Abstract
Objective
To investigate changes in quality of life (QoL), cognition and functional status according to arrhythmia recurrence after atrial fibrillation (AF) ablation.
Methods
We compared QoL, cognition and functional status in patients with recurrent atrial tachycardia (AT)/AF versus those without recurrent AT/AF in the AXAFA–AFNET 5 clinical trial. We also sought to identify factors associated with improvement in QoL and functional status following AF ablation by overall change scores with and without analysis of covariance (ANCOVA).
Results
Among 518 patients who underwent AF ablation, 154 (29.7%) experienced recurrent AT/AF at 3 months. Patients with recurrent AT/AF had higher mean CHA2DS2-VASc scores (2.8 vs 2.3, p<0.001) and more persistent forms of AF (51 vs 39%, p=0.012). Median changes in the SF-12 physical (3 (25th, 75th: −1, 8) vs 1 (−5, 8), p=0.026) and mental scores (2 (−3, 9) vs 0 (−4, 5), p=0.004), EQ-5D (0 (0,2) vs 0 (−0.1, 0.1), p=0.027) and Karnofsky functional status scores (10 (0, 10) vs 0 (0, 10), p=0.001) were more favourable in patients without recurrent AT/AF. In the overall cohort, the proportion with at least mild cognitive impairment (Montreal Cognitive Assessment <26) declined from 30.3% (n=157) at baseline to 21.8% (n=113) at follow-up. ANCOVA identified greater improvement in Karnofsky functional status (p<0.001) but not SF-12 physical (p=0.238) or mental scores (p=0.065) in those without recurrent AT/AF compared with patients with recurrent AT/AF.
Conclusions
Patients without recurrent AT/AF appear to experience greater improvement in functional status but similar QoL as those with recurrent AT/AF after AF ablation.
Keywords: atrial arrhythmia ablation procedures, atrial fibrillation, quality and outcomes of care
Introduction
Catheter ablation is increasingly employed treatment option for rhythm control in patients with atrial fibrillation (AF).1 While some clinical trials have shown that catheter ablation can improve cardiovascular outcomes in certain patient groups, the primary indication for catheter ablation in current practice is to improve symptoms and quality of life (QoL).1–4 Interestingly, improvements in symptom burden and QoL have been observed even in patients with recurrent AF.5
Despite evidence that catheter ablation is more effective than antiarrhythmic drug therapy for the treatment of recurrent AF, catheter ablation is associated with infrequent but measurable periprocedural risks, including stroke. Moreover, high-resolution diffusion weighted brain MRI identifies acute brain lesions without neurological symptoms in 10%–40% of patients undergoing catheter ablation.6 7 MRI-detected acute brain lesions may contribute to cognitive decline in patients who are treated with catheter ablation. These potential adverse effects of catheter ablation are concerning, especially in view of the mainly symptomatic benefits for patients.8 9
In order to examine the impact of the results of catheter ablation on symptoms, QoL, and cognition, we compared these important outcomes in patients with and without recurrent AF in the AXAFA clinical trial.10
Methods
The rationale and design of AXAFA–AFNET 5 (Anticoagulation using the direct factor Xa inhibitor apixaban during Atrial Fibrillation catheter Ablation: Comparison to vitamin K antagonist therapy) have been described previously.10 In brief, AXAFA-AFNET 5 was an investigator-initiated, prospective, parallel-group, randomised, open, blinded outcome assessment study comparing continuous apixaban therapy to vitamin K antagonist therapy during ablation. AXAFA-AFNET 5 was conducted in Europe and North America. The trial sponsor was AFNET, Münster, Germany (www.kompetenznetz-vorhofflimmern.de). AXAFA–AFNET 5 was designed by the steering committee in cooperation with AFNET and conducted in accordance with the declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines. The protocol was approved by ethical review boards at all institutions. The Clinical Research Institute (CRI, Munich, Germany) executed the study in cooperation with the steering committee and the sponsor. Data collection and entry was performed using the MARVIN eCRF system.10 An independent steering committee and an independent data and safety monitoring board guided the trial. Patients were not involved in the design of the trial. All adverse events were adjudicated by an independent endpoint review committee blind to study group and INR values. The Duke Clinical Research Institute served as the statistical core and performed the statistical analyses for the trial. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. This manuscript was written by the authors.
Study population
AXAFA–AFNET 5 enrolled patients scheduled for a de novo/first AF ablation with at least one established stroke risk factor (age >65 years, heart failure, hypertension, diabetes or prior stroke). For the purpose of this analysis, the study population included all patients from the AXAFA trial population who were randomised, underwent catheter ablation and had available baseline and follow-up QoL data.
Measures and outcomes
Several QoL and cognitive function measures were prospectively collected in AXAFA at baseline and 3-month follow-up, including EQ-5D, SF-12, modified European Heart Rhythm Association (mEHRA) classification, Karnofsky performance status, and Montreal Cognitive Assessment (MoCA) Test scores.
The EQ-5D was developed by the EuroQol group and is a short, standardised measure of generic health. The EQ-5D assesses five dimensions across three response levels: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. For the purpose of this analysis, the summary index was used in which the score ranges from 0 to 1, where higher scores reflect better health status.
The SF-12 is an abbreviated 12-item generic health related QoL measure derived from the Short Form 36. The measure evaluates physical functioning, limitations due to physical health problems, bodily pain, energy/fatigue, social functioning, limitations due to emotional problems, and psychological distress and well being. The SF-12 is reported as the physical component summary (PCS) and mental component summary (MCS). Higher scores indicate better health status. Samsa and colleagues defined the minimal clinically important difference (MCID) for the PCS as 3 in a cardiovascular (CV) population11 and Clement and colleagues12 have calculated the MCID as 2.7 (in a non-CV population). MCID values for the MCS are more variable and have not been well-quantified in patients with cardiovascular disease. In patients with joint disease, the reported absolute MCID values range from 1.4 to 4.5.13
The mEHRA classification assesses symptoms and functional limitation due to AF. The score is ordinal where class 1 represents no symptoms, class 2a represents mild symptoms (not troublesome to patient), class 2b represents moderate symptoms (troublesome to patient), class 3 represents severe symptoms (impacts normal daily activity) and class 4 represents disabling symptoms (normal daily activity is discontinued).14
The Karnofsky performance score is an instrument used to evaluate functional capacity. Originally developed in oncology, the score has been used in assessments of cardiovascular interventions.15 The Karnofsky score ranges from 100 to 0, where 100 is ‘perfect’ health and 0 is death.
The MoCA evaluates global cognition by assessing short term memory, visuospatial abilities, executive function, attention, concentration and working memory, language and orientation to time and place. The MoCA has 30 test items and can be administered in approximately 10 min. It is scored between 0 and 30 with higher values indicating better cognitive function. A score of 26 or higher indicates normal cognitive function.16
Changes in quality-of-life and cognitive function compared with baseline were prespecified secondary outcomes in AXAFA. As per study design, we also assessed freedom from atrial tachycardia (AT) or AF postablation (referred to as AF hereafter) after a 3-month blanking period as per consensus definition.
Statistical analysis
Changes in QoL, cognitive function and functional status were assessed at 3 months compared with baseline using the EQ-5D and SF-12 questionnaires, MoCA, and Karnofsky scale. Changes in QoL and cognitive function were evaluated separately by analysis of covariance (ANCOVA) models (SF-12 physical component scores, SF-12 mental component scores, Karnofsky scores and MoCA). The ANCOVA model included the recurrent AT/AF status as an indicator variable and adjustment for the baseline quality-of-life values. Multiple linear regression was conducted on changes in 3-month SF-12 scores (MCS and PCS) using clinically important baseline measures. These measures included age (years); sex; weight (kg); body mass index (kg/m2); systolic and diastolic blood pressure (mm Hg); Cockcroft-Gault estimated creatinine clearance (mg/dL); New York Heart Association functional classification; prior history of diabetes, stroke or transient ischaemic attack, vascular disease (coronary, peripheral, or carotid), mitral valve disease, aortic valve disease or chronic obstructive pulmonary disease; prior major bleeding; type of AF (paroxysmal versus persistent/long-standing persistent AF); modified EHRA (I, IIa, IIb, III, IV); MoCA; and rhythm at start of ablation (sinus rhythm, AF, atrial flutter, pacing, or other). A sensitivity analysis was conducting using multiple logistic regression assessing the difference in the proportion of patients showing a 2.5 point or more improvement in their MCS and PCS scores. Descriptive statistics for continuous and categorical variables were summarised as means (SDs), median (25th, 75th percentiles) and counts (percentages), respectively. Unadjusted statistical comparisons between continuous variables were performed using the Wilcoxon rank-sum test or two-sample t-test depending on normality; comparisons between nominal variables were performed using the Pearson’s χ² test or Fisher’s exact test, depending on expected cell sizes (n<5). All analyses were two-sided and tested at the nominal 0.05 significance level, and no adjustment was made for multiple testing.
Statistical analyses were performed with SAS V.9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Baseline characteristics
Among 518 patients undergoing ablation with available QoL data (82% of all patients undergoing AF ablation), 154 (29.7%) experienced recurrent AT/AF at the end of follow-up (3 months). Patients with recurrent AT/AF had higher CHA2DS2-VASc scores (3 (25th, 75th: 2, 3) vs 2 (1,3), p<0.001), more frequently had heart failure (37.7% vs 29.1%, p=0.04), prior stroke or transient ischaemic attack (11.7% vs 6.0%, p=0.028), coronary artery disease (17.5% vs 10.7%, p=0.033) and prior major bleeding (5.2% vs 0.8%, p=0.004) compared with patients who had no recurrent AT/AF (table 1). In terms of medical therapy, patients with recurrent AT/AF were less likely to be on flecainide (13.6% vs 22.3%, p=0.024). Patients with recurrent AT/AF were less likely to have paroxysmal AF (49.4% vs 61.3%, p=0.012) or to be in sinus rhythm at the time of ablation (56.5% vs 74.5%, p<0.001).
Table 1.
Baseline characteristics in those with and without recurrent AT/AF*
| All patients | Recurrent AT/AF | No recurrent AT/AF | P value | |
| n=518 | n=154 | n=364 | ||
| Age, median (q1, q3) | 64 (58 to 70), | 65 (60 to 70), | 64 (57 to 70), | 0.076 |
| Female | 174 (33.6%) | 61 (39.6%) | 113 (31.0%) | 0.059 |
| Weight, median (q1, q3) | 87 (76 to 98) | 88 (77 to 101) | 86 (76 to 97) | 0.231 |
| BMI, median (q1, q3) | 28 (25 to 31) | 29 (26 to 32) | 28 (25 to 31) | 0.184 |
| CHA2DS2VASc score, median (q1, q3) | 2 (2 to 3) | 3 (2 to 3) | 2 (1 to 3) | <0.001 |
| Hypertension, n (%) | 468 (90.3%) | 136 (88.3%) | 332 (91.2%) | 0.307 |
| Systolic blood pressure, median (q1, q3) | 140 (125 to 151) | 139 (125 to 151) | 140 (125 to 150) | 0.990 |
| Diastolic blood pressure, median (q1, q3) | 83 (76 to 90) | 85 (76 to 92) | 81 (75 to 90) | 0.145 |
| COPD, n (%) | 33 (6.4%) | 8 (5.2%) | 25 (6.9%) | 0.476 |
| Heart failure | 164 (31.7%) | 58 (37.7%) | 106 (29.1%) | 0.040 |
| NYHA I | 48 (9.3%) | 18 (11.7%) | 30 (8.2%) | |
| NYHA II | 98 (18.9%) | 30 (19.5%) | 68 (18.7%) | |
| NYHA III | 18 (3.5%) | 10 (6.5%) | 8 (2.2%) | |
| Diabetes mellitus, n (%) | 58 (11.2%) | 23 (14.9%) | 35 (9.6%) | 0.079 |
| Prior stroke or transient ischaemic attack, n (%) | 40 (7.7%) | 18 (11.7%) | 22 (6.0%) | 0.028 |
| Coronary Artery Disease, n (%) | 66 (12.7%) | 27 (17.5%) | 39 (10.7%) | 0.033 |
| Prior major bleeding | 11 (2.1%) | 8 (5.2%) | 3 (0.8%) | 0.004 |
| Paroxysmal AF, n (%) | 299 (57.7%) | 76 (49.4%) | 223 (61.3%) | 0.012 |
| Persistent or long-standing persistent AF, n (%) | 219 (42.3%) | 78 (50.6%) | 141 (38.7%) | 0.012 |
| Concomitant therapy | ||||
| Amiodarone | 86 (16.6%) | 20 (13.0%) | 66 (18.1%) | 0.15 |
| Dronedarone | 11 (2.1%) | 4 (2.6%) | 7 (1.9%) | 0.74 |
| Flecainide | 102 (19.7%) | 21 (13.6%) | 81 (22.3%) | 0.024 |
| Propafenone | 14 (2.7%) | 4 (2.6%) | 10 (2.7%) | 1.000 |
| Sotalol | 16 (3.1%) | 4 (2.6%) | 12 (3.3%) | 0.787 |
| ACE inhibitor or angiotensin receptor blocker | 309 (59.7%) | 102 (66.2%) | 207 (56.9%) | 0.047 |
| Calcium channel blocker | 122 (23.6%) | 33 (21.4%) | 89 (24.5%) | 0.459 |
| Diuretic | 179 (34.6%) | 62 (40.3%) | 117 (32.1%) | 0.076 |
| Statin | 186 (35.9%) | 51 (33.1%) | 135 (37.1%) | 0.389 |
| Beta blocker | 364 (70.3%) | 110 (71.4%) | 254 (69.8%) | 0.708 |
| Digoxin | 22 (4.3%) | 10 (6.5%) | 12 (3.3%) | 0.099 |
| Modified EHRA scale at baseline | 0.096 | |||
| mEHRA I, n (%) | 35 (6.8%) | 10 (6.5%) | 25 (6.9%) | |
| mEHRA IIa, n (%) | 129 (24.9%) | 28 (18.2%) | 101 (27.7%) | |
| mEHRA IIb, n (%) | 164 (31.7%) | 49 (31.8%) | 115 (31.6%) | |
| mEHRA III, n (%) | 180 (34.7%) | 62 (40.3%) | 118 (32.4%) | |
| mEHRA IV, n (%) | 10 (1.9%) | 5 (3.2%) | 5 (1.4%) | |
| Rhythm at time of ablation | ||||
| Sinus rhythm, n (%) | 358 (69.1%) | 87 (56.5%) | 271 (74.5%) | <0.001 |
| Atrial fibrillation, n (%) | 144 (27.86%) | 63 (40.9%) | 81 (22.3%) | |
| Atrial flutter, n (%) | 9 (1.7%) | 3 (1.9%) | 6 (1.6%) | |
| Pacing, n (%) | 7 (1.4%) | 1 (0.6%) | 6 (1.6%) | |
| Type of ablation | 0.129 | |||
| Pulmonary vein isolation | 476 (91.9%) | 147 (95.5%) | 329 (90.4%) | |
| Pulmonary vein isolation with adjunctive ablation | 39 (7.5%) | 7 (4.5%) | 32 (8.8%) | |
| Other | 3 (0.6%) | 0 (0%) | 3 (0.8%) | |
| Ablation energy source | 0.319 | |||
| Radiofrequency, n (%) | 323 (62.4%) | 100 (64.9%) | 223 (61.3%) | |
| Cryoablation, n (%) | 154 (29.7%) | 46 (29.9%) | 108 (29.7%) | |
| Other | 41 (7.9%) | 8 (5.2%) | 33 (9.1%) | |
*Fisher exact test was used when cell size was small, that is, n<5; otherwise Pearson’s χ² was used for categorical and Wilcoxon rank-sum test was used when data is not normally distributed; otherwise two-sample t-test used for continuous.
AF, atrial fibrillation; AT, atrial tachycardia; BMI, body mass index; COPD, chronic obstructive pulmonary disease; mEHRA, modified European Heart Rhythm Association.
Quality of life according to recurrent AT/AF
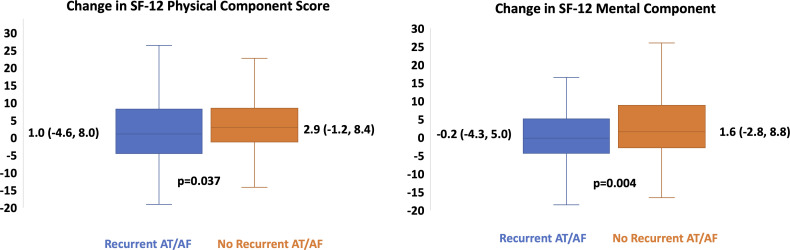
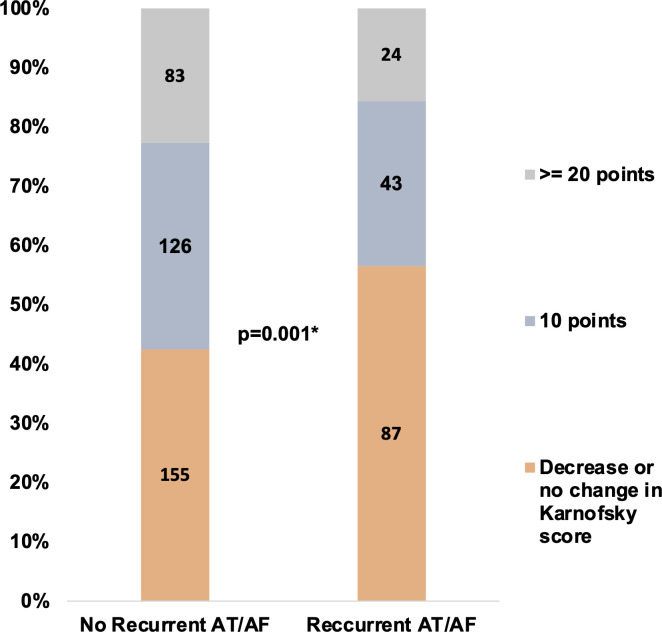
At baseline (before ablation), patients with recurrent AT/AF had lower SF-12 physical component scores (42 (37, 49) vs 45 (39,52), p=0.004) and marginally higher MoCA scores (28 (26,29) vs 27 (25,29), p=0.002, table 2). After ablation, changes in QoL and cognitive function were better in those patients without recurrent AT/AF, including the median change in SF-12 physical component scores, SF-12 mental component scores, EQ-5D scores, and Karnofsky scores. Figure 1 illustrates the change in the SF-12 physical and mental component scores according to the presence or absence of recurrent AT/AF. Figure 2 illustrates the change in Karnofsky Index categories between those patients with and without recurrent AT/AF. Notably, in the overall cohort, the proportion with at least mild cognitive impairment (MoCA <26) declined from 30.3% (n=157) at baseline to 21.8% (n=113) at follow-up.
Table 2.
Change in quality of life from baseline to end of study*
| All patients (n=518) | Recurrent AT/AF (n=154) | No recurrent AT/AF (n=364) | P value | |
| SF-12 physical component | ||||
| Baseline, median (q1, q3) | 45 (38 to 52) | 42 (37 to 49) | 45 (39 to 52) | 0.004 |
| End of study, median (q1, q3) | 49 (42 to 54) | 45 (38 to 52) | 50 (44 to 55) | <0.001 |
| Change, median (q1, q3) | 3 (−2 to 8) | 1 (−5 to 8) | 3 (−1 to 8) | 0.026 |
| SF-12 mental component | ||||
| Baseline, median (q1, q3) | 51 (43 to 58) | 51 (45 to 58) | 51 (43 to 58) | 0.762 |
| End of study, median (q1, q3) | 54 (46 to 59) | 52 (42 to 57) | 55 (48 to 60) | 0.003 |
| Change, median (q1, q3) | 1 (−3 to 8) | 0 (−4 to 5) | 2 (−3 to 9) | 0.004 |
| EQ-5D scores | ||||
| Baseline median (q1, q3) | 0.8 (0.7 to 1.0) | 0.8 (0.7 to 1.0) | 0.8 (0.7 to 1.0) | 0.457 |
| End of study, median (q1, q3) | 0.8 (0.7 to 1.0) | 0.8 (0.7 to 1.0) | 0.9 (0.7 to 1.0) | <0.001 |
| Change, median (q1, q3) | 0.0 (0.0 to 0.1) | 0.0 (−0.1 to 0.1) | 0.0 (0.0 to 0.2) | 0.027 |
| Karnofsky scale | ||||
| Baseline, median (q1, q3) | 90 (80 to 90) | 90 (80 to 100) | 90 (80 to 90) | 0.678 |
| End of study, median (q1, q3) | 100 (90 to 100) | 90 (80 to 100) | 100 (90 to 100) | <0.001 |
| Change, median (q1, q3) | 10 (0 to 10) | 0 (0 to 10) | 10 (0 to 10) | 0.001 |
| MoCA | ||||
| Baseline, median (q1, q3) | 27 (25 to 29) | 28 (26 to 29) | 27 (25 to 29) | 0.002 |
| End of study, median (q1, q3) | 28 (26 to 29) | 28 (27 to 30) | 28 (26 to 29) | 0.003 |
| Change, median (q1, q3) | 1 (−1 to 2) | 1 (−1 to 2) | 1 (−1 to 2) | 0.628 |
| At least mild cognitive impairment (MoCA <26) | ||||
| Baseline, n (%) | 157 (30.3%) | 33 (21.4%) | 124 (34.1%) | 0.004 |
| End of study, n(%) | 113 (21.8%) | 22 (14.3%) | 91 (25%) | 0.007 |
*Fisher exact test was used when cell size was small, that is, n<5; otherwise Pearson’s χ² was used for categorical and Wilcoxon rank-sum test was used when data is not normally distributed; otherwise two-sample t-test used for continuous.
AF, atrial fibrillation; AT, atrial tachycardia; MoCA, Montreal Cognitive Assessment.
Figure 1.
Change in the SF-12 physical and mental component scores according to the presence or absence of recurrent AT/AF. Shown in each box plot are the median changes with 25th and 75th percentiles in the SF-12 physical and mental component scores. The whiskers illustrate the maximum and minimum values. AF, atrial fibrillation; AT, atrial tachycardia.
Figure 2.
Shown in the bar graph are the changes in Karnofsky score categories according to the presence or absence of recurrent AT/AF. *P value is for the comparison in the change in median Karnofsky scores from baseline to follow-up. AF, atrial fibrillation; AT, atrial tachycardia.
ANCOVA demonstrated that the mean changes in QoL scores were greater among patients without recurrent AT/AF, when controlling for baseline measures as shown in table 3. The mean differences between those with and without recurrent AF, were not statistically significant at the p=0.05 level for any of the change in QoL measures except the Karnofsky score (4.9 (9.6) vs 7.9 (9.6), p<0.001). We also conducted a sensitivity analysis that assessed the proportion of patients with at least a 2.5 point increase in the PCS score. The proportion with a 2.5 point increase in the PCS was 44.8% (n=69/154) in those with recurrent AT/AF vs 52.2% (190/364) in those without recurrent AT/AF (p for difference after adjusting for baseline PCS=0.002). The proportion with a 2.5 point increase in the MCS was 35.7% (n=55/154) in those with recurrent AT/AF vs 46.7% (170/364) in those without recurrent AT/AF (p for difference after adjusting for baseline MCS=0.021).
Table 3.
ANCOVA results comparing baseline and 3-month follow-up scores for QoL measures
| All patients (n=518) |
Recurrent AT/AF (n=154) |
No recurrent AT/AF (n=364) |
P value | |
| EQ-5D total | ||||
| Baseline | 0.79 (0.22) | 0.78 (0.21) | 0.79 (0.22) | |
| End of study | 0.83 (0.20) | 0.78 (0.21) | 0.84 (0.20) | |
| Change | 0.04 (0.21) | 0.00 (0.21) | 0.05 (0.21) | 0.131 |
| SF-12 mental component | ||||
| Baseline | 49.78 (9.61) | 50.07 (9.22) | 49.67 (9.78) | |
| End of study | 51.79 (9.24) | 49.94 (9.72) | 52.58 (8.93) | |
| Change | 2.01 (9.26) | −0.12 (9.09) | 2.91 (9.19) | 0.065 |
| SF-12 physical component | ||||
| Baseline | 44.31 (9.07) | 42.57 (9.07) | 45.04 (8.98) | |
| End of study | 47.40 (8.69) | 44.43 (9.33) | 48.66 (8.09) | |
| Change | 3.10 (8.24) | 1.86 (8.93) | 3.62 (7.88) | 0.238 |
| Karnofsky | ||||
| Baseline | 86.3 (10.2) | 85.8 (11.2) | 86.5 (9.8) | |
| End of study | 93.2 (9.7) | 90.6 (11.4) | 94.3 (8.6) | |
| Change | 7.0 (9.7) | 4.9 (9.6) | 7.9 (9.6) | <0.001 |
| MoCA | ||||
| Baseline | 26.6 (2.8) | 27.1 (2.5) | 26.3 (2.9) | |
| End of study | 27.2 (2.8) | 27.7 (2.6) | 27.0 (2.9) | |
| Change | 0.6 (2.5) | 0.6 (2.3) | 0.7 (2.6) | 0.924 |
The p value in this table corresponds to the effect of recurrent AT/AF group in an ANCOVA model with change from baseline as the dependent variable, and baseline values and recurrent AT/AF group as covariates.
AF, atrial fibrillation; ANCOVA, analysis of covariance; AT, atrial tachycardia; MoCA, Montreal Cognitive Assessment; QoL, quality of life.
Associations between functional assessment and patient reported outcomes
In order to assess how improvements in functional status as measured by physician-determined mEHRA and Karnofsky functional status scores correlate with patient-reported outcomes (SF-12 and EQ-5D scores), we compared changes in these metrics. Table 4 details changes in QoL scores according to changes in Karnofsky scores. In patients with a decrease or no change in the Karnofsky score, the median change in the SF-12 physical component was 0.8 (−3.3, 6.5) compared with 3.3 (−0.4, 9.9) in those with a≥10 point Karnofsky improvement (p=0.002) and 5.4 (1.3, 11.2) in those with a≥20 point improvement in the Karnofsky score (p<0.001). Similarly, in patients with a decrease or no change in the Karnofsky score, the median change in the SF-12 mental component was 0.0 (−5.6, 5.8) compared with 1.6 (−1.9, 8.8) in those with a≥10 point Karnofsky improvement (p=0.035), and 2.2 (1.6, 10.0) in those with a≥20 point improvement in the Karnofsky score (p=0.013). Notably, the median change in the EQ-5D score was not materially different according to changes in the Karnofsky scores despite small but statistically significant improvements. The changes in patient-reported outcomes according to changes in mEHRA scores are shown in table 5. Patients with >1 class improvement in the mEHRA had greater increase in median SF-12 physical component scores at the end of the study compared with those with decreased or no change in mEHRA classification (p=0.033).
Table 4.
Changes in quality of life according to changes in Karnofsky score*
| Decrease or no change in Karnofsky score (n=242) |
≥10 point improvement in Karnofsky score (n=276) |
≥20 point improvement in Karnofsky score (n=107) |
|
| Change in SF-12 physical component score compared with baseline (Δ PCS) | |||
| Mean (SD) | 1.5 (8.2) | 4.5 (8.0) | 6.5 (7.9) |
| Median (25th, 75th) | 0.8 (−3.3 to 6.5) | 3.3 (−0.4 to 9.9) | 5.4 (1.3 to 11.2) |
| Min, Max | −19 to 30 | −17 to 26 | −16 to 26 |
| P value (compared with decrease or no change) | 0.002 | <0.001 | |
| Change in SF-12 mental component score compared with baseline (Δ MCS) | |||
| Mean (SD) | 0.3 (9.4) | 3.5 (8.9) | 4.4 (9.3) |
| Median (25th, 75th) | 0.0 (−5.6 to 5.8) | 1.6 (−1.9 to 8.8) | 2.2 (−1.6 to 10.0) |
| Min, Max | −29 to 26 | −22 to 44 | −21 to 44 |
| P value (compared with decrease or no change) | 0.035 | 0.013 | |
| Change in EQ-5D score compared with baseline (Δ EQ-5D) | |||
| Mean (SD) | 0.0 (0.2) | 0.1 (0.2) | 0.1 (0.2) |
| Median (25th, 75th) | 0.0 (−0.1 to 0.1) | 0.0 (0.0 to 0.2) | 0.0 (0.0 to 0.2) |
| Min, Max | −1 to 1 | −1 to 1 | −1 to 1 |
| P value (compared with decrease or no change) | 0.040 | 0.001 | |
The patients with ≥20 point improvement in the Karnofsky score (n=107) are also included in the group of patients with ≥10 point improvement (n=267). Unadjusted p values are from median comparison with ‘Decrease or No Change in Karnofsky Score’ group.
Table 5.
Changes in quality of life according to changes in mEHRA*
| Decrease or no change in mEHRA (n=81) |
Any improvement in mEHRA (n=437) |
1 Class improvement in mEHRA (n=146) |
>1 Class Improvement in mEHRA (n=291) |
|
| Change in SF-12 physical component score compared with baseline (Δ PCS) | ||||
| Mean (SD) | 0.6 (8.3) | 3.6 (8.2) | 1.7 (7.7) | 4.5 (8.2) |
| Median (25th, 75th) | 0.9 (−5.8 to 5.8) | 2.7 (−1.4 to 8.8) | 0.6 (−2.2 to 5.5) | 3.8 (−1.0 to 9.6) |
| Min, Max | −19 to 26 | −18 to 18 | −19 to 26 | −18 to 30 |
| P value (compared with decrease or no change) | 0.277 | 0.851 | 0.033 | |
| Change in SF-12 mental component score compared with baseline (Δ MCS) | ||||
| Mean (SD) | 0.9 (8.9) | 2.2 (9.3) | 1.7 (8.0) | 2.5 (9.9) |
| Median (25th, 75th) | 0.0 (−3.3 to 5.0) | 1.4 (−3.1 to 8.3) | 1.6 (−2.4 to 7.3) | 1.2 (−3.2 to 8.9) |
| Min, Max | −29 to 27 | −22 to 44 | −22 to 22 | −24 to 44 |
| P value (compared with decrease or no change) | 0.116 | 0.08 | 0.259 | |
| Change in EQ-5D score compared with baseline (Δ EQ-5D) | ||||
| Mean (SD) | 0.0 (0.2) | 0.0 (0.2) | 0.0 (0.2) | 0.0 (0.2) |
| Median (25th, 75th) | 0.0 (−0.1 to 0.1) | 0.0 (0.0 to 0.1) | 0.0 (0.0 to 0.1) | 0.0 (0.0 to 0.2) |
| Min, Max | −1 to 1 | −1 to 1 | 0 to 1 | −1 to 1 |
| P value (compared with decrease or no change) | 0.343 | 0.879 | 0.179 | |
*Unadjusted p values from median comparisons with 'Decrease or No Change in mEHRA' group.
mEHRA, modified European Heart Rhythm Association.
Discussion
Catheter ablation is performed to reduce arrhythmia burden, minimise symptoms and to improve QoL. While there are several trials that examined the impact of catheter ablation on QoL compared with medical therapy, including the recent CABANA trial,3 few have examined the impact of recurrent AT/AF after ablation on changes in QoL, functional status and patient reported outcomes. In our analysis of recurrent AT/AF after ablation in the AXAFA trial, we found that the raw changes in QoL (SF-12 physical and mental scores), EQ-5D and functional (Karnofsky status scores) improved more in patients without recurrent AF. After adjustment using ANCOVA, similar trends were observed although statistically significant improvement was greater only as measured with the Karnofsky score. Finally, we found that cognitive function as assessed by MoCA scores improved slightly after ablation regardless of recurrent AF.
Catheter ablation is an established therapy for patients with medically refractory symptomatic AF. While catheter ablation has led to improved cardiovascular outcomes in patients with heart failure and left ventricular dysfunction, the primary indication for ablation is to reduce symptoms and improve QoL. The aggregate evidence available from clinical trials demonstrates improvement in QoL after ablation. In the ThermoCool AF trial, patients who underwent ablation experienced significant improvement in SF-36 scores (mean+6.9 points for mental, +6.6 for physical) at 9 months of follow-up.2 Similarly, in the Catheter Ablation compared with optimised Pharmacological Therapy for Atrial Fibrillation (CAPTAF) trial, in which QoL was the primary endpoint, catheter ablation led to superior QoL compared with medical therapy at 12 months of follow-up.4 Most recently, the CABANA trial demonstrated that catheter ablation led to significant improvements in QoL that were maintained at 5 years after ablation when compared with medical therapy.3 Despite randomised trials comparing QoL with ablation versus medical therapy, few data are available comparing QoL, functional status and patient-reported outcomes in patients according to recurrence of atrial arrhythmias after ablation.
Consistent with our hypothesis, we observed that improvement in functional status was greater in those patients without recurrent AT/AF, as reflected by highly significant improvement in Karnofsky scores. However, we did not identify greater improvements in QoL in those without recurrent AT/AF. While the median changes in the SF-12 physical and mental scores were more favourable in patients without recurrent AT/AF, ANCOVA did not identify statistically significant greater improvement SF-12 physical (p=0.238) or mental scores (p=0.065) in those without recurrent AT/AF compared with patients with recurrent AT/AF. Finally, while the change in MoCA scores was not different between those with and without recurrent AT/AF after the 3-month follow-up, the proportion of the overall cohort with mild or greater cognitive impairment decreased from 30% to 22%. This cognitive improvement could also be related to an increased familiarity of the test by patients. Nonetheless, this finding is reassuring, particularly in light of findings of asymptomatic cerebral emboli in patients undergoing catheter ablation.6 7
Patient-reported outcomes are defined as any report of the status of a patient’s health that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else. Patient-reported outcomes have been increasingly emphasised by regulatory bodies, including the Food and Drug Administration. AXAFA included several patient-reported outcomes including the physical and mental components of the SF-12 and EQ-5D. There are few data regarding how clinician-derived and patient-reported assessments of improvement in functional status and QoL relate to one another. Results from the ORBIT registry suggest that changes in patient-reported QoL with the AFEQT score do correlate with the EHRA scores.17 In AXAFA, we found that improvements to Karnofsky score appeared to be reflected by improvements in patient-reported outcomes including the SF-12 physical and mental components.
Limitations
There are several limitations that should be kept in mind when considering these data. This is a secondary analysis of data from a randomised controlled trial and QoL data were not available for all patients. While the hypothesis tested in our analysis was whether changes in QoL after ablation differ according to the presence or absence of recurrent AT/AF, we did not have a comparator group who did not undergo ablation. Additionally, the changes in QoL were analysed at 3 months and therefore reflect intermediate term outcomes after AF ablation. Furthermore, despite of using different test versions, a learning effect by serial testing has to be taken into account.
Conclusion
AF ablation resulted in very good functional status in this large cohort of patients with stroke risk factors. Patients without recurrent AT/AF experience greater improvement in functional status after AF ablation. Future studies should examine how changes in QoL, cognition and functional status vary according to arrhythmia recurrence in long-term follow-up.
Key messages.
What is already known on this subject?
Catheter ablation of atrial fibrillation (AF) improves quality of life.
What might this study add?
This study examines how quality of life changes according to outcomes after AF ablation.
How might this impact on clinical practice?
Patients without recurrent atrial tachycardia/AF experience greater improvement in functional status after AF ablation. Assessing patient-reported outcomes after ablation is important to help guide treatment and improve outcomes.
Footnotes
Contributors: Conceived and designed the analysis: JPP, DMT, HA, PK. Collected the data: BB, TF. Performed the analysis: TM, AL, HA. Wrote the first draft of the paper: JPP. Revised the paper based on intellectual contribution: JPP, DMT, TM, AL, KGH, BB, JDB, DJC, AE, TF, IVG, PG, MG, JH, GH, HA, LM, JCN, GN, TDP, DS, US, ST, JV, LDB, PK.
Funding: AXAFA-AFNET 5 was an investigator initiated trial. The study was sponsored by the AF-NET. AXAFA-AFNET5 was partially funded by BMS/Pfizer and by the German Centre for Cardiovascular Research (DZHK) to AFNET. Further support came from European Union (grant agreement No 633196 (CATCH ME) to BB, PK, LM, US, AFNET), British Heart Foundation (FS/13/43/30324 and AA/18/2/34218 to PK), Leducq Foundation to PK and the Netherlands Heart Foundation (RACE V) to US.
Competing interests: The study was sponsored by the AF-NET with funding from BMS-Pfizer and the European Union. JPP receives grants for clinical research from Abbott, ARCA biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals and NHLBI and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Bayer, Biotronik, GSK, Johnson & Johnson, Medtronic, Motif Bio, Sanofi and Phillips. DMT receives speaking honoraria from Boston Scientific, Medtronic and Abbott. KGH reports study grants by Bayer and Sanofi-Aventis, lecture fees/advisory board fees from Sanofi-Aventis, Pfizer, Bristol-Myers-Squibb, Boehringer Ingelheim, Daiichi Sankyo, Edwards Lifesciences, Biotronik and Medtronic. JV reports speaker honoraria from Abbott. PK receives research support for basic, translational and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK) and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past. JCN is supported by a grant from the Novo Norvodisk Foundation (NNF16OC0018658). PK is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Duke IRB and all participating hospitals/institutions.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available. The data are not publicly available.
References
- 1. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. J Arrhythm 2017;33:369–409. 10.1016/j.joa.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. 10.1001/jama.2009.2029 [DOI] [PubMed] [Google Scholar]
- 3. Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275-1285. 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blomström-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–68. 10.1001/jama.2019.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wokhlu A, Monahan KH, Hodge DO, et al. Long-Term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol 2010;55:2308–16. 10.1016/j.jacc.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 6. Haeusler KG, Kirchhof P, Endres M. Left atrial catheter ablation and ischemic stroke. Stroke 2012;43:265–70. 10.1161/STROKEAHA.111.627067 [DOI] [PubMed] [Google Scholar]
- 7. Herm J, Fiebach JB, Koch L, et al. Neuropsychological effects of MRI-detected brain lesions after left atrial catheter ablation for atrial fibrillation: long-term results of the MACPAF study. Circ Arrhythm Electrophysiol 2013;6:843–50. 10.1161/CIRCEP.113.000174 [DOI] [PubMed] [Google Scholar]
- 8. Medi C, Evered L, Silbert B, et al. Subtle post-procedural cognitive dysfunction after atrial fibrillation ablation. J Am Coll Cardiol 2013;62:531–9. 10.1016/j.jacc.2013.03.073 [DOI] [PubMed] [Google Scholar]
- 9. Schwarz N, Kuniss M, Nedelmann M, et al. Neuropsychological decline after catheter ablation of atrial fibrillation. Heart Rhythm 2010;7:1761–7. 10.1016/j.hrthm.2010.07.035 [DOI] [PubMed] [Google Scholar]
- 10. Di Biase L, Callans D, Hæusler KG, et al. Rationale and design of AXAFA-AFNET 5: an investigator-initiated, randomized, open, blinded outcome assessment, multi-centre trial to comparing continuous apixaban to vitamin K antagonists in patients undergoing atrial fibrillation catheter ablation. Europace 2017;19:132–8. 10.1093/europace/euw368 [DOI] [PubMed] [Google Scholar]
- 11. Samsa G, Edelman D, Rothman ML, et al. Determining clinically important differences in health status measures: a general approach with illustration to the health Utilities index mark II. Pharmacoeconomics 1999;15:141–55. 10.2165/00019053-199915020-00003 [DOI] [PubMed] [Google Scholar]
- 12. Clement ND, Makaram N, Bell J, et al. Columbus® computer navigated total knee arthroplasty: gap balancing versus measured resection. Knee 2017;24:1442–7. 10.1016/j.knee.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 13. Nwachukwu BU, Chang B, Voleti PB, et al. Preoperative short form health survey score is predictive of return to play and minimal clinically important difference at a minimum 2-year follow-up after anterior cruciate ligament reconstruction. Am J Sports Med 2017;45:2784–90. 10.1177/0363546517714472 [DOI] [PubMed] [Google Scholar]
- 14. Wynn GJ, Todd DM, Webber M, et al. The European heart rhythm association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace 2014;16:965–72. 10.1093/europace/eut395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boucher JM, Dupras A, Jutras N, et al. Long-Term survival and functional status in the elderly after cardiac surgery. Can J Cardiol 1997;13:646–52. [PubMed] [Google Scholar]
- 16. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 17. Freeman JV, Simon DN, Go AS, et al. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the outcomes Registry for better informed treatment of atrial fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes 2015;8:393–402. 10.1161/CIRCOUTCOMES.114.001303 [DOI] [PubMed] [Google Scholar]