrinolysis in COVID-19 remain to be defined. Complex interactions between inflammation and the coagulation and fibrinolytic system have been examined and controversially discussed for decades [24–26]. One potential mechanism may be the production of alpha defense in neutrophils, which are known to promote fibrin polymerization and block fibrinolysis in vitro [27].
In our cohort, we found markedly elevated markers of inflammation, including interleukin-6, CRP and ferritin; however, only the maximum CRP level differed significantly between patients with and without thromboembolic complications. We could not detect significant differences among additional individual analytes (i.e., tPA or PAI concentrations) between both groups; however, we did not evaluate the effect of the complement or bradykinin system, which are both known to play crucial roles in connecting the inflammatory response and fibrinolytic activity. Future clinical trials should also focus on the role of thrombin-activatable fibrinolysis inhibitor (TAFI), plasmin-alpha-2-antiplasmin (PAP) complexes and antiplasmin, which would give valuable insights into the mechanisms of COVID-19-induced hypofibrinolysis. Furthermore, endothelial dysfunction is likely involved but was not assessed.
ROC analyses provided an AUC for ML in EXTEM of 0.8. As such, it might be a candidate as prediction marker of future thromboembolic complications. Zhou et al. reported D-dimers to be one of the most sensitive and specific factors predicting mortality in a large cohort of COVID-19-patients in China [14]. Cui et al. found a good sensitivity and specificity using a cutoff of 1.5 ng/ml for predicting thrombotic events in COVID-19 patients [8]. D-dimers were also markedly elevated in our cohort and were found to be significantly higher in the subgroup with thromboembolic events. ROC analysis for D-dimers revealed an AUC of 0.78. The combination of the maximum D-dimer and ML in EXTEM (D-dimer—ML) improved the AUC to 0.92, with a cutoff of 3.7 for a sensitivity of 94% and specificity > 90%. The predictive value of this D-dimer–ML parameter, however, requires validation in a second cohort.
In addition to providing insights in the mechanism of thrombus formation, our results may underline the possible therapeutic option of specific fibrinolytic therapy for ARDS caused by COVID-19. Administration of recombinant t-PA has already been suggested as a potential treatment and has shown promising results in a previous study independent of COVID-19 [28]. Currently, a phase IIa trial is underway to examine the effect of thrombolytics in COVID-19 patients with hypoxemic lung injury (ClinicalTrials.gov, NCT 04357730) [29].
There are several limitations to our study. First, ROTEM measurements were performed when patients were transferred to our ICUs after different treatment periods in other hospitals. Thus, the ROTEM results reflect different stages of the disease. Also, many, but not all patients, were previously treated with heparin when thromboelastometry measurements were performed. Second, the study is monocentric, performed in a tertiary care center, and the generalizability to other settings and patients with a less severe course and earlier stages of the disease needs to be tested. Third, our prediction models based on associations between poor clot lysis, D-dimers and the presence of thromboembolic events are hypotheses and require validation in independent patient cohorts and prospective observational studies. Fourth, thromboembolic events may have been underdiagnosed, as only ultrasound was routinely performed, while CT scans to exclude pulmonary embolism were only performed in some patients. Fifth, our results are descriptive in nature and do not provide explanatory models for the observed hypofibrinolysis. Future studies should focus on the examination of possible mechanisms.
Sixth, 25% of patients of our cohort received ECMO therapy, which may itself have had a thrombogenic effect and in part may have contributed to the high rates of thrombosis. However, the current literature points into the direction that in some cases ECMO rather leads to hyperfibrinolysis [30]. An ECMO-side effect as an explanation for a systematic hypofibrinolysis as observed in our cohort thus appears rather unlikely. Seventh, even though the statistical analysis showed robust values for our analysis, it may be difficult to guide clinical decision based on these values, as the difference in maximum lysis is 2%.
In summary, we found substantially reduced fibrinolysis in COVID-19 patients, which was more pronounced in patients with thromboembolic events. Clot ML time, as assessed by ROTEM as a single parameter, or in combination with D-dimers may prove valuable for thromboembolic risk stratification in COVID-19 patients and aid in decision-making regarding anticoagulation strategies.
Conclusions
ROTEM revealed severe hypofibrinolysis in COVID-19 patients. Maximum lysis, especially following stimulation of the extrinsic coagulation system, was inversely associated with an enhanced risk of thromboembolic complications. The combination of maximum lysis with D-dimer concentrations revealed high sensitivity and specificity of thromboembolic risk prediction. Hence, ROTEM may help to identify patients benefiting from therapeutic anticoagulation.
Acknowledgements
Not applicable.
Abbreviations
- ROTEM
Rotational thromboelastometry
- COVID-19
Coronavirus 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ARDS
Acute respiratory distress syndrome
- ICU
Intensive care units
- MCF
Maximum clot firmness
- ML
Maximum lysis
- PT
Prothrombin time
- INR
International normalized ratio
- aPTT
Activated partial thromboplastin time
- t-PA
Tissue-type plasminogen activator
- PAI-1
Plasminogen activator inhibitor-1
- IQR
Interquartile range
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- CI
Confidence intervals
- PCT
Procalcitonin
- CRP
C-reactive protein
Authors’ contributions
The study was designed by Dr. Zickler and Dr. Kruse. Dr. Magomedov, Dr. Kamhieh-Milz, Dr. Körner and Dr. Kahl contributed to study design, data collection, data analyses and interpretation, Dr. Münch and Dr. Kurreck performed data collection. Dr. Piper performed statistical analyses. Dr. Dörner contributed to study design and interpretation. Drs. Zickler, Eckardt and Gotthardt wrote the first draft of the manuscript and revised subsequent versions. All authors read and approved the final manuscript..
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The study was approved by the ethics committees of Charité – Universitätsmedizin Berlin (EA4/115/20).
Consent for publication
Not applicable.
Competing interests
Dr. Kruse, Dr. Magomedov, Dr. Kurreck, Dr. Münch, Dr. Koerner, Dr. Kamhieh-Milz, Dr. Kahl, Dr. Piper and Dr. Dörner have nothing to disclose. Dr. Eckardt reports personal fees from Akebia, grants from Amgen, grants from Astra Zeneca, grants and personal fees from Bayer, grants from Fresenius, from Genzyme, from Shire and grants and personal fees from Vifor, outside the submitted work. Dr. Zickler reports grants and personal fees from Baxter, outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jan Matthias Kruse and Abakar Magomedov have contributed equally to this work
References
- 1.Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020. [DOI] [PMC free article] [PubMed]
- 5.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;4:1–10. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher TE, Leblebicioglu H, Bozkurt I, et al. Rotational thromboelastometry alongside conventional coagulation testing in patients with Crimean-Congo haemorrhagic fever: an observational cohort study. Lancet Infect Dis. 2019;19:862–871. doi: 10.1016/S1473-3099(19)30112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorlinger K, Bhardwaj V, Kapoor PM. Simulation in coagulation testing using rotational thromboelastometry: a fast emerging, reliable point of care technique. Ann Card Anaesth. 2016;19:516–520. doi: 10.4103/0971-9784.185546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care. 2014;18:549. doi: 10.1186/s13054-014-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akay OM, Ustuner Z, Canturk Z, Mutlu FS, Gulbas Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Med Oncol. 2009;26:358–364. doi: 10.1007/s12032-008-9129-0. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt FCF, Manolov V, Morgenstern J, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9:19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67:125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 19.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1714350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50:1–6. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibanez C, Perdomo J, Calvo A, et al. High D dimers and low global fibrinolysis coexist in COVID19 patients: what is going on in there? J Thromb Thrombolysis. 2020;15:1–5. doi: 10.1007/s11239-020-02226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts I. Fibrinolytic shutdown: fascinating theory but randomized controlled trial data are needed. Transfusion. 2016;56(Suppl 2):S115–S118. doi: 10.1111/trf.13490. [DOI] [PubMed] [Google Scholar]
- 24.Lupu F, Keshari RS, Lambris JD, Coggeshall KM. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133(Suppl 1):S28–31. doi: 10.1016/j.thromres.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost. 2007;5(Suppl 1):24–31. doi: 10.1111/j.1538-7836.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- 26.Gando S, Wada H, Thachil J. Scientific, Standardization Committee on DICotISoT, Haemostasis. Differentiating disseminated intravascular coagulation (DIC) with the fibrinolytic phenotype from coagulopathy of trauma and acute coagulopathy of trauma-shock (COT/ACOTS) J Thromb Haemost. 2013;11:826–835. doi: 10.1111/jth.12190. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Fanne R, Stepanova V, Litvinov RI, et al. Neutrophil alpha-defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood. 2019;133:481–493. doi: 10.1182/blood-2018-07-861237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardaway RM, Harke H, Tyroch AH, Williams CH, Vazquez Y, Krause GF. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg. 2001;67:377–382. [PubMed] [Google Scholar]
- 29.Barrett CD, Moore HB, Moore EE, et al. Fibrinolytic therapy for refractory COVID-19 acute respiratory distress syndrome: Scientific rationale and review. Res Pract Thromb Haemost. 2020;4:524–531. doi: 10.1002/rth2.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durila M, Smetak T, Hedvicak P, Berousek J. Extracorporeal membrane oxygenation-induced fibrinolysis detected by rotational thromboelastometry and treated by oxygenator exchange. Perfusion. 2019;34:330–333. doi: 10.1177/0267659118824218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.