Abstract
An 11-year-old Toy Poodle underwent a computed tomography examination with contrast (iohexol) enhancement under anesthesia. Heart rate and R-wave amplitude on electrocardiogram (ECG) increased 2.5 min after iohexol administration, and end-tidal carbon dioxide decreased to 12 mmHg. A progressive ST segment depression was observed on ECG. Subsequently, the ECG waveform changed to ventricular fibrillation. However, spontaneous circulation returned following cardiopulmonary resuscitation. Myocardial ischemia or anaphylactic shock was suspected in the dog, which explains the ST segment depression observed on ECG. When performing radiological examinations with a contrast agent, the ECG waveform changes, such as an increase in heart rate, R-wave amplitude, or ST segment depression, should be carefully monitored. This might enable early detection of cardiac dysfunction and the ensuing cardiac arrest in dogs.
Keywords: contrast agent, dog, ST segment depression
In dogs, ST segment depression on an electrocardiogram (ECG) is indicative of myocardial ischemia [14]. Blood to the myocardium is supplied from the epicardial to the endocardial side. Myocardial ischemia is transmurally heterogeneous; the subendocardium is at a higher risk for ischemia than the mid-wall or epicardium [7]. Data have suggested that lower blood supply to the subendocardium, rather than the higher oxygen demand, induces subendocardial vulnerability [8]. In case of partial occlusion, subendocardial blood supply is compromised to a higher extent than subepicardial flow [1]. When the coronary vessels are highly constricted, blood perfusion might occur on the epicardial side; however, blood supply may be insufficient on the endocardial side [15]. Subsequently, the membrane potential of the endocardial cardiomyocytes becomes shallow and the duration of action potential also shortens; this generates a potential difference in comparison to the epicardial cardiomyocytes [14]. Hence, the ST segment that reflects the repolarization process is depressed.
Iohexol, a nonionic and water-soluble radiographic contrast medium, is often used in veterinary medicine. It is widely known that administration of a contrast agent, including iohexol, causes renal dysfunction [5]. Kløw et al. [13] conducted experimental trials on dogs and observed that iohexol caused coronary vasospasms. Furthermore, in clinical cases, it was reported that administration of iothalamate meglumine caused acute severe systemic reactions occurred in two anesthetized dogs [16]. Moreover, in another study, 0.8% of the dogs and cats administered with iohexol developed severe reactions, such as convulsions and tachycardia [19]. To the best of our knowledge, there are no reports of contrast agents causing changes in ECG waveform in clinical situations. Herein, we report our experience with a dog that presented with ST segment depression and ventricular fibrillation after iohexol administration.
An 11-year-old, 5.2-kg, spayed, female Toy Poodle with tumors of the tongue root, was referred to Rakuno Gakuen University Animal Medical Center. Twelve days before admission, a biopsy was performed under general anesthesia. Midazolam (Midazolam, Sandoz Co., Ltd., Tokyo, Japan) (0.1 mg/kg) and tramadol (Tramal Injection 100, Nippon Shinyaku Co., Ltd., Kyoto, Japan) (4 mg/kg) were mixed and administered intravenously as premedication. After 7 min, anesthesia was induced with propofol (Propoflo 28, Zoetis Japan Co., Ltd., Tokyo, Japan) (total 9 mg/kg) and the oral cavity was observed. Two tumors located at the base of the tongue were biopsied, following which the dog recovered from anesthesia. There were no complications during anesthesia and the dog was discharged on the day of the procedure. Squamous cell carcinoma was diagnosed on histopathological examination.
On the day of admission, computed tomography (CT) was performed for evaluation of tumor margins and confirmation of lung metastasis. The general condition of the patient before anesthesia was good, except dyslipidemia (triglyceride: 1,530 mg/dl), which was observed on a blood biochemistry test. Auscultation revealed no abnormalities in heart or lung sounds. A chest X-ray revealed Grade I tracheal collapse [25]; however, exercise intolerance and cough were not observed. According to the American Society of Anesthesiologists physical status (ASA-PS) classification system, the dog was grades as ASA-PS Class-2.
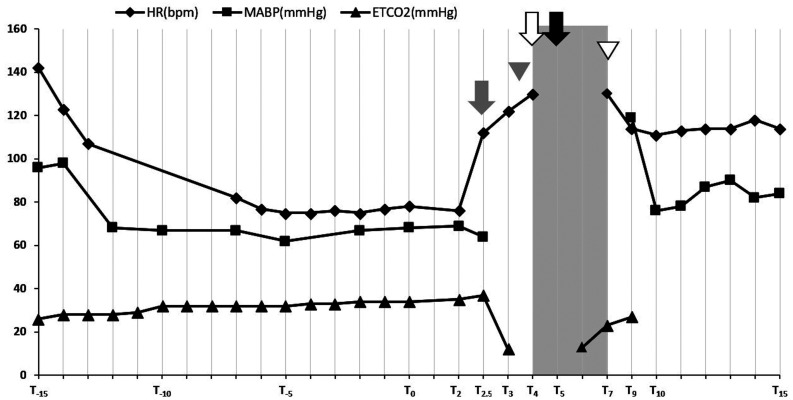
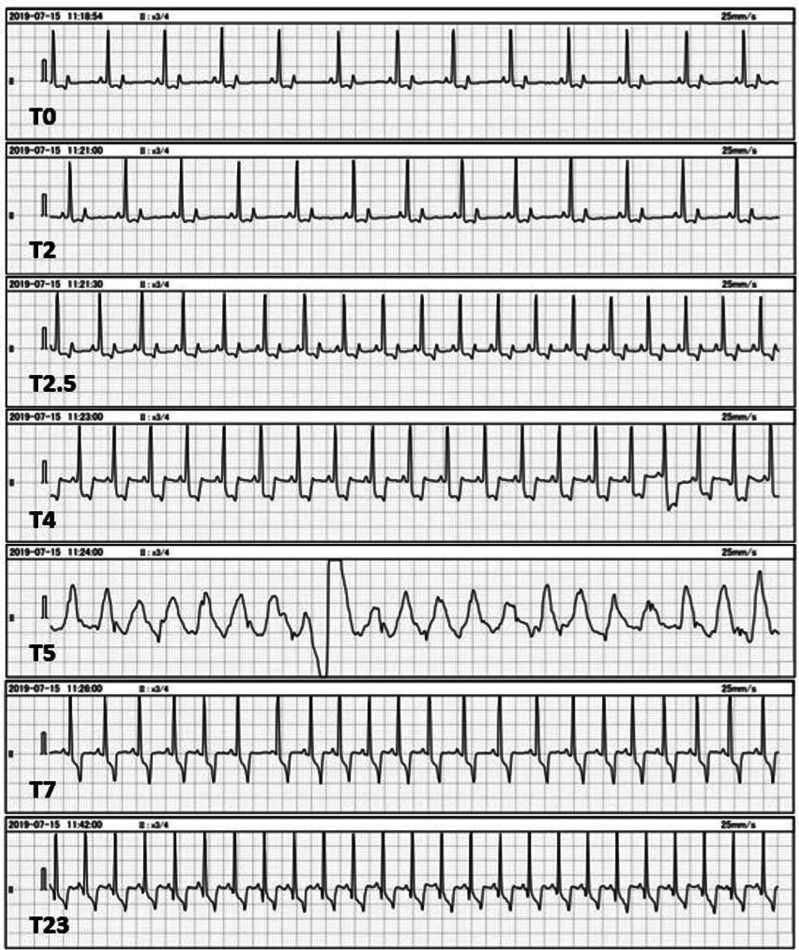
A 24-gauge intravenous catheter was placed in the cephalic vein. Propofol was slowly administered intravenously as an anesthetic induction agent. After observing the oral cavity, a total of 13 mg/kg of propofol was administered. The eyelid reflex and the jaw tension disappeared; and endotracheal intubation was performed. Intubation was maintained with 3.0% sevoflurane in 2 l/min of oxygen administered using a semi-closed circle system (Fancy 80M, Kimura Medical Instrument Co., Ltd., Tokyo, Japan.); thereafter, pressure-controlled ventilation was started (Compos β-EV, Metran Co., Ltd., Saitama, Japan) with a respiratory rate of 12 breaths/min. The peak inspiratory pressure was set at 8 cmH2O. Lead II of ECG (heart rate, HR; beats/min), percutaneous arterial oxygen saturation (SpO2; %), and partial pressure of end-tidal carbon dioxide (ETCO2; mm Hg) by side-stream capnography were recorded using a patient monitoring machine (BSM-3562 Life Scope VS, Nnihon Kohden Co., Ltd., Tokyo, Japan), and an oscillometric, noninvasive, mean arterial blood pressure (MABP; mmHg) with continuous measurement intervals was recorded using a blood pressure monitor (BP-88v, Omron Healthcare Co., Ltd., Kyoto, Japan). Lactated Ringers solution was administered intravenously at 5 ml/kg/hr. Since body movement was observed during positioning for the CT examination, rocuronium (Eslax, MSD Co., Ltd., Tokyo, Japan) (0.5 mg/kg), a peripheral neuromuscular blocking agent, was administered intravenously. After 30 min of induction, iohexol (Omnipaque; 300 mgI/ml, GE Healthcare Co., Ltd., Tokyo, Japan) (2 ml/kg) was administered for a duration 20 sec (T0). Description after administration of iohexol was recorded as follows: 1 min as T1, 2 min as T2, etc. Changes in the HR, MABP, and ETCO2 before and after administration of the iohexol are shown in Fig. 1 and the digital ECG waveforms extracted from the patient monitor are shown in Fig. 2. Initially, there was an increase in amplitude of the R-wave at T2, followed by a large increase in heart rate (T0: 78 beats/min to T2.5: 112 beats/min). A slight decrease in MABP was observed (T0: 68 mm Hg to T2.5: 64 mm Hg) (T2.5). Subsequently, ETCO2 suddenly decreased to 12 mm Hg (T3) and ST segment depression (defined as 0.2 mV lower than the baseline [23]) was observed (T3.5). Since we could not palpate a pulse in the femoral artery, we assumed that a reduction in cardiac output and pulseless electrical activity had occurred. Hence, sevoflurane inhalation was discontinued (T4). Immediately, chest compression and cardiopulmonary resuscitation with epinephrine IV (Bosmin, Daiichi Sankyo Co., Ltd., Tokyo, Japan) (0.01 mg/kg) were performed because ETCO2 was no longer detected (T4). The ECG waveform shifted to ventricular fibrillation (VF) (T5), but chest compressions continued until a defibrillator was ready (T4–T7). The ETCO2 suddenly increased to 23 mm Hg, and then heart sounds could be auscultated by a stethoscope (T7). Simultaneously, spontaneous circulation returned; hence, the chest compressions were terminated. Subsequently, sugammadex (Bridion, MSD Co., Ltd., Tokyo, Japan) (4 mg/kg) was administered intravenously to counteract the effects of rocuronium (T9). Extubation was done as spontaneous breathing resumed immediately after injection of sugammadex and cardiorespiratory functions were stable without additional cardiovascular agonists (T10). Despite the ST segment depression at T23, cardiorespiratory function was stable and there were no symptoms associated with anaphylactic shock or allergies after recovery from anesthesia. The dog was observed overnight and discharged three days after admission. Echocardiography revealed no cardiac dysfunction at revisit.
Fig. 1.
Changes in heart rate, mean arterial blood pressure, and end-tidal carbon dioxide during anesthesia. Shading: chest compression, Gray arrow: increase in amplitude of the R-wave, Gray arrowhead: ST segment depression confirming, White arrow: epinephrine administration, Black arrow: VF confirming, White arrowhead: return of spontaneous circulation. The time frame is relative to the administration of iohexol (T0) where 1 min since administration is T1, 2 min is T2, etc.
Fig. 2.
Changes in the waveform of electrocardiograms after administration of iohexol. Observed changes to lead II ECG waveforms immediately after (T0), and 2, 2.5, 4, 5, 7, and 23 min (T2, T2.5, T4, T5, T7, T23, respectively) after iohexol administration. R-wave amplitude increases at T2 and heart rate increases at T2.5. The ST segment depression occurs from T3.5 and visually reveals at T4, and then ventricular fibrillation occurred at T5. The return of spontaneous circulation occurs at T7. The ST segment depression remains at T23 after recovery from anesthesia.
To the best of our knowledge, this is the first clinical report observing changes in ST segment depression on the ECG waveform in a dog administered with iohexol. In human medicine, it has been reported that coronary angiography with iohexol causes coronary vasospasm and ST segment depression [24]. Coronary angiography with iohexol in experimental trials on dogs has shown to increase the R-wave amplitude and ST segment depression [13]. Similar changes were noted in this case. Although coronary angiography was not performed in this case, one cause for the cardiovascular spasm could have been the administration of iohexol.
The development of a life-threatening anaphylactic reaction after contrast agent administration has been described previously in veterinary patients. Skin and mucous membrane edema were frequently observed in anaphylactic reactions caused by contrast agents [4]. In another case report of cardiac arrest due to suspected anaphylactic reaction after iodinated contrast agent administration, a severe crackle was heard on auscultation of the thorax [17]. In anaphylactic shock, systemic vasodilation and tachycardia occur because of baroreceptor reflex, and epinephrine is considered the drug of choice for treatment [22]. Although skin and mucous membrane edema and crackle sounds were not observed in our case, an increase in HR and a decrease in MABP at T2.5 may have been caused by systemic vasodilation and baroreceptor reflex. These responses could be the initial cardiovascular response to anaphylactic shock. Therefore, this could be another reason for the anaphylactic shock following iohexol administration.
In experimental regional ischemia, an increasing HR reflects subendocardial flow and contraction, and increases myocardial oxygen consumption [10]. A change in ETCO2 reflects cardiac output if the tidal volume is constant in the ventilation setting [11, 21]. ETCO2 had shown slight increase initially (T2.5) followed by an increase in HR, and then it suddenly decreased (T3) after iohexol administration. The initial increase in HR may have been due to myocardial ischemia or anaphylaxis. We used a pressure-controlled ventilator for this dog. The tidal volume in a pressure-controlled ventilator is not constant due to changes in airway resistance or lung-thoracic compliance. Since the dog was not interfered with during CT examination, changes in tidal volume were minimal. Therefore, it was considered that the administration of iohexol had caused a decrease in cardiac output following acute myocardial ischemia. Alternatively, bronchospasm due to anaphylaxis may have caused severe hypoventilation. In contrast, the rise of ETCO2 during cardiopulmonary resuscitation is a useful indicator of the resumption of self-heartbeat [18]. Herein, the ETCO2 suddenly rose during cardiopulmonary resuscitation at T7; and we confirmed the return of spontaneous circulation by ECG and the resumption of self-heartbeat by auscultation.
The recommended treatment of coronary vasospasm after administration of contrast agent in human medicine is calcium antagonists, not beta-adrenergic blockers [9]. In cardiac arrest caused by coronary vasospasms, epinephrine may maintain or exacerbate coronary vasospasm; hence, calcium antagonists and/or nitrates have been reported as the recommended treatment [12, 26]. However, we could not confirm that the cause of cardiac arrest in this case was cardiovascular spasm caused by iohexol administration. Therefore, the dog was judged to be in cardiac arrest, and chest compression and intravenous administration of low-dose epinephrine (0.01 mg/kg) [2] were started. As a result, spontaneous circulation returned in this case. However, further studies are required for finding an appropriate treatment modality for iohexol-induced coronary vasospasms.
Epinephrine administration is recommended during cardiopulmonary resuscitation and anaphylactic shock [2, 22]. However, epinephrine can induce arrhythmias, including VF [6]. In addition, ischemia of the cardiomyocytes may enhance the epinephrine-induced arrhythmias [20]. VF is a dangerous rhythm that causes cardiac arrest and significantly reduces cardiac output. In human medicine, it was reported that iohexol rarely caused VF and its incidence was reported to be 0.4–1.0% [3]. The use of defibrillators for the treatment of VF is recommended [2], though, in this case, we did not use defibrillators because spontaneous circulation resumed before the equipment was ready. Defibrillation immediately after confirmation of VF is recommended for electrical phases within 4 min of cardiac arrest [2]. However, we could not clarify the cause of VF in this case. Therefore, it may be necessary to prepare for emergency treatment when administering a contrast agent that may cause myocardial ischemia or anaphylactic shock.
In conclusion, when performing a contrast agent examination using iohexol in dogs, careful attention to the changes in ECG waveform, such as an increase in HR and R-wave amplitude and ST segment depression, may allow for early detection of cardiac dysfunction leading to cardiac arrest.
REFERENCES
- 1.Bache R. J., Schwartz J. S.1982. Effect of perfusion pressure distal to a coronary stenosis on transmural myocardial blood flow. Circulation 65: 928–935. doi: 10.1161/01.CIR.65.5.928 [DOI] [PubMed] [Google Scholar]
- 2.Fletcher D. J., Boller M., Brainard B. M., Haskins S. C., Hopper K., McMichael M. A., Rozanski E. A., Rush J. E., Smarick S. D., American College of Veterinary Medicine, Veterinary Emergency and Critical Care Society. 2012. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 7: Clinical guidelines. J. Vet. Emerg. Crit. Care (San Antonio) 22 Suppl 1: S102–S131. doi: 10.1111/j.1476-4431.2012.00757.x [DOI] [PubMed] [Google Scholar]
- 3.Flinck A., Gottfridsson B.2001. Experiences with iohexol and iodixanol during cardioangiography in an unselected patient population. Int. J. Cardiol. 80: 143–151. doi: 10.1016/S0167-5273(01)00460-0 [DOI] [PubMed] [Google Scholar]
- 4.Girard N. M., Leece E. A.2010. Suspected anaphylactoid reaction following intravenous administration of a gadolinium-based contrast agent in three dogs undergoing magnetic resonance imaging. Vet. Anaesth. Analg. 37: 352–356. doi: 10.1111/j.1467-2995.2010.00545.x [DOI] [PubMed] [Google Scholar]
- 5.Goic J. B., Koenigshof A. M., McGuire L. D., Klinger A. C., Beal M. W.2016. A retrospective evaluation of contrast-induced kidney injury in dogs (2006–2012). J. Vet. Emerg. Crit. Care (San Antonio) 26: 713–719. doi: 10.1111/vec.12511 [DOI] [PubMed] [Google Scholar]
- 6.Hayashi Y., Sumikawa K., Tashiro C., Yamatodani A., Yoshiya I.1988. Arrhythmogenic threshold of epinephrine during sevoflurane, enflurane, and isoflurane anesthesia in dogs. Anesthesiology 69: 145–147. doi: 10.1097/00000542-198807000-00035 [DOI] [PubMed] [Google Scholar]
- 7.Hoffman J. I.1987. Transmural myocardial perfusion. Prog. Cardiovasc. Dis. 29: 429–464. doi: 10.1016/0033-0620(87)90016-8 [DOI] [PubMed] [Google Scholar]
- 8.Hoffman J. I., Baer R. W., Hanley F. L., Messina L. M.1985. Regulation of transmural myocardial blood flow. J. Biomech. Eng. 107: 2–9. doi: 10.1115/1.3138516 [DOI] [PubMed] [Google Scholar]
- 9.Hung M. J., Hu P., Hung M. Y.2014. Coronary artery spasm: review and update. Int. J. Med. Sci. 11: 1161–1171. doi: 10.7150/ijms.9623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indolfi C., Ross J., Jr.1993. The role of heart rate in myocardial ischemia and infarction: implications of myocardial perfusion-contraction matching. Prog. Cardiovasc. Dis. 36: 61–74. doi: 10.1016/0033-0620(93)90022-6 [DOI] [PubMed] [Google Scholar]
- 11.Isserles S. A., Breen P. H.1991. Can changes in end-tidal PCO2 measure changes in cardiac output? Anesth. Analg. 73: 808–814. doi: 10.1213/00000539-199112000-00023 [DOI] [PubMed] [Google Scholar]
- 12.Kiss G., Corre O., Gueret G., Nguyen Ba V., Gilard M., Boschat J., Arvieux C. C.2009. Management of cardiac arrest caused by coronary artery spasm: epinephrine/adrenaline versus nitrates. Heart Lung 38: 228–232. doi: 10.1016/j.hrtlng.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 13.Kløw N. E., Tande P. M., Hevrøy O., Refsum H.1990. Mechanism of ECG changes and arrhythmogenic properties of low osmolality contrast media during coronary arteriography in dog. Cardiovasc. Res. 24: 303–308. doi: 10.1093/cvr/24.4.303 [DOI] [PubMed] [Google Scholar]
- 14.Mirvis D. M.1988. Physiologic bases for anterior ST segment depression in patients with acute inferior wall myocardial infarction. Am. Heart J. 116: 1308–1322. doi: 10.1016/0002-8703(88)90456-5 [DOI] [PubMed] [Google Scholar]
- 15.Mirvis D. M., Ramanathan K. B., Wilson J. L.1986. Regional blood flow correlates of ST segment depression in tachycardia-induced myocardial ischemia. Circulation 73: 365–373. doi: 10.1161/01.CIR.73.2.365 [DOI] [PubMed] [Google Scholar]
- 16.Pollard R. E., Pascoe P. J.2008. Severe reaction to intravenous administration of an ionic iodinated contrast agent in two anesthetized dogs. J. Am. Vet. Med. Assoc. 233: 274–278. doi: 10.2460/javma.233.2.274 [DOI] [PubMed] [Google Scholar]
- 17.Rodrigo-Mocholí D., Willems A., Schauvliege S., Bosmans T.2015. Cardiopulmonary arrest in a cat as a result of a suspected anaphylactic reaction to an intravenously administered iodinated contrast agent. Vet. Anaesth. Analg. 42: 554–555. doi: 10.1111/vaa.12268 [DOI] [PubMed] [Google Scholar]
- 18.Sandroni C., De Santis P., D’Arrigo S.2018. Capnography during cardiac arrest. Resuscitation 132: 73–77. doi: 10.1016/j.resuscitation.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 19.Scarabelli S., Cripps P., Rioja E., Alderson B.2016. Adverse reactions following administration of contrast media for diagnostic imaging in anaesthetized dogs and cats: a retrospective study. Vet. Anaesth. Analg. 43: 502–510. doi: 10.1111/vaa.12335 [DOI] [PubMed] [Google Scholar]
- 20.Schömig A.1990. Catecholamines in myocardial ischemia. Systemic and cardiac release. Circulation 82 Suppl: II13–II22. [PubMed] [Google Scholar]
- 21.Shibutani K., Muraoka M., Shirasaki S., Kubal K., Sanchala V. T., Gupte P.1994. Do changes in end-tidal PCO2 quantitatively reflect changes in cardiac output? Anesth. Analg. 79: 829–833. doi: 10.1213/00000539-199411000-00002 [DOI] [PubMed] [Google Scholar]
- 22.Shmuel D. L., Cortes Y.2013. Anaphylaxis in dogs and cats. J. Vet. Emerg. Crit. Care (San Antonio) 23: 377–394. doi: 10.1111/vec.12066 [DOI] [PubMed] [Google Scholar]
- 23.Smith F. W. K., Jr., Tilley L. P.2016. Electrocardiography. pp. 49–76. In: Manual of Canine and Feline Cardiology, 5th ed. (Smith, Jr. F. W. K., Tilley, L. P., Oyama, M. A. and Sleeper, M. M. eds.), Elsevier, St. Louis. [Google Scholar]
- 24.Sullivan I. D., Wainwright R. J., Reidy J. F., Sowton E.1984. Comparative trial of iohexol 350, a non-ionic contrast medium, with diatrizoate (Urografin 370) in left ventriculography and coronary arteriography. Br. Heart J. 51: 643–647. doi: 10.1136/hrt.51.6.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tappin S. W.2016. Canine tracheal collapse. J. Small Anim. Pract. 57: 9–17. doi: 10.1111/jsap.12436 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z. P., Su X., Yang Y. C., Wu M. X., Liu B., Liu C. W.2015. Cardiac arrest with coronary artery spasm: does the use of epinephrine during cardiopulmonary arrest exacerbate the spasm? Am. J. Emerg. Med. 33: 479.e5–479.e6. doi: 10.1016/j.ajem.2014.08.052 [DOI] [PubMed] [Google Scholar]