Abstract
The purpose of this study was to investigate the neuroprotective potential of submicron (milled) and blended Lycium barbarum (LB) in glaucomatous retinal neuropathy using a rat model of high intraocular pressure (HIOP) induced retinal ischemia. The rats were treated with 500, 250, 100 mg/kg LB (submicron or blended form) orally once daily for 56 days respectively after 1 week of retinal ischemia induction. We conducted electroretinography (ERG), histopathological analysis in retina and antioxidative level assays, such as total glutathione (GSH (glutathione) + reduced glutathione) + GSSH (glutathione disulfide), catalase activity, SOD (superoxide dismutase) activity, and lipid peroxidant malondialdehyde (MDA) in the retina and plasma of test rats. The results indicated that the amplitudes of a and b wave of ERG were preserved in rats treated with submicron and blended LB groups, the best protective effect on ERG b wave amplitudes was observed at the dosage of 250 mg/kg of both forms of LB. Retinal thickness was best preserved, particularly significant in the retinal inner nuclear layer in submicron 250 mg/kg LB group. The levels of antioxidant GSSH+GSH, SOD and catalase activity in the retina were higher in blended 500 mg/kg and submicron 250 mg/kg groups than other groups, while the MDA level was lower in submicron LB groups than that in blended LB and non-LB IR group. In the plasma, there was no significant difference in the levels of GSSH+GSH and catalase activity between treated groups, but higher levels of SOD and lower levels of MDA were observed in 250 mg/kg submicron and 500 mg/kg submicron LB groups than the blended LB and non-LB IR groups. Generally better antioxidative effects were observed in the submicron LB than blended LB among treated groups, especially the 250 mg/kg submicron LB, providing good retinal neuroprotection by preserving retinal structure and function with improved antioxidative capacity. The submicron LB may have clinical implication as an adjuvant therapy of oxidative stress and retinal damage caused by HIOP induced retinal ischemia and reperfusion injury.
Keywords: antioxidative, Lycium barbarum, neuroprotective, rat, retinal ischemia
During normal cellular metabolism, free radicals and reactive oxygen species were formed. The lowered capacity of cellular mechanism to defend against these pathological conditions are referred to as oxidative stress; which these conditions had been related to a variety of ocular diseases, such as glaucoma, cataract, and age-related macular degeneration. Ischemia induced oxidative stress [9], whereas post-ischemic reperfusion injury results in a further increase of oxidative molecules and the depletion of endogenous free radical scavengers [23]. The subsequent caspase-dependent and caspase-independent mitochondrial pathways cause cell death and tissue damage, e.g. the death of retinal ganglion cells [11].
Lycium barbarum (LB) contains a variety of natural compounds, including alkaloids, steroids, carotenoids, polysaccharides, and volatile oils; which known to protect the retina from oxidative stress [1]. Numerous literatures had demonstrated that LB contains polysaccharides, betaine, betacarotine, and zeaxanthin that plays an anti-oxidative role [4, 17, 22, 24, 30]. Chan et al., 2007 documented that LB provide a protective effect on retinal ganglion cells in a glaucomatous ocular hypertension model [5]. Li et al., 2011 further confirmed that LB results in a reduction of neuronal damage, blood-retinal barrier disruption and oxidative stress [19].
Advancement in submicron technology has a pharmaceutical implication due to its specificity and effectiveness in drug delivery. Submicron technology has been used in the treatment of cancer and cardiovascular diseases. Submicron particles including liposomes, polymers, micelles, dendrimers, quantum dots, submicron shells, or gold submicron particles are commonly tested and used as a carrier for pharmaceutically active compounds and drugs [3, 7, 8, 18]. According to one of our studies by Chang et al., 2018, LB provided protective and antioxidative effects on the rat retina with light-induced retinal degeneration. And the submicron LB protected degenerative retina better than blended LB [6]. However, the antioxidative effects of submicron particles derived from LB on the ischemia and reperfusion injury has not been delineated. Multiple researches had proven that high intro-ocular pressure provides a good model to study retinal ischemia and reperfusion injury [10, 16, 26, 28]. Hence, we conducted an experiment with a rat model with transiently elevated intra-ocular pressure treated with submicron LB and blended LB to analyze their protective effects on retinal function, structure and antioxidation, in terms of the level of glutathione and oxidized glutathione (GSH and GSSH) ratio, catalase or superoxide dismutase (SOD) and the peroxidants levels of malondialdehyde (MDA).
MATERIALS AND METHODS
Animals
Experiments were performed on adult male Spraque-Dawley (SD) rats obtained from the Center of Laboratory Animal, National Yang Ming University, each aged 4–5 weeks and weighing 180–220 g. All rats were housed in a standard animal room with 50–100 lux illumination on a 12-hr light/12-hr dark cyclic light schedule for 1–2 weeks before the beginning of the experiments. Animals had free access to commercial rat diet and water. The eyes of all animals were examined by slit-lamp biomicroscopy and indirect ophthalmoscopy prior to experiments and during recovery stages of the experiments. Rats were anesthetized with isoflurane by mask during the operation and electroretinography (ERG) recording; then they were euthanized by overdose of thiamylal sodium (Citosol® Shinlin Sinseng, Taipei, Taiwan). All experimental procedures involving animals were performed in accordance with both the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research and the guideline of the Animal Care and Use Committee of the National Taiwan University.
Rat model of high intraocular pressure induced retinal ischemia-reperfusion
Six weeks old male Spraque-Dawley (SD) rats were injected with 130 mmHg isotonic saline solution (0.9% NaCl) into the right eye for 50 min under general anesthesia. General anesthesia was induced and maintained by mask using 2–3% isoflurane (delivered in 100% oxygen). Topical 0.5% proparacaine hydrochloride (Alcaine®, Alcon Laboratories Inc., Fort Worth, TX, USA) was applied for corneal anesthesia. No procedure was done on the contralateral eye (left eye), served as a control. As a prevention against bleeding from iris, the pupils were dilated with topical 0.5% tropicamide (Mydriacyl®, Alcon). The intraocular pressure (IOP) was raised to 130 mmHg by cannulation of the anterior chamber with a 30-gauge needle connected to a hydrostatic pressure device containing sterile 0.9% NaCl (isotonic saline solution) for 50 min [27].
Preparation of Lycium barbarum
LB was prepared by Institute of Food Science and Technology, National Taiwan University laboratory of nano-technologic research. Briefly, 5 g dried fruits of LB was prepared by boiling in 500 ml distilled water then blended with high speed homogenizer stirrer (Polytron PT 3100) to obtain the blended aqueous extract with the particle size of 3.58 ± 3.8 µm. In addition, the blended LB was milled with Minipur to obtain a submicron extract of LB with a particle size of 100 ± 70 nm. The particle sizes were all measured by Flow CytoMetry to make sure the quality of the test material was consistent.
Preparation of retinal tissues and plasma, and erythrocytes (RBCs) lysis
The retina was excised immediately and washed twice with phosphate buffered saline (PBS). Then the excised retina was frozen in liquid nitrogen, to ground in a pestle and molar with liquid nitrogen to prepare a fine powder, stored it immediately at −80°C. Followed the instructions of antioxidant and lipid peroxidation analysis kits (Glutathione Assay kit, SOD determination kit, Catalase Assay kit and TBARS (MDA) Assay kit), to homogenize the retinal tissues. An aliquot power was taken and buffers from different analysis kits were added to homogenize the retinal tissues with a polytetrafluoroethylene (PTFE) pestle in glass tube until an even suspension was achieved. Blood was collected in heparin tube from jugular vein of rats with 23 Gauge needle by 1 ml syringe under anesthesia. Separated the red blood cells from plasma by centrifugation at 3,000 rpm for 10 min at 4°C. Took plasma and stored it immediately at −80°C. Each RBCs sample for CAT analysis was washed 3 times with PBS by centrifugation at 3,000 rpm for 10 min at 4°C. Resuspended the RBCs in 5X volumes of iced cold distilled water. Centrifuged at 3,000 rpm for 10 min. Store the supernatant at −80°C until analysis.
Experimental designs
The animals were divided into eight groups, consisting of six groups with 6 animals each (all underwent high IOP induced retinal ischemia damage) to be treated with different dosage and particle sizes of LB, and one no-IR control group and one induced ischemia reperfusion but no treatment (IR-injury) group. IR-injury group underwent high IOP induced retinal ischemia reperfusion damage received no LB treatment (fed with isotonic saline solution). Among the 6 treatment groups, 3 groups were Blended LB-treated: fed with 100, 250, 500 mg/kg blended LB respectively; while another 3 groups were submicron LB-treated: fed with 100, 250, 500 mg/kg submicron (milled) LB, respectively. All the blended or submicron LB or isotonic saline solution were administered via oral gavage to the Sprague-Dawley rats daily for 56 days after 1 week of HIOP induced retinal ischemia reperfusion according to the respective group.
Antioxidant & lipid peroxidation analysis
The levels of total glutathione (GSH + GSSH) in retinal homogenate of experimental rats’ eyes were measured by Glutathione Assay Kit (CS0260, Sigma-Aldrich Inc., St. Louis, MO, USA). An aliquot power of retina was taken for being deproteinized. 5% 5-Sulfosalicylic Acid Solution (SSA) was added and vortexed. The retinal tissues were homogenized with a polytetrafluoroethylene (PTFE) pestle in glass tube on ice until an even suspension was achieved. Left the suspension at 4°C for 10 min and then centrifuged at 10,000 rpm for 10 min at 4°C to remove the precipitated protein. Took a 200 µl plasma and add 200 µl 5% SSA Solution and vortexed. Centrifuged at 10,000 rpm for 10 min at 4°C. Measured the volume of the supernatant and used it as the original sample volume in the calculation for glutathione determination. Stored the samples at −80°C until analysis. The level of total glutathione was measured spectrophotometrically at 412 nm and calculated according to the equation and instruction of the assay kit.
The superoxide dismutase was measured by SOD determination Kit (19160, Sigma-Aldrich Inc.). An aliquot power of retina was taken and ice cold 0.1 M Tris/HCl, pH 7.4 containing 0.5% Triton X-100, 5 mM β-ME, 0.1 mg/ml PMSF was added to homogenize the retinal tissues with a polytetrafluoroethylene (PTFE) pestle in glass tube on ice until an even suspension was achieved. Centrifuged the crude tissue homogenate at 14,000 rpm for 5 min at 4°C and discarded the cell debris. Stored the samples at −80°C until analysis. Minus eighty°C stored plasma was taken for analysis. Following the SOD Assay Protocol, the samples were placed in the 96-wells microplates and incubated the plates at 37°C for 20 min. The SOD activity was measured spectrophotometrically at 450 nm and calculated according to the instruction and equation of the assay kit.
The catalase was measured by Catalase Assay Kit (CAT100, Sigma-Aldrich Inc.). An aliquot power of retina was taken and 1x Assay Buffer was added to homogenize the retinal tissues with a polytetrafluoroethylene (PTFE) pestle in glass tube on ice until an even suspension was achieved. Centrifuged at 10,000 rpm for 15 min at 4°C. Collected supernatant and stored the samples at −80°C until analysis. Plasma was tested directly. The level of CAT was measured spectrophotometrically at 520 nm and calculated according to the equation and instruction of the assay kit.
The peroxidants of malondialdehyde was measured by OxiSelectTM TBARS Assay Kit (MDA Quantitation, STA-330, Cell Biolabs Inc., San Diego, CA, USA). An aliquot power of retina was taken and 1x BHT was added to homogenize the retinal tissues with a polytetrafluoroethylene (PTFE) pestle in glass tube on ice until an even suspension was achieved. Centrifuged at 10,000 rpm for 5 min at 4°C. Collected supernatant and stored the samples at −80°C until analysis. The supernatant was assayed directly for its TBARS (MDA) level and results were normalized based on its protein concentration. 1x BHT was added to plasma samples to prevent further oxidation. Plasma was assayed directly without prior processing. The concentration of MDA was expressed as µM/g. The level of MDA was measured spectrophotometrically at 532 nm according to the equation and instruction of the assay kit.
Electroretinography recording
Rats were dark adapted for over 12 hr and prepared under dim red light. Scotopic ERGs were recorded with Acrivet RETIport system (ERG-VEP-AEP, Acrivet An-Vision GmbH, Hennigsdorf, Germany) before, and 3, 7, 14, 28 and 56 days after high IOP induced retinal ischemia reperfusion damage in experimental eyes and contralateral control eyes. The data were recorded following general anesthesia with isoflurane (AttaneTM) and corneal anesthesia with 0.5% proparacaine (Alcaine, Alcon). All manipulations were performed under dim red illumination (λmax=659 nm) following overnight dark adaptation (>12 hr). Flash ERG was performed after pupillary dilation with 0.5% tropicamide (Mydriacyl®, Alcon) using an LED light source and responses were recorded and using a contact lens electrode (ERG-jet®, Universo Plastique Inc., Le Crêt-du-Locle, Switzerland) referenced to a subcutaneous needle electrode (platinum subcutaneous needle electrode F-E2, Grass-Telefactor Division, Astro-Med Inc., West Warwick, RI, USA) and a ground electrode on the ear. The intensity of light used to record the retinal responses to light stimulus was 1 cd-s/m2. Each amplitude result was the average of two 10-sec flash light stimuli [6].
Histopathology
The rats were euthanized with an overdose of thiamylal sodium (Citosol®) 56 days after high IOP induced retinal ischemia reperfusion damage. Both eyes were enucleated through conjunctivae and fixed in 10% formalin for two days and then embedded in paraffin. Paraffin embedded tissues were sagittal sectioned vertically to optic discs, cut at 4 µm thickness, and stained with hematoxylin and eosin (H&E) stain. The cell layers of retinal thickness, including inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor segment layer (PSL), and photoreceptor segment layer−inner limiting membrane (PSL-ILM, retinal full thickness), were measured under light microscopy [6]. The thickness of twelve areas of each retinal specimen was measured beginning from the optic disc in the center and moving peripherally in increments of 250 µm in 2 opposite ways, dorsally and ventrally, which included central and peripheral retina. The average measured values of the IPL/ONL ratio from twelve measurements of each eye were used to evaluate the extent of ischemia-reperfusion damage of retina [25].
Statistical analysis
All value was expressed as mean ± SD. Statistical significance was analyzed either by paired t-test or one-way ANOVA followed by Scheffe’s multiple comparison test, as indicated. Values of P<0.05 were taken to be statistically significant.
RESULTS
Electroretinography
The changes of retinal function following high IOP induced retinal ischemia reperfusion damage (IR injury) before, and in 3, 7, 14, 28 and 56 days in untreated IR injury group are shown in Table 1. Eyes of test rats in untreated IR injury group showed reduced amplitudes in a-wave and b-wave after IR injury compared with those of the same eyes before IR injury. The amplitudes in a-wave and b-wave were increased over time, but there was no significantly statistical difference between different days after high IOP induced ischemia reperfusion damage in untreated IR injury group.
Table 1. The electroretinography amplitudes before retinal ischemia reperfusion (IR) injury, and 3, 7, 14, 28 and 56 days after high intraocular pressure induced retinal IR injury in untreated IR injury group (n=6).
| Retinal amplitude (μV/div) | Pre | Post-3 days | Post-7 days | Post-2 wks | Post-4 wks | Post-8 wks |
|---|---|---|---|---|---|---|
| a wave | 209.1 ± 10.74 a) | 61.54 ± 10.16 b) | 85.66 ± 16.29 b) | 74.56 ± 33.29 b) | 67.00 ± 42.2 b) | 73.4 ± 11.62 b) |
| b wave | 384.7 ± 61.99 a) | 59.56 ± 21.42 b) | 60.44 ± 10.86 b) | 61.58 ± 14.11 b) | 97.9 ± 43.5 b) | 103.5 ± 11.37 b) |
Pre, before IR injury; post-3 days, 3 days after IR injury; post-7 days, 7 days after IR injury; post-2 weeks, 2 weeks after IR injury; post-4 weeks, 4 weeks after IR injury; post-8 weeks, 8 weeks after IR injury. Data are shown as Mean ± SD and evaluation by one-way ANOVA followed by Scheffe’s multiple comparison test. n=6 in each group. The data with different superscript alphabetical letters indicate significantly statistical difference (P<0.05) between groups. The data with the same superscript letters indicate no significant difference (P>0.05) between groups.
The preservation effect of retinal function following high IOP induced retinal ischemia reperfusion damage in 56 days in experimental eyes are shown in Table 2. Submicron or blended LB groups revealed less reduction in a-wave and b-wave amplitudes compared with the rat groups without LB treatment on day 56 after IR injury. The ERG amplitudes of a and b waves were preserved in both submicron and blended LB treatment groups. The ERG amplitude of a waves were not significantly different among the different particle sizes nor different dosage treatment groups. However, the best ERG amplitudes of b waves preservation rate were observed in the 250 mg/kg submicron LB group. The 250 mg/kg submicron LB group had significant protective effect on retinal function after IR injury (P<0.05).
Table 2. Comparison of electroretinography a and b wave amplitudes among eight experimental groups.
| Experimental groups | Pre | Post-8 wks | Preservation | Pre | Post-8 wks | Preservation |
|---|---|---|---|---|---|---|
| a wave (μV) | a wave (μV) | a wave (%) | b wave (μV) | b wave (μV) | b wave (%) | |
| Normal control (n=6) | 183.2 ± 40.24 a) | 210.3 ± 24.48 a) | 114.79 ± 10.18 a) | 365.2 ± 68.56 a) | 346.5 ± 53.48 a) | 94.88 ± 3.95 a) |
| Untreated IR injury (n=6) | 209.1 ± 10.74 a) | 73.4 ± 11.62 b) | 35.10 ± 7.53 b) | 384.7 ± 61.99 a) | 103.5 ± 11.37 b) | 26.90 ± 4.42 b) |
| Blended (500 mg/kg) (n=6) | 183.2 ± 45.61 a) | 122.1 ± 18.62 c) | 66.65 ± 6.75 cd) | 365.2 ± 64.50 a) | 212.6 ± 46.64 c) | 58.21 ± 7.26 c) |
| Blended (250 mg/kg) (n=6) | 173.8 ± 22.63 a) | 105.8 ± 4.57 c) | 60.87 ± 6.17 cd) | 374.5 ± 54.65 a) | 163.2 ± 35.78 d) | 43.58 ± 6.71 d) |
| Blended (100 mg/kg) (n=6) | 157.5 ± 32.72 a) | 93.3 ± 12.59 c) | 59.24 ± 9.06 c) | 348.4 ± 63.92 a) | 198.4 ± 35.22 cd) | 56.95 ± 5.18 cd) |
| Milled (500 mg/kg) (n=6) | 186.5 ± 32.34 a) | 103.5 ± 20.32 c) | 55.5 ± 7.09 c) | 386.7 ± 44.88 a) | 193.6 ± 19.47 cd) | 50.06 ± 5.68 d) |
| Milled (250 mg/kg) (n=6) | 194.6 ± 25.43 a) | 132.7 ± 15.04 c) | 68.19 ± 5.11 cd) | 405.6 ± 37.30 a) | 253.8 ± 27.92 e) | 62.57 ± 4.80 e) |
| Milled (100 mg/kg) (n=6) | 202.4 ± 29.65 a) | 137.3 ± 22.81 c) | 67.84 ± 3.08 cd) | 397.4 ± 55.81 a) | 217.5 ± 14.18 cd) | 54.73 ± 4.14 d) |
Eight experimental groups include normal control group, untreated IR injury group, blended groups: 500 mg/kg, 250 mg/kg, 100 mg/kg; and milled (submicron) groups: 500 mg/kg, 250 mg/kg, 100 mg/kg. pre, before IR injury; post-8 wks, 8 weeks after IR injury; preservation, the preservation ratio of a or b wave amplitudes in LB treatment groups, untreated IR injury group and normal control group in 8 weeks after IR injury (The preservation ratio=a or b wave amplitude of post-8 wks/ a or b wave amplitude before IR injury). Data are shown as Mean ± SD and evaluation by one-way ANOVA followed by Scheffe’s multiple comparison test. n=6 in each group. The data with different superscript alphabetical letters indicate significantly statistical difference (P<0.05) between groups. The data with the same superscript letters indicate no significant difference (P>0.05) between groups.
Retinal thickness
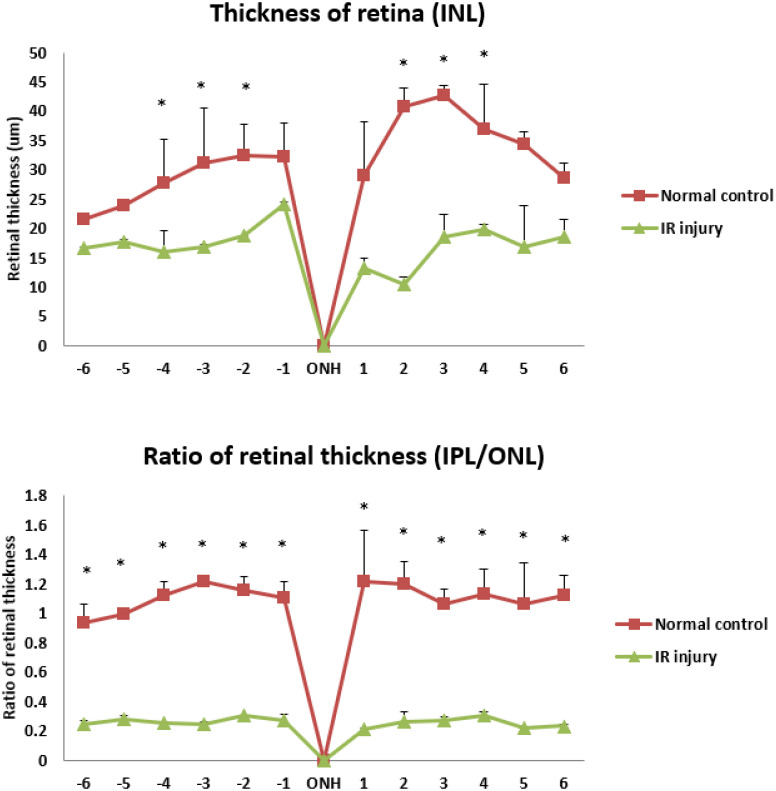
In histopathologic evaluation, the retinal thickness of INL and the IPL/ONL ratio was lower on day 56 after high IOP induced retinal ischemia reperfusion damage (IR injury) in experimental eyes compared with normal control group are shown in Fig. 1. The thickness of INL decreased significantly following IR injury compared with the normal control group, and the IPL/ONL ratio were lower (0.21 ± 0.01 ~ 0.3 ± 0.03) following IR injury compared with the normal control group (1.21 ± 0.02 ~ 0.93 ± 0.13). All LB treatment groups showed protective effect on retinal thickness of INL and the IPL/ONL ratio on day 56 post-IR injury.
Fig. 1.
The retinal thickness of inner nuclear layer (INL) and the inner plexiform / outer nuclear layer (IPL/ONL) ratio on day 56 after high intraocular pressure induced retinal ischemia reperfusion (IR) injury and its comparison to the normal control eyes. INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; ONH, optic nerve head. The numbers of the horizon axis of the Figures are the increments of 250 µm in 2 opposite ways away from the central optic disc when measuring retinal thickness. The positions of number −1– −6 follow the direction of ventral retina, while the positions of number 1-6 follow the direction of dorsal retina. Data are shown as Mean ± SD and evaluation by one-way ANOVA followed by Scheffe’s multiple comparison test. n=6 in each group. *P<0.05 indicate significantly statistical difference versus IR injury group.
The preservation of retinal thickness was best demonstrated in the 250 mg/kg submicron LB group in which retinal inner nuclear layer (INL) were significantly preserved. In contrast, other treatment groups of retinal inner nuclear layer showed no significant difference. Similarly, the IPL/ONL showed no significant difference between all LB treatment groups (Table 3).
Table 3. Comparison of thickness of different retinal layers (INL and IPL/ONL) on day 56 after high intraocular pressure induced retinal ischemia reperfusion damage (IR injury) in eight experimental groups.
| Retinal thickness (μM) | −6 | −5 | −4 | −3 | −2 | −1 | ONH | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INL | Normal control (n=6) | 21.52 ± 0.62 a) | 23.91 ± 0.48 a) | 27.71 ± 7.38 a) | 31.09 ± 9.38 a) | 32.47 ± 5.37 a) | 32.21 ± 5.74 a) | 0 | 28.99 ± 9.22 a) | 40.73 ± 3.31 a) | 42.61 ± 1.70 a) | 36.89 ± 7.79 a) | 34.26 ± 2.24 a) | 28.56 ± 2.60 a) |
| IR injury (n=6) | 16.63 ± 0.19 a) | 17.69 ± 0.33 a) | 15.88 ± 3.67 b) | 16.80 ± 0.56 b) | 18.75 ± 0.11 b) | 24.10 ± 0.35 a) | 0 | 13.26 ± 1.75 a) | 10.46 ± 1.22 b) | 18.61 ± 3.86 b) | 19.81 ± 0.86 b) | 16.74 ± 7.23 a) | 18.54 ± 3.06 a) | |
| Blended (500 mg/kg, n=6) | 26.12 ± 8.24 a) | 36.45 ± 5.42 b) | 36.12 ± 6.5 a) | 40.56 ± 8.10 a) | 34.50 ± 4.50 a) | 32.10 ± 6.41 a) | 0 | 31.54 ± 5.10 a) | 35.16 ± 6.40 a) | 42.13 ± 4.12 a) | 34.55 ± 3.75 a) | 30.45 ± 5.40 a) | 27.23 ± 7.45 a) | |
| Blended (250 mg/kg, n=6) | 28.64 ± 12.18 a) | 33.46 ± 13.58 b) | 37.37 ± 3.21 a) | 47.25 ± 7.29 a) | 36.34 ± 3.06 a) | 30.08 ± 4.53 a) | 0 | 32.94 ± 2.40 a) | 37.55 ± 7.17 a) | 40.89 ± 12.08 a) | 36.59 ± 1.93 a) | 29.95 ± 3.78 a) | 25.11 ± 8.60 a) | |
| Blended (100 mg/kg, n=6) | 25.41 ± 4.23 a) | 36.45 ± 5.73 b) | 37.98 ± 5.44 a) | 35.24 ± 4.12 a) | 34.60 ± 2.30 a) | 33.41 ± 7.40 a) | 0 | 36.78 ± 3.21 a) | 39.43 ± 6.78 a) | 40.12 ± 3.48 a) | 35.45 ± 5.52 a) | 34.60 ± 3.11 a) | 35.10 ± 5.40 a) | |
| Milled (500 mg/kg, n=6) | 33.12 ± 6.14 b) | 34.64 ± 5.30 b) | 34.56 ± 4.70 a) | 37.10 ± 3.45 a) | 36.41 ± 6.44 a) | 36.56 ± 4.17 a) | 0 | 40.20 ± 7.10 a) | 49.50 ± 5.14 a) | 36.40 ± 6.42a) | 37.90 ± 2.41 a) | 34.10 ± 3.80 a) | 33.24 ± 4.10 a) | |
| Milled (250 mg/kg, n=6) | 34.20 ± 3.33 b) | 32.82 ± 1.64 b) | 33.70 ± 3.25 a) | 36.97 ± 2.79 a) | 37.51 ± 5.08 a) | 40.57 ± 7.95 a) | 0 | 60.25 ± 13.32 b) | 56.16 ± 5.34 c) | 52.64 ± 9.24 c) | 54.14 ± 9.24 c) | 49.95 ± 7.82 b) | 51.85 ± 8.03 b) | |
| Milled (100 mg/kg, n=6) | 30.40 ± 6.10 b) | 33.54 ± 5.40 b) | 36.12 ± 3.40 a) | 37.56 ± 4.76 a) | 37.43 ± 6.30 a) | 38.23 ± 5.40 a) | 0 | 43.15 ± 10.30 a) | 47.50 ± 8.10 a) | 42.68 ± 6.40 a) | 42.12 ± 3.47 a) | 37.45 ± 4.22 a) | 32.12 ± 6.41 a) | |
| IPL/ONL | Normal control (n=6) | 0.93 ± 0.13 a) | 0.99 ± 0.01 a) | 1.12 ± 0.09 a) | 1.21 ± 0.02 a) | 1.15 ± 0.10 a) | 1.10 ± 0.11 a) | 0 | 1.21 ± 0.35 a) | 1.20 ± 0.15 a) | 1.06 ± 0.10 a) | 1.13 ± 0.17 a) | 1.06 ± 0.28 a) | 1.12 ± 0.14 a) |
| IR injury (n=6) | 0.24 ± 0.03 b) | 0.28 ± 0.02 b) | 0.25 ± 0.01 b) | 0.24 ± 0.02 b) | 0.30 ± 0.00 b) | 0.27 ± 0.04 b) | 0 | 0.21 ± 0.01 b) | 0.26 ± 0.07 b) | 0.27 ± 0.02 b) | 0.30 ± 0.03 b) | 0.22 ± 0.00 b) | 0.23 ± 0.01 b) | |
| Blended (500 mg/kg, n=6) | 0.88 ± 0.21 a) | 0.86 ± 0.09 a) | 1.02 ± 0.12 a) | 0.97 ± 0.34 a) | 0.95 ± 0.17 a) | 0.97 ± 0.12 a) | 0 | 0.93 ± 0.17 a) | 0.94 ± 0.11 a) | 0.99 ± 0.08 a) | 1.04 ± 0.06 a) | 0.97 ± 0.22 a) | 0.93 ± 0.18 a) | |
| Blended (250 mg/kg, n=6) | 0.89 ± 0.09 a) | 1.05 ± 0.19 a) | 1.07 ± 0.32 a) | 1.08 ± 0.29 a) | 0.95 ± 0.22 a) | 0.96 ± 0.22 a) | 0 | 0.89 ± 0.15 a) | 0.97 ± 0.07 a) | 1.07 ± 0.13 a) | 1.14 ± 0.09 a) | 1.12 ± 0.12 a) | 1.06 ± 0.26 a) | |
| Blended (100 mg/kg, n=6) | 0.96 ± 0.13 a) | 1.01 ± 0.17 a) | 1.04 ± 0.07 a) | 0.97 ± 0.24 a) | 0.92 ± 0.17 a) | 0.86 ± 0.15 a) | 0 | 0.87 ± 0.23 a) | 0.94 ± 0.14 a) | 1.03 ± 0.30 a) | 1.12 ± 0.20 a) | 1.09 ± 0.14 a) | 1.03 ± 0.19 a) | |
| Milled (500 mg/kg, n=6) | 0.75 ± 0.16 a) | 0.73 ± 0.17 a) | 0.80 ± 0.20 a) | 0.71 ± 0.18 a) | 0.76 ± 0.23 a) | 0.83 ± 0.08 a) | 0 | 0.84 ± 0.04 a) | 0.82 ± 0.18 a) | 0.79 ± 0.13 a) | 0.75 ± 0.06 a) | 0.81 ± 0.12 a) | 0.84 ± 0.04 a) | |
| Milled (250 mg/kg, n=6) | 0.89 ± 0.18 a) | 0.82 ± 0.13 a) | 0.74 ± 0.18 a) | 0.77 ± 0.17 a) | 0.83 ± 0.29 a) | 0.79 ± 0.23 a) | 0 | 0.81 ± 0.14 a) | 0.84 ± 0.23 a) | 0.76 ± 0.25 a) | 0.90 ± 0.33 a) | 0.77 ± 0.20 a) | 0.95 ± 0.10 a) | |
| Milled (100 mg/kg, n=6) | 0.73 ± 0.08 a) | 0.76 ± 0.15 a) | 0.81 ± 0.07 a) | 0.76 ± 0.21 a) | 0.73 ± 0.19 a) | 0.82 ± 0.12 a) | 0 | 0.83 ± 0.14 a) | 0.87 ± 0.16 a) | 0.74 ± 0.04 a) | 0.73 ± 0.16 a) | 0.74 ± 0.15 a) | 0.78 ± 0.20 a) | |
Eight experimental groups include control group, IR injury group, blended groups: 500 mg/kg, 250 mg/kg, 100 mg/kg; and milled (submicron) groups: 500 mg/kg, 250 mg/kg, 100 mg/kg. IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer, IPL/ONL, the ratio of IPL/ONL. ONH, optic nerve head. Data are shown as Mean ± SD and evaluation by one-way ANOVA followed by Scheffe’s multiple comparison test. n=6 in each group. The data with different superscript alphabetical letters indicate significantly statistical difference (P<0.05) between groups. The data with the same superscript letters indicate no significant difference (P>0.05) between groups.
Antioxidative capacity & lipid peroxidation analysis
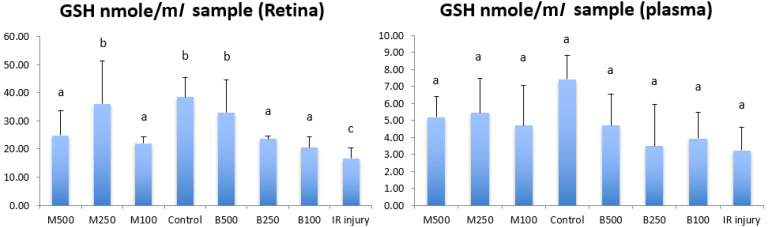
To understand antioxidative capacity and lipid peroxidation of retina and plasma/RBC, total glutathione (GSSH+GSH, GSH) level, SOD, catalase activity (CAT) and of lipid peroxidation malondialdehyde (MDA) in the retina and peripheral blood were examined on day 56 in rats with or without IR injury. Figure 2 shows the concentration of GSH in retina and plasma of all LB treatment groups, control group and IR injury group. GSH concentration in retina in IR injury group was 16.76 ± 3.76 nmol/ml, that was significant lower (P<0.05) than normal control group (38.47 ± 6.87 nmol/ml) and all LB treatment groups. The 250 mg/kg submicron LB group (36.09 ± 15.17 nmol/ml) and 500 mg/kg blended LB group (33.06 ± 11.49 nmol/ml) had best antioxidative effects in retina compared with other treatment groups. And the GSH concentration of these 2 groups in retina was similar to normal control group. In plasma, there was no significant difference of GSH concentration between all treatment groups, control group and IR injury group.
Fig. 2.
Comparison of the level of total glutathione (GSH (glutathione) + GSSG (glutathione disulfide)) in the retina and plasma between all experimental groups in 8 weeks after high intraocular pressure induced retinal ischemia reperfusion (IR) injury. GSH, GSH+GSSG; M500, milled (submicron) 500 mg/kg; M250, milled (submicron) 250 mg/kg; M100, milled (submicron) 100 mg/kg; Control, normal control; B500, blended 500 mg/kg; B250, blended 250 mg/kg; B100, blended 10 mg/kg. Data are shown as Mean ± SD and evaluation by one-way ANOVA followed by Scheffe’s multiple comparison test. n=6 in each group. The data with different superscript letters indicate significantly statistical difference (P<0.05) between groups. The data with the same superscript letters indicate no significant difference (P>0.05) between groups.
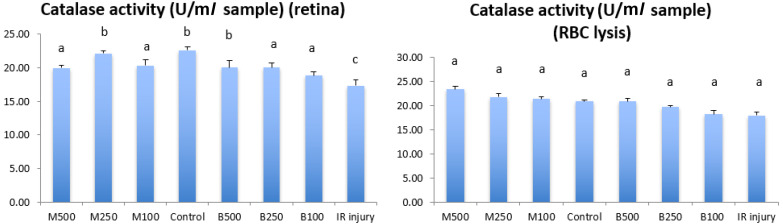
Figure 3 shows the catalase activity (CAT) in retina and RBC in all LB treatment groups, control group and IR injury group. The CAT concentration in retina of IR group was 17.31 ± 0.84 U/ml, that was significant lower (P<0.05) than normal control group (22.61 ± 0.46 U/ml) and all LB treatment groups. The 250 mg/kg submicron LB group (20.06 ± 0.98 U/ml) and 500 mg/kg blended LB group (22.13 ± 0.35 U/ml) had best antioxidative effects in retina compared with other treatment groups. And the CAT concentrations of these 2 LB groups in retina were similar to normal control group. In RBC samples, there were no significant difference of CAT concentration between all treatment groups, control group and IR injury group.
Fig. 3.
Comparison of the levels of catalase activity in retina and plasma between all experimental groups in 8 weeks after high intraocular pressure induced retinal ischemia reperfusion (IR) injury. M500, milled (submicron) 500 mg/kg; M250, milled (submicron) 250 mg/kg; M100, milled (submicron) 100 mg/kg; Control, normal control; B500, blended 500 mg/kg; B250, blended 250 mg/kg; B100, blended 100 mg/kg. Data are shown as Mean ± SD and evaluation by one-way ANOVA followed by Scheffe’s multiple comparison test. n=6 in each group. The data with different superscript letters indicate significantly statistical difference (P<0.05) between groups. The data with the same superscript letters indicate no significant difference (P>0.05) between groups.
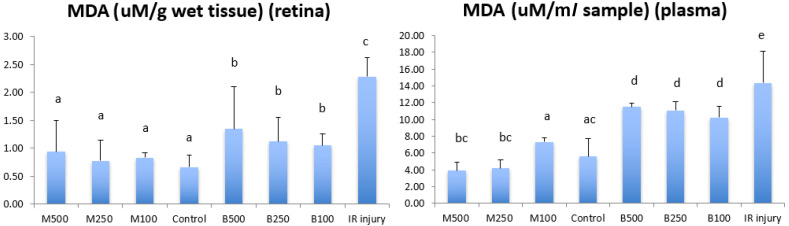
Figure 4 shows the concentration of lipid peroxidation malondialdehyde (MDA) in retina and plasma of all LB treatment groups, control group and IR injury group. In IR injury group, MDA levels as an indicator of oxidative stress was increased significantly to 2.29 ± 0.35 µM/g wet tissue in retina and 14.37 ± 3.73 µM/g wet tissue in plasma (P<0.05) compared with normal control group (0.66 ± 0.21 µM/g wet tissue) in the retina and (5.59 ± 2.13 µM/g wet tissue) plasma. All submicron LB groups (in retina: dose 100 mg/kg, 0.83 ± 0.08 µM/g wet tissue; dose 250 mg/kg, 0.78 ± 0.37 µM/g wet tissue ; does 500 mg/kg, 0.94 ± 0.55 µM/g wet tissue; in plasma: dose 100 mg/kg, 7.33 ± 0.45 µM/g wet tissue; dose 250 mg/kg, 4.17 ± 1.05 µM/g wet tissue; dose 500 mg/kg, 3.87 ± 1.03 µM/g wet tissue) had significantly reduced peroxidation than that of blended LB groups (in retina: dose 100 mg/kg, 1.05 ± 0.21 µM/g wet tissue; dose 250 mg/kg, 1.12 ± 0.44 µM/g wet tissue ; dose 500 mg/kg, 1.35 ± 0.76 µM/g wet tissue; in plasma: dose 100 mg/kg, 10.22 ± 1.32 µM/g wet tissue; dose 250 mg/kg, 11.05 ± 1.06 µM/g wet tissue; dose 500 mg/kg, 11.50 ± 0.42 µM/g wet tissue) in retina and plasma.
Fig. 4.
Comparison of the levels of a biomarker of lipid peroxidation malondialdehyde (MDA) in retina and plasma between all experimental groups in 8 weeks after high intraocular pressure induced retinal ischemia reperfusion (IR) injury. M500, milled (submicron) 500 mg/kg; M250, milled (submicron) 250 mg/kg; M100, milled (submicron) 100 mg/kg; Control, normal control; B500, blended 500 mg/kg; B250, blended 250 mg/kg; B100, blended 100 mg/kg. Data are shown as Mean ± SD and evaluation by one-way ANOVA followed by Scheffe’s multiple comparison test. n=6 in each group. The data with different superscript letters indicate significantly statistical difference (P<0.05) between groups. The data with the same superscript letters indicate no significant difference (P>0.05) between groups.
There was no significant difference in peroxidation between different dosage of both submicron and blended LB groups in retina, and the blended LB group in plasma. However, higher doses of submicron LB, such as 500 mg/kg and 250 mg/kg, had significant reduced peroxidation in plasma compared with that of submicron dose 100 mg/kg.
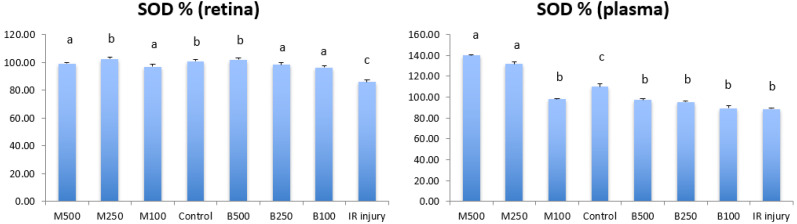
Figure 5 shows the levels of superoxide dismutase (SOD) in retina and plasma of all LB treatment groups, control group and IR injury group. SOD level of retina in IR injury group was 85.97 ± 1.57%, that was significant lower (P<0.05) than normal control group (100 ± 1.13%) and all LB treatment groups. The 250 mg/kg submicron LB group (102.50 ± 1.37%) and 500 mg/kg blended LB group (102.00 ± 1.43%) had best antioxidative effects in retina compared to other treatment groups. And the SOD levels of these 2 groups in retina were similar to normal control group. In plasma, SOD level in IR injury group was 88.47 ± 1.15%, it was significant lower (P<0.05) than normal control group (110.00 ± 2.86%). The 500 mg/kg submicron LB group (139.95 ± 0.71%) and 250 mg/kg submicron LB group (131.87 ± 2.05%) had best antioxidative effects in plasma compared with other treatment groups.
Fig. 5.
Comparison of the levels of superoxide dismutase (SOD) in retina and plasma between all experimental groups in 8 weeks after high intraocular pressure induced retinal ischemia reperfusion (IR) injury. M500, milled (submicron) 500 mg/kg; M250, milled (submicron) 250 mg/kg; M100, milled (submicron) 100 mg/kg; Control, normal control; B500, blended 500 mg/kg; B250, blended 250 mg/kg; B100, blended 100 mg/kg. Data are shown as Mean ± SD and evaluation by one-way ANOVA followed by Scheffe’s multiple comparison test. n=6 in each group. The data with different superscript letters indicate significantly statistical difference (P<0.05) between groups. The data with the same superscript letters indicate no significant difference (P>0.05) between groups.
DISCUSSION
High intraocular pressure (HIOP) induced retinal ischemia is an animal model frequently used to study retinal ischemia–reperfusion injury and also a model for acute-angle closure glaucoma [12, 15]. Degeneration of retinal ganglion cells, thinning of retinal inner nuclear layer and damage of retinal function measured by electroretinography were observed in this model [2, 12, 15, 31]. In this model, retinal ischemia induced oxidative stress, and meanwhile post-ischemia reperfusion injury caused further oxidative molecules accumulation and the depletion of endogenous free radical scavengers. The free radical burst from the early stage of reperfusion overwhelms normal cellular antioxidant defense mechanisms caused oxidative stress and retinal injury [12, 23, 31]. In this study, we indicated that 50 min HIOP induced retinal ischemia –reperfusion injury caused a retinal functional reduction of a- and b-wave amplitudes, a significant decreased thickness of retinal inner nuclear layer (INL), and the levels of GSH, SOD and CAT in retina significant decreased, the levels of peroxidant MDA significant increased. We found the amplitudes in a-wave and b-wave tended to increase over time after high IOP induced ischemia reperfusion damage in untreated IR injury group, but there was no significantly statistical difference between different days. The retinal function was improved gradually after we removed the HIOP induced retinal ischemia-reperfusion injury in 8 weeks. We also found that the oxidative stresses were not only in retina locally but were also present in systemic system. The levels of GSH and SOD were decreased in plasma and CAT was decreased in RBC samples, but without significant statistical difference compared to control group. The level of MDA was significant increasing in plasma of IR rats too.
To our knowledge, this is one of the first few studies addressing the in vivo antioxidant effects of submicron and blended LB during retinal ischemia and reperfusion injury induced by intraocular hypertension. The first study in this field showed that laser ocular hypertension rat model fed with LB extracts had significant reduction of retinal ganglion cell (RGC) loss in eyes in 28 days [5]. The level of RGC loss was nearly undetectable in adult female Sprague-Dawley Rats fed with 10 mg/kg LB crude extract, supporting the claim that LB had protective effect on retinal damage.
In previous studies on rat models of diabetic retinopathy and light-induced retinopathy by our research team, LB showed functional protection of the affected retina in about 8 weeks [6, 14]. The later study, 250 mg/kg LB exhibited significant protective effects on both the ONL and PSL retinal segments regardless of the forms of LB given, and documented with significantly thicker RGC and a significant reduction in the MDA levels with increased antioxidative capacity. The results revealed submicron LB protected degenerative retina much better than blended LB. In this study on retinal IR injury, 2 forms of LB (blended and submicron forms) and three different doses (100 mg/kg, 250 mg/kg and 500 mg/kg) of each form of LB were fed to experimental rats in 8 weeks, and we recorded the ERG a wave and b wave amplitudes of experimental groups on day 56 after IR injury, and then compared these data with the ERG a wave and b wave amplitudes before IR injury of each experimental groups. In order to know if different forms or different doses of LB have long-term neuroprotective effect of retinal function, day 56 rather than day 3, 7, 14 or 28 was chosen for recording. Even the retinal function has slightly recovered after IR injury over time in 56 days, the data showed all LB treated groups have significant neuroprotective effects compared with IR injury groups without treatments on day 56. In this result, we found both forms of LB do have the long-term neuroprotective effect of retinal function. We observed that both forms of LB treatment groups had protective effects in retinal function and antioxidant effects in retina, compared with IR groups without treatments. However, submicron LB performed better than blended LB in general. The results of this study are similar to Chang’s study even though the submicron and blended LB are used to treat different retinal diseases. The preservation ratio of ERG a wave and b wave amplitude of blended LB treated group and submicron LB treated group on day 56 have significant neuroprotective effects compared with untreated group in Chang’s study. The retinal degeneration model was induced by experimental light-induced phototoxicity. The intensive light induced oxidative stress in retina leading to photoreceptors degeneration [6]. The HIOP induced retinal ischemia model also induces oxidative stress, but it causes thinning of retinal inner nuclear layer not the outer nuclear layer. However, both of these 2 disease models caused retinal ganglion cell death and oxidative stress in our studies. Lycium Barbarum (LB), as an antioxidant, provides neuroprotection by down-regulating RAGE (the receptor for advanced glycation endproducts), ET-1 (Endothelin-1), and AGE in the retina, as well as their related signaling pathways, which was related to inhibiting vascular damages and the neuronal degeneration in acute ocular hypertension insults [21]. However, the protective role of LB on retinal ischemia or retinal degeneration remained to understand in details.
Different methods of LB preparation showed different antioxidative effects. Luo et al., 2004 and Ho et al., 2009 confirmed that methanol and hot water extracts of LB fruits showed a better total antioxidant capacity and oxygen radical absorbance capacity, while fruit water decoction and crude polysaccharide extracts exhibited stronger antioxidant activity than major purified polysaccharide fractions, which were isolated and identified by HPLC. The study also found that LB fruits were rich in carotene, riboflavin, ascorbic acid, thiamine, nicotinic acid, betaine, coumarin (scopoletin), zeaxanthin, cryptoxanthin, etc., most of which are antioxidants and were responsible for antioxidant properties of LB [13, 20].
Association between submicronized ingredients and improved antioxidant effects of total carotenoids (ex: beta-carotene or zeaxanthin) and crude polysaccharides have been demonstrated in study conducted by Institute of Food Science and Technology, National Taiwan University (unpublished data). Our study reveals that modification of LB into submicron particles increased its neuroprotective effect significantly. The detailed mechanism of neuroprotective effects of submicron LB is not clear to date. The particles of submicron LB are smaller than blended LB and are close to nanomaterial which may improve the absorption from intestinal mucosa or make the transport of nanoparticles to target cells via blood and lymph vessels more easily. Different dosage regimen may also have impact on the antioxidant effect of LB. Chan et al. confirmed that the dose-response curve for the neuroprotective effects of LB on RGC appeared to be a U-shaped curve. The best neuroprotective effect of LB on RGC was at the dose of 10 mg/kg [5]. Ho et al. showed that exposure to LB markedly reduced glutamate neurotoxicity in a concentration-dependent manner [13]. The dose dependent effect among sub-lethal dose of controlled LB was similar to previous studies. The neuroprotective effect was better in all groups treated with different doses of submicron LB than that in non-submicronized LB, and the neuroprotective outcome of submicronized LB was not dose-dependent. The U-shaped dose related effect has been demonstrated in another study where rats were treated with overdosed LB [5]. Therefore, whether the high efficiency of submicronized LB produced similar effect caused by overdosed LB or whether this paradoxical effect is by chance due to relatively small size is not known. Further studies are needed to delineate the effects of higher dose submicronized LB.
The usage of LB as a medical treatment for post-HIOP-IR condition, or glaucoma cases, is still considered as an alternative choice. Several of other drugs such as lidocaine (LDC) and methylprednisolone (MP) have been documented to possess retinal neuroprotective effects studied by our research team, in which MP showed an excellent preservation in IPL/ONL ratio and ERG b wave amplitudes. [29] Our study provides more experimental data to prove neuroprotective and antioxidative effects of two forms of LB, bringing LB one step closer to be utilized as a more routine auxiliary treatment for retinal protection in glaucoma patients.
Taking together, our study revealed that submicron LB, especially at the dose of 250 mg/kg, provided a significantly better retinal neuroprotective effect, in terms of preservation of both retinal structure as well as retinal function with improved antioxidative capacity in rats with retinal ischemia induced by high intraocular pressure (HIOP). The submicron LB may have clinical implication as an adjuvant therapy of oxidative stress and retinal damage caused by HIOP induced retinal ischemia and reperfusion injury. The detailed mechanism of improved antioxidation of submicron LB requires more investigations.
Acknowledgments
The authors would like to thank Drs. Nien-Chen Lin and An-I Yeh for assistance of preparing submicron and blended LB. The study was supported by research grants 98 Agri-3.1.3-Food-Z2, and NSC98-2313-B-002-027-MY3 (National Science Council, Taiwan). There is no potential conflict relative to this work.
REFERENCES
- 1.Amagase H., Nance D. M.2008. A randomized, double-blind, placebo-controlled, clinical study of the general effects of a standardized Lycium barbarum (Goji) Juice, GoChi. J. Altern. Complement. Med. 14: 403–412. doi: 10.1089/acm.2008.0004 [DOI] [PubMed] [Google Scholar]
- 2.Adachi M., Takahashi K., Nishikawa M., Miki H., Uyama M.1996. High intraocular pressure-induced ischemia and reperfusion injury in the optic nerve and retina in rats. Graefes Arch. Clin. Exp. Ophthalmol. 234: 445–451. doi: 10.1007/BF02539411 [DOI] [PubMed] [Google Scholar]
- 3.Baran E. T., Ozer N., Hasirci V.2002. In vivo half life of nanoencapsulated L-asparaginase. J. Mater. Sci. Mater. Med. 13: 1113–1121. doi: 10.1023/A:1021125617828 [DOI] [PubMed] [Google Scholar]
- 4.Breithaupt D. E., Weller P., Wolters M., Hahn A.2004. Comparison of plasma responses in human subjects after the ingestion of 3R,3R′-zeaxanthin dipalmitate from wolfberry (Lycium barbarum) and non-esterified 3R,3R′-zeaxanthin using chiral high-performance liquid chromatography. Br. J. Nutr. 91: 707–713. doi: 10.1079/BJN20041105 [DOI] [PubMed] [Google Scholar]
- 5.Chan H. C., Chang R. C., Koon-Ching Ip A., Chiu K., Yuen W. H., Zee S. Y., So K. F.2007. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp. Neurol. 203: 269–273. doi: 10.1016/j.expneurol.2006.05.031 [DOI] [PubMed] [Google Scholar]
- 6.Chang J. S., Lee Y. J., Wilkie D. A., Lin C. T.2018. The Neuroprotective and antioxidative effects of submicron and blended Lycium barbarum in experimental retinal degeneration in rats. J. Vet. Med. Sci. 80: 1108–1115. doi: 10.1292/jvms.17-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Jong W. H., Borm P. J.2008. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomedicine 3: 133–149. doi: 10.2147/IJN.S596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan R.2003. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2: 347–360. doi: 10.1038/nrd1088 [DOI] [PubMed] [Google Scholar]
- 9.Flammer J., Haefliger I. O., Orgül S., Resink T.1999. Vascular dysregulation: a principal risk factor for glaucomatous damage? J. Glaucoma 8: 212–219. doi: 10.1097/00061198-199906000-00012 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Valenzuela E., Shareef S., Walsh J., Sharma S. C.1995. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp. Eye Res. 61: 33–44. doi: 10.1016/S0014-4835(95)80056-5 [DOI] [PubMed] [Google Scholar]
- 11.Gilgun-Sherki Y., Rosenbaum Z., Melamed E., Offen D.2002. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol. Rev. 54: 271–284. doi: 10.1124/pr.54.2.271 [DOI] [PubMed] [Google Scholar]
- 12.Grozdanic S. D., Sakaguchi D. S., Kwon Y. H., Kardon R. H., Sonea I. M.2003. Functional characterization of retina and optic nerve after acute ocular ischemia in rats. Invest. Ophthalmol. Vis. Sci. 44: 2597–2605. doi: 10.1167/iovs.02-0600 [DOI] [PubMed] [Google Scholar]
- 13.Ho Y. S., Yu M. S., Yik S. Y., So K. F., Yuen W. H., Chang R. C.2009. Polysaccharides from wolfberry antagonizes glutamate excitotoxicity in rat cortical neurons. Cell. Mol. Neurobiol. 29: 1233–1244. doi: 10.1007/s10571-009-9419-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu C. K., Lee Y. J., Colitz C. M., Chang C. J., Lin C. T.2012. The protective effects of Lycium barbarum and Chrysanthemum morifolum on diabetic retinopathies in rats. Vet. Ophthalmol. 15 Suppl 2: 65–71. doi: 10.1111/j.1463-5224.2012.01018.x [DOI] [PubMed] [Google Scholar]
- 15.Hughes W. F.1991. Quantitation of ischemic damage in the rat retina. Exp. Eye Res. 53: 573–582. doi: 10.1016/0014-4835(91)90215-Z [DOI] [PubMed] [Google Scholar]
- 16.Johnson E. C., Morrison J. C., Farrell S., Deppmeier L., Moore C. G., McGinty M. R.1996. The effect of chronically elevated intraocular pressure on the rat optic nerve head extracellular matrix. Exp. Eye Res. 62: 663–674. doi: 10.1006/exer.1996.0077 [DOI] [PubMed] [Google Scholar]
- 17.Junghans A., Sies H., Stahl W.2001. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 391: 160–164. doi: 10.1006/abbi.2001.2411 [DOI] [PubMed] [Google Scholar]
- 18.Kipp J. E.2004. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 284: 109–122. doi: 10.1016/j.ijpharm.2004.07.019 [DOI] [PubMed] [Google Scholar]
- 19.Li S. Y., Yang D., Yeung C. M., Yu W. Y., Chang R. C., So K. F., Wong D., Lo A. C.2011. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS One 6: e16380. doi: 10.1371/journal.pone.0016380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Q., Cai Y., Yan J., Sun M., Corke H.2004. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 76: 137–149. doi: 10.1016/j.lfs.2004.04.056 [DOI] [PubMed] [Google Scholar]
- 21.Mi X. S., Feng Q., Lo A. C. Y., Chang R. C. C., Lin B., Chung S. K., So K. F.2012. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS One 7: e45469. doi: 10.1371/journal.pone.0045469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami T., Nagamura Y., Hirano K.1998. The recovering effect of betaine on carbon tetrachloride-induced liver injury. J. Nutr. Sci. Vitaminol. (Tokyo) 44: 249–255 (Tokyo). doi: 10.3177/jnsv.44.249 [DOI] [PubMed] [Google Scholar]
- 23.Osborne N. N., Casson R. J., Wood J. P., Chidlow G., Graham M., Melena J.2004. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 23: 91–147. doi: 10.1016/j.preteyeres.2003.12.001 [DOI] [PubMed] [Google Scholar]
- 24.Peng Y., Ma C., Li Y., Leung K. S., Jiang Z. H., Zhao Z.2005. Quantification of zeaxanthin dipalmitate and total carotenoids in Lycium fruits (Fructus Lycii). Plant Foods Hum. Nutr. 60: 161–164. doi: 10.1007/s11130-005-9550-5 [DOI] [PubMed] [Google Scholar]
- 25.Saito S., Ohashi M., Naito A., Fukaya Y., Suzuki Y., Araie M.2005. Neuroprotective effect of the novel Na+/Ca2+ channel blocker NS-7 on rat retinal ganglion cells. Jpn. J. Ophthalmol. 49: 371–376. doi: 10.1007/s10384-005-0210-3 [DOI] [PubMed] [Google Scholar]
- 26.Sawada A., Neufeld A. H.1999. Confirmation of the rat model of chronic, moderately elevated intraocular pressure. Exp. Eye Res. 69: 525–531. doi: 10.1006/exer.1999.0732 [DOI] [PubMed] [Google Scholar]
- 27.Sellés-Navarro I., Villegas-Pérez M. P., Salvador-Silva M., Ruiz-Gómez J. M., Vidal-Sanz M.1996. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest. Ophthalmol. Vis. Sci. 37: 2002–2014. [PubMed] [Google Scholar]
- 28.Shareef S. R., Garcia-Valenzuela E., Salierno A., Walsh J., Sharma S. C.1995. Chronic ocular hypertension following episcleral venous occlusion in rats. Exp. Eye Res. 61: 379–382. doi: 10.1016/S0014-4835(05)80131-9 [DOI] [PubMed] [Google Scholar]
- 29.Tasi W. C., Petersen-Jones S. M., Huang P. Y., Lin C. T.2012. The neuroprotective effects of lidocaine and methylprednisolone in a rat model of retinal ischemia-reperfusion injury. J. Vet. Med. Sci. 74: 307–313. doi: 10.1292/jvms.11-0099 [DOI] [PubMed] [Google Scholar]
- 30.Wang N. T., Lin H. I., Yeh D. Y., Chou T. Y., Chen C. F., Leu F. C., Wang D., Hu R. T.2009. Effects of the antioxidants lycium barbarum and ascorbic acid on reperfusion liver injury in rats. Transplant. Proc. 41: 4110–4113. doi: 10.1016/j.transproceed.2009.08.051 [DOI] [PubMed] [Google Scholar]
- 31.Zheng L., Liu S., Sun M. Z., Chang J., Chance M. R., Kern T. S.2009. Pharmacologic intervention targeting glycolytic-related pathways protects against retinal injury due to ischemia and reperfusion. Proteomics 9: 1869–1882. doi: 10.1002/pmic.200701071 [DOI] [PMC free article] [PubMed] [Google Scholar]