Abstract
FUT8-CDG is a severe multisystem disorder caused by mutations in FUT8, encoding the α-1,6-fucosyltransferase. We report on dizygotic twins with FUT8-CDG presenting with dysmorphisms, failure to thrive, and respiratory abnormalities. Due to the severe phenotype, oral L-fucose supplementation was started. Glycosylation analysis using mass spectrometry indicated a limited response to fucose therapy while the clinical presentation stabilized. Further research is needed to assess the concept of substrate supplementation in FUT8-CDG.
Keywords: Congenital disorders of glycosylation, Fucose, Mass spectrometry, Therapy
1. Introduction
Fucosylation, the enzymatic addition of L-fucose to nascent N- or O-glycans using the donor guanosine diphosphate (GDP)-L-fucose, is a major step in the glycosylation of many proteins and lipids. Mediated by a group of enzymes called fucosyltransferases, this process has been shown to have functional importance in immunity [1], infection [2], and neoplastic diseases [3,4].
Congenital disorders of glycosylation (CDG) are inborn errors of metabolism affecting the glycosylation of various macromolecules and presenting as multisystem disorders [5,6] affecting a plethora of organ systems. Several subtypes affecting fucosylation have been identified [7,8]. In SLC35C1-CDG, mutations in the gene encoding the Golgi localized GDP-fucose transporter lead to a syndrome characterized by psychomotor retardation, dysmorphic features, and immunodeficiency due to a lack of selectin-mediated leucocyte extravasation [9]. This is caused by a lack of fucosylated selectin ligands impairing the proper binding of leucocytes to endothelial selectins [10]. Consequently, highly elevated peripheral neutrophil counts are a hallmark of the syndrome which is also known as leukocyte adhesion deficiency II (LADII) [11]. Some variants of this disorder are amenable to treatment by oral fucose substitution [9,12] which results in normalization of neutrophile counts, improved immune response, and improved psychomotor functions [12].
FUT8-CDG (MIM: 618005) is a recently discovered CDG caused by mutations in FUT8 (HGNC:4019), which encodes an α-1,6-fucosyltransferase [13,14]. This enzyme catalyzes the transfer of L-fucose from GDP-fucose to the most proximal N-acetylglucosamine (GlcNAc) residue of N-linked glycans, a process named core fucosylation [15]. In affected individuals, core fucosylation is impaired, leading to a decrease or loss of core-fucosylated N-glycans in both, serum and fibroblasts [14]. The resulting phenotype is marked by developmental delay, microcephaly, dysmorphism, skeletal pathologies, gastrointestinal and respiratory abnormalities, feeding problems, and muscular hypotonia. All reported patients had epileptic seizures.
Here, we report on dizygotic twins with FUT8-CDG who received oral fucose supplementation as a therapeutic trial due to the severe phenotype. Detailed glycan studies using matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS), electrospray ionization (quadrupole) time of flight mass spectrometry (ESI-(Q)TOF MS), and lectin blotting were employed to characterize the effect of fucose supplementation on the N-glycome in order to allow an initial evaluation of this treatment approach.
2. Materials and methods
2.1. Data acquisition and compliance with ethical standards
Written informed consent was obtained prior to data acquisition and experimental studies. Data acquisition and analysis was approved by the relevant ethical committee (Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster, 2019-199f-s). Fucose supplementation was started as part of an individual treatment attempt (Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster, 31.05.2013, and § 4 AMG).
2.2. Genetic analyses
Exome sequencing of the patients and their parents was performed as detailed previously [16]. FUT8 variants were confirmed by Sanger sequencing.
2.3. Glycosylation analysis
Glycosylation analysis of the standard biomarker serum transferrin by isoelectric focusing (IEF) and high-performance liquid chromatography (HPLC) was performed following previously published protocols [17].
N-glycome profiling using MALDI-TOF MS and MS/MS was performed as previously described [18,19].
Serum N-glycan profiles from both patients were measured before and during fucose supplementation with a clinically validated semi-quantitative N-glycan assay using flow injection ESI-QTOF MS [20]. Carbohydrate deficient transferrin testing (CDT) was performed using accurate mass analysis of affinity purified transferrin protein using a LC-ESI-TOF MS system [21].
2.4. Lectin blot analysis
Serum was diluted 1:10 in lysis buffer (50 mM TRIS, 150 mM NaCl, 1.0% w/v Triton-X, pH 7.6) with protease inhibitor (Roche #46931320019) and denatured for 10 min at 70 °C. After cooling to room temperature, 2 μL of diluted serum (~ 15 μg protein) was treated with or without 1 μL PNGase F (New England Biolabs, #P0704S) at 37 °C for 1 h according to manufactures protocols. Samples were prepared with 4× Sample Loading Buffer (Li-COR, 928–40004) with 10% v/v β-mercaptoethanol, denatured for 10 min at 95 °C, cooled to room temperature, and loaded in to the gel (NuPAGE 4 to 12% Bis-Tris, 1.0 mm, Mini Protein Gel, 12-well, ThermoFisher, NP0322) alongside 2 μL Chameleon Duo Pre-Stained Protein Ladder (LiCOR, 928–60000). Gels were run using the MiniProtean Tetra Electrophoresis System (BioRAD, 1658004) at 140 mV for 1 h. Proteins were transferred to nitrocellulose membranes (ThermoFisher, IB23003) using the iBlot Dry Blotting System (ThermoFisher, IB1001). Total protein was measured using the Revert 700 Total Protein Stain (LiCOR 926-11011). Membranes were then incubated in 5% BSA in TBS-Tween 0.1% for 1 h, followed by incubation with biotinylated lectins (Vector Labs: LCA B-1045, AAL B-1395, SNA B-1305, PHA-E B-1125, RCA B-1085, Con A B-1105) at a 1:1000 dilution (1:20,000 for Con A) and 1:2000 dilution of Mouse anti-Human Alpha 1-Acid Glycoprotein antibody (R&D Systems, MAB2694) in 5% BSA in TBS-Tween 0.1%, overnight at 4 °C on a rocking platform shaker. Membranes were washed three times in TBS-Tween 0.1% for 5 min, and then incubated with fluorescent conjugated streptavidin IRDye 800CW (LiCOR, 926-32230), Goat anti-Mouse IgG IRDye 680RD (LiCOR, 925-68070), or Goat anti-Human IgG IRDye 800CW (LiCOR, 926-32232) at 1:25,000 dilution in 5% BSA in TBS-Tween 0.1% for 30 min protected from light. Membranes were again washed three times in TBS-Tween 0.1% for 5 min and imaged using a LiCOR Odyssey CLx Imaging System and analyzed using LiCOR Image Studio Software. A comprehensive characterization of biotinylated lectin binding specificity by glycan microarray can be found on the National Center for Functional Glycomics website (www.ncfg.hms.harvard.edu).
2.5. Fucosylation analysis of transferrin and IgG
Fucosylation of IgG and transferrin was analyzed by mass spectrometry of purified glycopeptides according to a previously reported method, with minor modifications [22,23]. Briefly, an affinity column for transferrin was prepared using a rabbit polyclonal antibody against human transferrin (Dako, Glostrup, Denmark) and a ligand-coupling Sepharose column (HiTrap NHS-activated HP, GE Healthcare, NJ, USA). For IgG, a protein G-coupled Sepharose column was purchased from GE Healthcare. The antibody or protein G-coupled Sepharose was recovered from the column. 10 μL of serum were mixed with a 20-μL slurry of Sepharose in 0.5 mL of phosphate-buffered saline (PBS) and the resulting solution incubated at 4 °C for 30 min. After washing the Sepharose, the protein was eluted in 0.1 M glycine-HCl buffer at pH 2.5. After drying by a vacuum concentrator, the purified protein was dissolved in 0.5 mL of 6 M guanidium hydrochloride, 0.25 M Tris–HCl, pH 8.5 and reduced by treatment with 5 mg of dithiothreitol at 60 °C for 30 min. A 10 mg portion of iodoacetamide was then added to achieve carbamidomethylation, and the resulting solution was incubated in the dark at room temperature for 30 min. The reagents were removed by a NAP-5 gel filtration column (GE Healthcare) equilibrated with 0.05 N HCl, and the recovered protein solution was adjusted to pH 8.5 with Tris. Digestion was performed using a mixture of trypsin (Sequencing Grade Modified Trypsin, Promega, Madison, MI, USA) and Acromobacter lysylendopeptidase (Wako Pure Chem Co., Osaka, Japan) at 37 °C for 12 h. For transferrin, the digest was desalted using a Millipore ZipTip C18 pipette tip and analyzed with a MALDI time-of-flight (TOF) mass spectrometer equipped with a 337-nm wavelength nitrogen laser (Voyager DE-Pro, SCIEX, MA, USA). The sample matrix was 20 mg/mL of 2,5-dihydroxybenzoic acid dissolved in 50% acetonitrile in water. Measurements were performed for positive ions, and the linear TOF mode was used to measure the average mass of ions. For IgG, the glycopeptides were purified by using G-tip C18 (Nikkyo Technos Co., Tokyo, Japan) according to the manufacturer's instruction and then analyzed by MALDI TOF MS as described above.
Fucosylation levels were calculated from the relative intensity of the glycopeptide ions with and without fucosylation; the ions at m/z 4868.9 (Fuc+) and m/z 4722.8 (Fuc-) containing the Asn611 with a di-sialylated biantennary oligosaccharide of transferrin and those at m/z 2602.5 (Fuc+) and m/z 2456.4 (Fuc-) from the Fc region of IgG2 (amino acid sequence of EEQYNSTYR with an agalactosylated biantennary oligosaccharide at 814.8306 m/z) were used for calculation. For IgG, MS measurement was performed five times and the results were expressed as mean ± standard deviation.
2.6. L-fucose supplementation
Due to the finding of severely impaired core fucosylation, fucose substitution has been hypothesized to be a potential therapy for FUT8-CDG [13,14]. Given the severe phenotype observed in the patients, we supplemented oral fucose as a therapy trial. Informed consent was obtained from both parents following detailed information.
Both patients were started on fucose supplementation administered in five equal doses per day via nasogastral or enteral tube. L-Fucose (pharmacy grade) was kindly supplied by Glycom (Hørsholm, Denmark). The starting dose of 100 mg/d (~15 mg/kg for patient A and ~ 19 mg/kg for patient B) was gradually increased to a final dose of 8 kg/d (~ 825 mg/kg for patients A and B). After five months of therapy, 10 g/d galactose were added (1.2 g/kg for patient A, 1.4 g/kg for patient B). This was done due to the finding of hypogalactosylation as demonstrated by an increase in glycans lacking one or more galactose residues (Supplementary Table 1). A detailed overview of the dosing is found in Supplementary Table 2).
Glycosylation of IgG and transferrin was analyzed on a regular basis. In addition, N-glycome profiling of serum glycoproteins using MALDI-TOF MS and MS/MS was performed on pretreatment samples and samples collected under the final dosages of both L-fucose and galactose.
3. Results
3.1. Patients
The patients are dizygotic twins that were conceived following intracytoplasmic sperm injection (ICSI). During pregnancy, the mother was diagnosed with gestational diabetes but did not require insulin treatment. In the 27th week of pregnancy, vaginal bleeding occurred such that respiratory distress syndrome (RDS) prophylaxis with corticosteroids was initiated. The children were born in the 37th week of pregnancy. They presented as floppy infants with a respiratory adaptation disorder requiring nasal continuous positive airway pressure (nCPAP) ventilation. Both children were born with their weights, lengths, and head circumferences all below the 3rd age adapted percentile. Due to a sucking weakness, they were fed via nasogastric and later a gastrojejunal tube. Muscular hypotonia as well as mild dysmorphic features were noted at birth. Patient A, the first-born twin, presented with an arched palate. Patient B also had an arched palate and small tag on the right ear. Echocardiography revealed a trabecular ventricular septal defect (VSD) in patient A and both patients were diagnosed with a patent foramen ovale (PFO). Electrocardiography (ECG) also identified a right bundle branch block in patient A. Standard screening for metabolic diseases as part of the German newborn screening program showed no abnormalities.
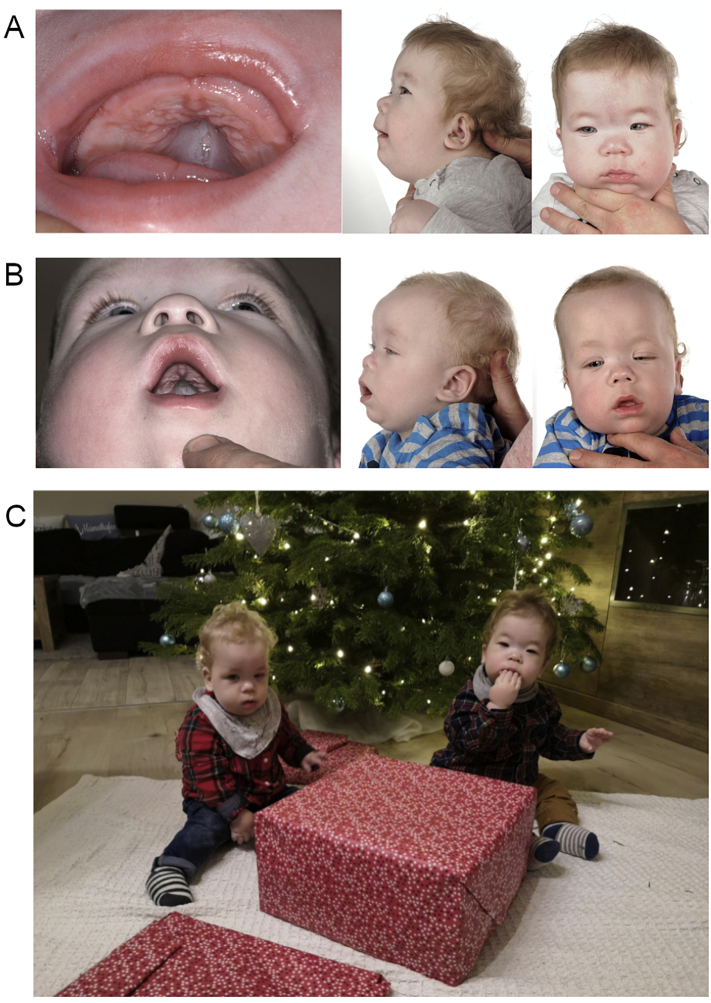
At the age of four months, the patients presented to our center for further diagnostic work-up. In addition to the previously mentioned dysmorphisms, a broad nasal bridge, epicanthal fold, and convergent strabismus were noted in both individuals (Fig. 1 A, B). Gradual reoccurrence of respiratory symptoms as indicated by recurrent airway infections and elevated apnea/hypopnea indices (AHI) in both patients necessitated the reintroduction of nCPAP ventilation. Due to the complex phenotype, exome sequencing of both patients and their parents was performed. With a Nijmegen Pediatric CDG rating Scale (NPCRS) [24] score of 28 in patient A and 29 in patient B corresponding to a severe burden of disease, CDG was suspected.
Fig. 1.
Phenotype of twins with FUT8-CDG.
Clinical presentation of patient A (A) and patient (B) pretreatment. Both patients show typical dysmorphisms with an arched palate, low- set ears, a broad nasal bridge, and a prominent epicanthal fold. Strabismus is present in both individuals. Note the muscular hypotonia necessitating support to keep an upright position. Over the course of L-fucose the treatment, the clinical presentation improved with improvement of muscle tone and discontinuation of ventilatory support (C).
3.2. Compound heterozygosity for two novel variants in FUT8 causing FUT8-CDG
Exome sequencing identified the same variants (c.1403del [p.Ser468Tyrfs*26]);(c.1418G > A [p.Arg473Gln]) in FUT8 (NM_176155.2) in both patients. While the c.1403del variant leads to a premature termination of translation with a consequently truncated protein, the c.1418G > A variant affects a highly conserved residue and has not been described previously either in the literature or in variant databases. The patients' mother was found to be heterozygous for the c.1403del variant while the father was heterozygous for c.1418G > A.
3.3. Fucosylation analysis confirms impaired core fucosylation in FUT8-CDG
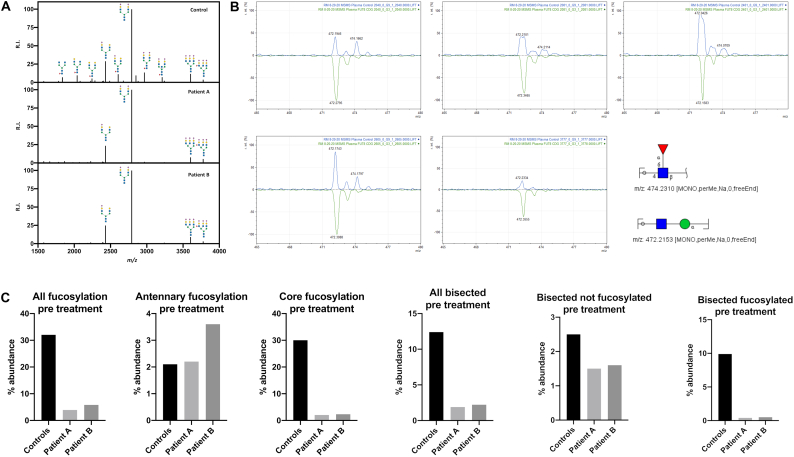
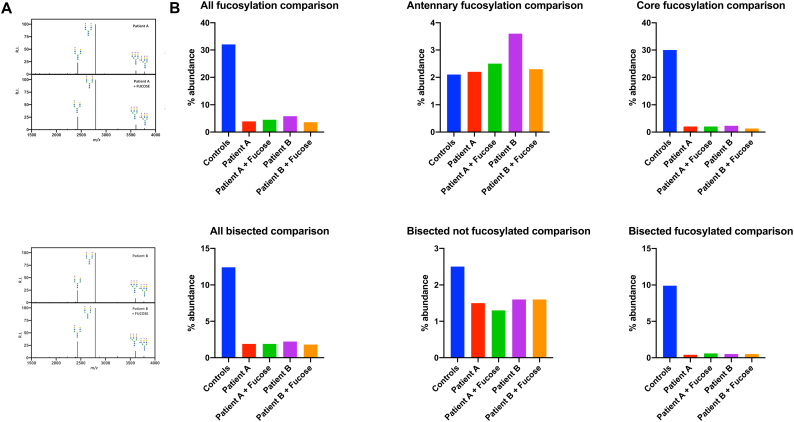
Analysis of transferrin N-glycosylation by IEF and HPLC showed no abnormalities in either child (data not shown). Using N-glycome profiling via MALDI TOF-MS, a reduction of total glycan diversity, characterized by a pronounced loss of core fucosylation, was identified (Fig. 2). In contrast to core fucosylated glycan species, antennary fucosylation, catalyzed by α-1,3-fucosyltransferases [25], was preserved in samples from both patients when compared to healthy controls (Fig. 2C). Interestingly, bisected glycans were dramatically reduced, while other glycan categories (high mannose, hybrid, etc.) did not show major differences between patient and control samples (Fig. 2D). Most bisected N-glycans in plasma are core fucosylated (~80%) [26], and the abundance of non-fucosylated bisected N-glycans did not change dramatically. Thus, we suspect the loss of bisected N-glycans is related to impaired FUT8 activity and not MGAT3, the enzyme that synthesizes bisected N-glycans.
Fig. 2.
FUT8-CDG leads to a lack of core fucosylation in MALDI TOF-MS N-glycome profiling.
A) 5 μg of human plasma protein was analyzed as previously described (Mealer et al., 2020). Permethylated N-glycome profiles from batch controls and two FUT8-CDGs are shown, with relative intensity (R.I) on the y-axis and mass-to-charge (m/z) ratio on the x-axis. Profiles with glycans greater than 3% relative abundance are illustrated. B) MS/MS fractionation confirms the absence of core fucosylation in FUT8-CDG samples. C) MALDI-MS profiling of FUT8-CDGs confirms a lack of core fucosylation with a preservation of antennary fucosylation in both patients. D) MALDI-MS profiling of FUT8-CDGs identifies a unique loss of bisected N-glycans in FUT8-CDG, the majority of which are also core fucosylated, and minimal change in high mannose or hybrid glycans.
To further characterize masses predicted to contain core fucose (m/z 2040, m/z 2081, m/z 2401, m/z 2605), MS/MS fractionation was performed (Fig. 2B), also of a glycan with antennary fucose (m/z 3777). The fragment ion corresponding to a core fucosylated N-glycan (-Fuc-GlcNAc-reducing end-) at m/z 474.2 was easily detected at all 4 masses in controls, and easily distinguished from the neighboring peak at m/z 472.2 (-GlcNAc-Man-). The core fucosylated fragment at m/z 474.2 was absent in FUT8-CDG, with residual signal representing isotopic C13 signal from the neighboring 472.2 fragment. Fragmentation of m/z 3777 did not produce the core fucosylated 474.2 fragment in controls or FUT8-CDG, but did produce a fragment at m/z 1021.5 consistent with antennary fucose (-NeuAc-Gal-(Fuc)-GalNAc-). Of note, ion fragments from alternate glycosidic bond breaks were not detected in any samples for the described masses. In sum, MS/MS fragmentation results are consistent with a lack of core fucosylation and preservation of antennary fucosylation in the plasma of FUT8-CDG.
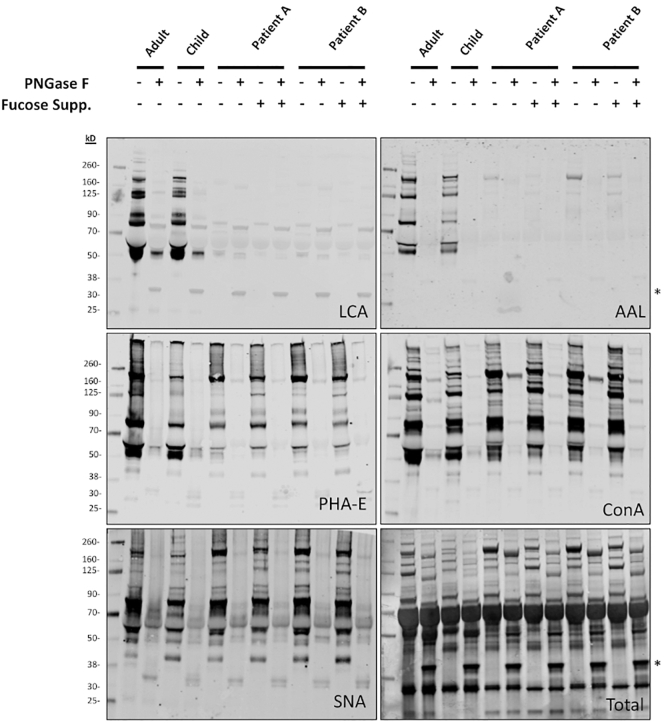
To further confirm the lack of core fucosylated species in serum, western blotting with biotinylated lectins that recognize specific glycosylation features was employed. A dramatic reduction in signal from fucose-binding lectins was observed in FUT8-CDG compared to an adult and age matched control, including LCA; which highly recognizes N-glycans with core fucose, and ALL, which generally recognizes terminal and core fucose residues (Fig. 3).
Fig. 3.
Lectin blotting confirms a loss of core fucosylation in FUT8-CDG.
FUT8-CDG has a near total absence of fucose-specific lectin binding, which does not improve with fucose supplementation, consistent with MALDI-MS glycomics results. Human plasma (0.5 μL) was loaded in each lane after treatment with no without PNGase F at 37 °C for 1 h. Protein visualized using biotinylated lectins (LCA, AAL, Con A, SNA) followed by fluorescently labelled streptavidin, and total protein stain (Total) using the LICOR system. Non-specific signal from PNGase F at 35kD is marked with an asterisk (*).
Binding of PHA-E, which has a preference for bisected N-glycans but is not specific, was reduced but not absent in FUT8-CDG, particularly the band at molecular weight of 50 kD, which corresponds to the IgG heavy chain. Additional lectins including ConA, which recognizes the α-mannose structure of many N-glycans without regard to fucosylation, SNA, which require terminal α2-6-linked sialic acid for binding, and RCA, which binds to glycans with terminal galactose, were unaltered in FUT8-CDGs (Supplementary Fig. 1). In addition, a decrease of IgG was noted.
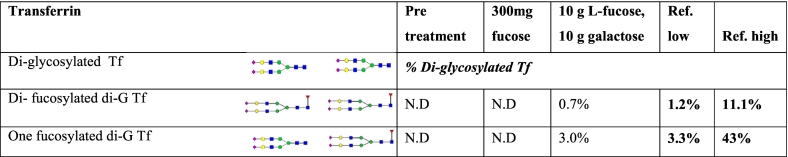
In order to assess the glycosylation changes in a more detailed way, targeted analysis of selected glycoproteins was performed. After affinity purification of transferrin, the intact transferrin glycoprotein and glycopeptides were subjected to both LC-ESI-TOF MS and MALDI-TOF to assess fucosylation. Using ESI-TOF MS, no di- or monofucosylated diglycosylated transferrin was detected in pre-treatment samples (Table 1). In contrast, MALDI-TOF MS identified severely reduced but nevertheless identifiable fucosylation well below the levels seen in healthy controls (Fig. 4D). Targeted analysis of IgG Fc fucosylation using MALDI-TOF MS indicated a severe hypofucosylation in pretherapeutic samples well below values seen in healthy controls (Fig. 5B).
Table 1.
Transferrin analysis of FUT8-CDG before and after fucose supplement. Ref: reference. Di-G Tf: diglycosylated transferrin.
Fig. 4.
N-glycome profiling using MALDI-TOF MS is unaltered following fucose supplementation in FUT8-CDG.
A) N-glycome profiling using MALDI-TOF MS did not improve following oral L-fucose supplementation. B) The abundance of fucosylated as well as bisected glycans showed fluctuations in relative abundance without improving substantially. Total abundance of core fucosylated glycans and core fucosylated bisected glycan structures remains severely reduced while fluctuations of antennary fucosylated glycans and non-fucosylated bisected glycans are present.
Fig. 5.
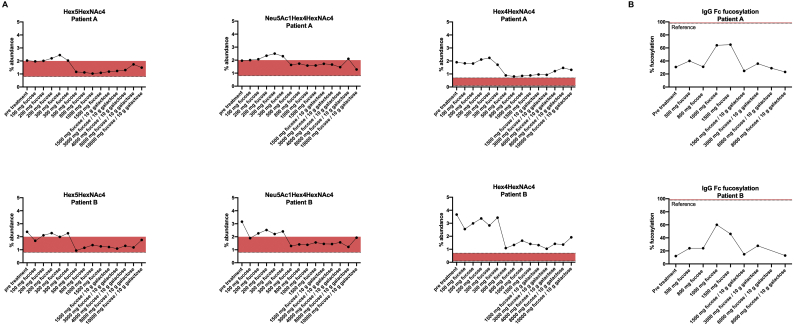
Targeted glycosylation analysis using ESI-TOF MS of immunopurified glycoproteins identifies subtle improvement of glycosylation following fucose substitution.
A) ESI-TOF MS identified truncated and non-fucosylated glycan species that decreased to or close to ranges seen in controls under fucose supplementation. Fucosylated glycan species showed fluctuations, remaining below reference ranges (Supplementary Fig. 2). B) Targeted analysis of fucosylation of an agalactosylated glycan on IgG Fc showed an initial increase while later falling to the ranges of initial values under higher doses of fucose and additional galactose. Fucosylation of serum transferrin at one of the two glycosylation site showed fluctuations in % fucosylation of a disialo-glycan without any clear effect of fucose supplementation.
3.4. Fucose supplementation has a moderate and protein specific effect on core fucosylation in FUT8-CDG
Following fucose supplementation in both twins, glycosylation assessment was again performed in order to detect any effects of the intervention. Total serum N-glycome profiling using MALDI TOF-MS did not show any increase in either core or antennary fucosylation after treatment. Similarly, no significant change was observed with regards to the severe lack of glycan diversity or bisected glycans compared to pretreatment samples (Fig. 4A, B). These findings were confirmed with lectin blotting, demonstrating no significant changes in LCA; ALL, PHA-E, and RCA binding following fucose supplementation in either patient (Fig. 3).
In contrast, targeted analysis of fucosylation on serum transferrin using ESI-TOF MS after protein affinity purification detected an increase in core fucosylation on serum transferrin with treatment. Both, di- and mono-fucosylated diglycosylated transferrin, increased under fucose supplementation while still remaining below ranges seen in unaffected controls (Table 1).
Importantly, analysis using ESI-QTOF MS showed improvement of selected glycan species of the serum N-glycome (Fig. 5A, Fig. 6B). Specifically, a decrease in truncated non-fucosylated glycan species to normal ranges (agalactosylated glycan Hex3HexNAc4 [814 m/z], asialo glycan Hex5HexNAc4 [976.8834 m/z], and mono-galactosylated, Neu5Ac1Hex4HexNAc4 [1041.4047 m/z]) could be observed. However, glycans with truncated and fucosylated glycan structures remained below reference ranges and showed little to no response to fucose supplementation (Supplementary Fig. 2). Interestingly, the abundance of a fucosylated disialo-biantennary glycan at 1341.0078 m/z, a fucosylated glycan with intact structure, was moderately improved with fucose therapy, while its non-fucosylated counterpart, the disialo-biantennary glycan at 1267.9788 m/z was also increased (Fig. 6 B). IgG Fc fucosylation as determined by analysis of an agalactosylated glycan structure at 887.8596 m/z showed a considerable increase under L-fucose doses of approximately 1 g/d (~ 100 mg/kg bw/d) but decreased to pre-treatment levels at higher doses and/or following the addition of galactose (Fig. 5B).
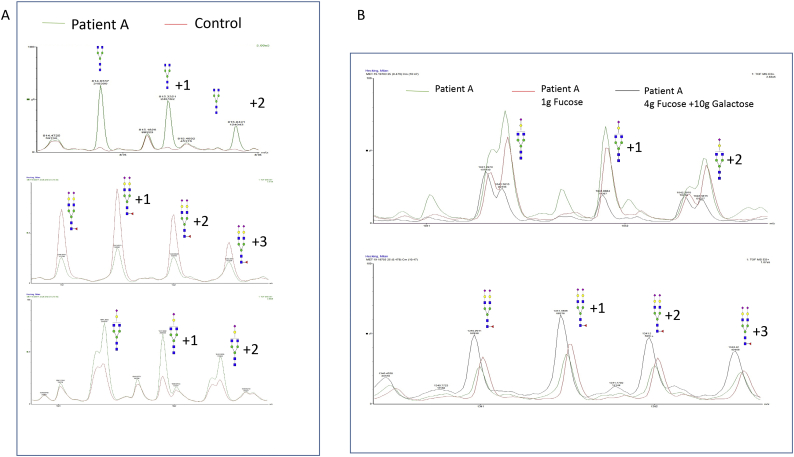
Fig. 6.
ESI-QTOF MS detects reduced dysglycosylation following fucose/galactose treatment in FUT8-CDG.
A) In exemplary pretreatment samples taken from Patient A, ESI-QTOF MS both show severe lack of fucosylation as well as hypogalactosylation based on analysis of selected reporter glycans. B) Following supplementation of 1 g/day fucose, a reduction in hypofucosylated glycans was noted while hypogalactosylated glycans remained elevated. Additional supplementation of 10 g galactose/day in conjunction with an increase of fucose supplementation lead to a further shift towards regular glycosylation and specifically a reduction in hypogalactosylation.
Clinically, the patients stabilized considerably during fucose supplementation. Ventilatory support could be discontinued and muscular hypotonia and motor abilities improved. At the age of 20 months the children are able to get to the sitting position without help (Fig. 1C). Intestinal tube feeding is still necessary. No adverse effects were noted during treatment with L-fucose, with regular monitoring of liver and kidney function tests remaining in the normal range.
4. Discussion
FUT8-CDG is a rare congenital disorders of glycosylation. Only seven patients have been reported in the literature. These showed a remarkably uniform phenotype consisting of intrauterine growth restriction, dysmorphisms, seizures, and failure to thrive [13]. The patients reported in this manuscript did not show any signs of seizure activity. A right bundle branch block in patient A is the first electrophysiological abnormality reported in FUT8-CDG.
Based on the pathophysiology of FUT8-CDG, the supplementation of fucose has been proposed as a potential treatment for this debilitating disorder [14]. Indeed, successful fucose treatment in some SLC35C1-CDG patients [12,27] demonstrated the amenability of disturbed fucose metabolism to substrate supplementation. Previous in vitro studies on skin fibroblasts derived from FUT8-CDG patients did not affect the severely disturbed N-glycan profile [14]. This was suspected to be – in part – due to the fact that these cells were carrying truncating protein variants while missense variants might be responsive to substrate supplementation [14]. Due to the severe phenotype observed in the patients presented herein, oral fucose was supplemented as an individual therapy trial based on treatment protocols for SLC35C1-CDG [9].
N-glycome profiling using MALDI-TOF MS readily identified severe hypofucosylation that was attributable to a near total absence of core fucosylation in pre-treatment samples and verified by MS/MS and lectin blotting. Bisected N-glycans, most of which are core-fucosylated, were also severely reduced (Fig. 2). MALDI-TOF MS did not identify any changes in core fucosylation or bisection following substitution of high doses of L-fucose.
In contrast, ESI-QTOF MS of post therapy serum glycans did identify a reduction in abundance of truncated non-fucosylated glycans (Fig. 5A), whereas their fucosylated counterpart glycan species remained below reference ranges (Supplementary Fig. 2). For glycan species with intact structure, both non-fucosylated and fucosylated glycans have mildly improved abundance upon fucose therapy, while % fucosylation of intact disialo-glycans remained unchanged (Fig. 6). This is indicative of moderate improvement of core fucosylation following oral fucose substitution in FUT8-CDG.
Targeted glycosylation analysis of selected glycoproteins was employed to evaluate the effect of L-fucose supplementation in greater detail. Analysis of immunopurified serum transferrin indicated improved fucosylation when analyzed by ESI-TOF MS (Table 1) but no significant improvement when % fucosylation was measured on a single glycosylation site by MALDI-TOF MS (Supplementary Fig. 3). Analysis of fucosylation of IgG Fc glycan structures indicated an increased fucosylation of an agalactosylated glycan in samples collected while the patients were receiving 1000–1500 mg L-fucose/day. Samples collected under higher fucose in combination with galactose supplementation had reduced levels of fucosylation similar to values from samples before therapy. This finding can be explained by a general reduction of hypo- or agalactosylated glycan species following galactose substitution as indicated by ESI-QTOF MS.
The somewhat conflicting results could reflect different levels of sensitivity of the methods used for analysis. In MALDI-TOF MS, normalization to a single “reporter glycan” during analysis with severely decreased glycan abundance will alter the relative intensity of the remaining glycan species and distort its overall representation that might account for the failure to detect subtle changes following fucose supplementation. However, N-glycome profiling using either MALDI-TOF MS or ESI-TOF MS of serum or plasma glycans is reliable in the diagnosis of FUT8-CDG and both methods have been applied in a plethora of clinical applications [28], stressing its utility for characterizing the general state of the glycome.
The results may also reflect a selective effect of oral fucose supplementation on the glycosylation of certain proteins in certain tissues as well as certain glycosylation sites of a glycoprotein. Transferrin, the principal iron transport protein in vertebrates, is synthesized in the liver [29] and has been used as a biomarker since the discovery of CDG [30]. The selective improvement observed in this sparsely fucosylated protein [31] at the intact protein level but not at one particular site may be related to site specific glycosylation of transferrin that has been demonstrated in various CDG subtypes [32,33]. In addition, increases of a truncated non-fucosylated glycan species were detected on IgG. The general reduction of agalactosylated glycans in serum and increase of fucosylation of this truncated glycan on a specific site of IgG upon fucose therapy might be indicative of tissue specific effects, either due to the tissue specific delivery of orally supplemented fucose or due to tissue-specific affinities of fucose transporters and enzymes upstream of α-1,6-fucosyltransferase. FUT8 levels are much lower in the liver compared to other tissues, which may reflect why proteins synthesized by the liver such as transferrin and alpha-1 acid glycoprotein generally have low levels of core fucosylation [34]. Why IgG, which makes up a larger portion of the total plasma N-glycome and at baseline is usually core fucosylated, did not show a greater change with fucose supplementation is unclear. Lymphocytes express higher levels of FUT8 but may have different pathways for uptake and use of fucose. Although plasma is easily accessible, it is unlikely to capture fucosylation changes in other organs, such as the brain. All FUT8-CDG patients reported to date have profound neurologic impairment, suggesting a critical role for core fucosylation in the nervous system. FUT8 is expressed in brain and peripheral nerves [34], and a large portion of brain N-glycans are core fucosylated [19]. Fucose supplementation improved hypotonia and respiratory drive in our FUT8-CDG patients, and may reflect restoration of pathway function in the nervous system that would not be detectable in plasma.
In summary, we describe two novel FUT8-CDG patients with dramatically impaired core fucosylation in plasma at baseline, confirmed by several methodologies. Under fucose supplementation, there was a moderate clinical effect. It is possible that the amount of given fucose was not high enough, and further analyses on a wider array of biofluids or derived cell lines might be needed to assess the tissue specific effects of supplementation therapy in the future. As some CDG stabilize over time without intervention [35], it cannot entirely be ruled out that the observed clinical improvement may be independent of fucose supplementation and thus part of the natural disease course. Given the currently lacking data on the natural history of FUT8-CDG, little is known about long-term development of the glycosylation phenotype. However, given the severe phenotype of FUT8-CDG including death in multiple individuals during childhood, this is unlikely. Fucose supplementation appeared well tolerated in our patients and may have clinical utility in the treatment of FUT8-CDG.
The following are the supplementary data related to this article.
Lectin western blotting does not show major differences in galactosylation in FUT8-CDG.
Glycans with truncated and fucosylated structures show no clear response to fucose supplementation.
Transferrin fucosylation remains severely reduced following oral fucose supplementation in FUT8-CDG.
Raw data of ESI-QTOF MS of serum samples in FUT8-CDG.
L-Fucose dosing in patient A and patient B.
Author contributions
Julien H. Park1,2 study design, supervision, aggregation of clinical data, writing
Janine Reunert1 molecular genetics, study design
Miao He3 mass spectrometry, glycosylation analysis, interpretation of data
Robert G. Mealer4,5,6 mass spectrometry, glycosylation analysis, interpretation of data
Maxence Noel6 glycosylation analysis, immunoblotting
Yoshinao Wada7 mass spectrometry, glycosylation analysis
Marianne Grüneberg1 glycosylation analysis
Judit Horváth8 molecular genetics
Richard D. Cummings6 study design, supervision, interpretation of data
Oliver Schwartz1 supervision, patient care, acquisition of clinical data
Thorsten Marquardt1 study design, supervision, patient care, writing
Acknowledgements
This study was in part funded by a personal grant of the fund “Innovative Medical Research” (IMF) of the University of Münster Medical School, Münster, Germany (to JHP; Project PA 5 2 19 01). We would like to thank Glycom (Hørsholm, Denmark) for generously supplying fucose used in supplementation.
References
- 1.Zhang Q., Joubert M.K., Polozova A. Glycan engineering reveals interrelated effects of terminal galactose and core fucose on antibody-dependent cell-mediated cytotoxicity. Biotechnol. Prog. 2020 doi: 10.1002/btpr.3045. [DOI] [PubMed] [Google Scholar]
- 2.Takamatsu S., Shimomura M., Kamada Y. Core-fucosylation plays a pivotal role in hepatitis B pseudo virus infection: a possible implication for HBV glycotherapy. Glycobiology. 2016;26:1180–1189. doi: 10.1093/glycob/cww067. [DOI] [PubMed] [Google Scholar]
- 3.Lu H.H., Lin S.Y., Weng R.R. Fucosyltransferase 4 shapes oncogenic glycoproteome to drive metastasis of lung adenocarcinoma. EBioMedicine. 2020;57:102846. doi: 10.1016/j.ebiom.2020.102846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanas A., Zaal A., van der Haar Àvila I. FUT9-driven programming of colon cancer cells towards a stem cell-like state. Cancers. 2020;12 doi: 10.3390/cancers12092580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeken J. Congenital disorders of glycosylation. Handb. Clin. Neurol. 2013;113:1737–1743. doi: 10.1016/B978-0-444-59565-2.00044-7. [DOI] [PubMed] [Google Scholar]
- 6.Jaeken J., Péanne R. What is new in CDG? J. Inherit. Metab. Dis. 2017;40:569–586. doi: 10.1007/s10545-017-0050-6. [DOI] [PubMed] [Google Scholar]
- 7.Ng B.G., Rosenfeld J.A., Emrick L. Pathogenic variants in fucokinase cause a congenital disorder of glycosylation. Am. J. Hum. Genet. 2018;103:1030–1037. doi: 10.1016/j.ajhg.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lübke T., Marquardt T., von Figura K., Körner C. A new type of carbohydrate-deficient glycoprotein syndrome due to a decreased import of GDP-fucose into the golgi. J. Biol. Chem. 1999;274:25986–25989. doi: 10.1074/jbc.274.37.25986. [DOI] [PubMed] [Google Scholar]
- 9.Marquardt T., Luhn K., Srikrishna G., Freeze H.H., Harms E., Vestweber D. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood. 1999;94:3976–3985. [PubMed] [Google Scholar]
- 10.Marquardt T., Brune T., Lühn K. Leukocyte adhesion deficiency II syndrome, a generalized defect in fucose metabolism. J. Pediatr. 1999;134:681–688. doi: 10.1016/S0022-3476(99)70281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker D.J., Lowe J.B. Leukocyte adhesion deficiency type II. Biochim. Biophys. Acta. 1999;1455:193–204. doi: 10.1016/s0925-4439(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 12.Wild M.K., Luhn K., Marquardt T., Vestweber D. Leukocyte adhesion deficiency II: therapy and genetic defect. Cells Tissues Organs. 2002;172:161–173. doi: 10.1159/000066968. [DOI] [PubMed] [Google Scholar]
- 13.Ng B.G., Dastsooz H., Silawi M. Expanding the molecular and clinical phenotypes of FUT8-CDG. J. Inherit. Metab. Dis. 2020;43:871–879. doi: 10.1002/jimd.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng B.G., Xu G., Chandy N. Biallelic mutations in FUT8 cause a congenital disorder of glycosylation with defective fucosylation. Am. J. Hum. Genet. 2018;102:188–195. doi: 10.1016/j.ajhg.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyoshi E., Noda K., Yamaguchi Y. The α1-6-fucosyltransferase gene and its biological significance. Biochim. Biophys. Acta Gen. Subj. 1999;1473:9–20. doi: 10.1016/s0304-4165(99)00166-x. [DOI] [PubMed] [Google Scholar]
- 16.Park J.H., Hogrebe M., Gruneberg M. SLC39A8 deficiency: a disorder of manganese transport and glycosylation. Am. J. Hum. Genet. 2015;97:894–903. doi: 10.1016/j.ajhg.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tegtmeyer L.C., Rust S., van Scherpenzeel M. Multiple phenotypes in phosphoglucomutase 1 deficiency. N. Engl. J. Med. 2014;370:533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mealer R.G., Jenkins B.G., Chen C.-Y. The schizophrenia risk locus in SLC39A8 alters brain metal transport and plasma glycosylation. Sci. Rep. 2020;10:13162. doi: 10.1038/s41598-020-70108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams S.E., Noel M., Lehoux S. The restricted nature of protein glycosylation in the mammalian brain. bioRxiv. 2020 2020.10.01.322537. [Google Scholar]
- 20.Chen J., Li X., Edmondson A. Increased clinical sensitivity and specificity of plasma protein N-glycan profiling for diagnosing congenital disorders of glycosylation by use of flow injection–electrospray ionization–quadrupole time-of-flight mass spectrometry. Clin. Chem. 2019;65:653–663. doi: 10.1373/clinchem.2018.296780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Raihan M.A., Reynoso F.J., He M. Glycosylation analysis for congenital disorders of glycosylation. Curr. Protoc. Hum. Genet. 2015;86 doi: 10.1002/0471142905.hg1718s86. 17.8.1-.8.22. [DOI] [PubMed] [Google Scholar]
- 22.Wada Y., Azadi P., Costello C.E. Comparison of the methods for profiling glycoprotein glycans—HUPO human disease glycomics/proteome initiative multi-institutional study. Glycobiology. 2007;17:411–422. doi: 10.1093/glycob/cwl086. [DOI] [PubMed] [Google Scholar]
- 23.Tajiri M., Kadoya M., Wada Y. Dissociation profile of protonated fucosyl glycopeptides and quantitation of fucosylation levels of glycoproteins by mass spectrometry. J. Proteome Res. 2009;8:688–693. doi: 10.1021/pr800727w. [DOI] [PubMed] [Google Scholar]
- 24.Achouitar S., Mohamed M., Gardeitchik T. Nijmegen paediatric CDG rating scale: a novel tool to assess disease progression. J. Inherit. Metab. Dis. 2011;34:923–927. doi: 10.1007/s10545-011-9325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries T., Knegtel R.M.A., Holmes E.H., Macher B.A. Fucosyltransferases: structure/function studies. Glycobiology. 2001;11 doi: 10.1093/glycob/11.10.119r. 119R-28R. [DOI] [PubMed] [Google Scholar]
- 26.Clerc F., Reiding K.R., Jansen B.C., Kammeijer G.S., Bondt A., Wuhrer M. Human plasma protein N-glycosylation. Glycoconj. J. 2016;33:309–343. doi: 10.1007/s10719-015-9626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etzioni A., Tonetti M. Fucose supplementation in leukocyte adhesion deficiency type II. Blood. 2000;95:3641–3643. [PubMed] [Google Scholar]
- 28.Wuhrer M. Glycomics using mass spectrometry. Glycoconj. J. 2013;30:11–22. doi: 10.1007/s10719-012-9376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawabata H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019;133:46–54. doi: 10.1016/j.freeradbiomed.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Jaeken J., van Eijk H.G., van der Heul C., Corbeel L., Eeckels R., Eggermont E. Sialic acid-deficient serum and cerebrospinal fluid transferrin in a newly recognized genetic syndrome. Clin. Chim. Acta. 1984;144:245–247. doi: 10.1016/0009-8981(84)90059-7. [DOI] [PubMed] [Google Scholar]
- 31.Nagae M., Morita-Matsumoto K., Arai S. Structural change of N-glycan exposes hydrophobic surface of human transferrin. Glycobiology. 2014;24:693–702. doi: 10.1093/glycob/cwu033. [DOI] [PubMed] [Google Scholar]
- 32.Wada Y. Mass spectrometry of transferrin glycoforms to detect congenital disorders of glycosylation: site-specific profiles and pitfalls. Proteomics. 2016;16:3105–3110. doi: 10.1002/pmic.201500551. [DOI] [PubMed] [Google Scholar]
- 33.Sun S., Hu Y., Jia L. Site-specific profiling of serum glycoproteins using N-linked glycan and glycosite analysis revealing atypical N-glycosylation sites on albumin and α-1B-glycoprotein. Anal. Chem. 2018;90:6292–6299. doi: 10.1021/acs.analchem.8b01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Consortium T.G. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiff M., Roda C., Monin M.-L. Clinical, laboratory and molecular findings and long-term follow-up data in 96 French patients with PMM2-CDG (phosphomannomutase 2-congenital disorder of glycosylation) and review of the literature. J. Med. Genet. 2017;54:843. doi: 10.1136/jmedgenet-2017-104903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lectin western blotting does not show major differences in galactosylation in FUT8-CDG.
Glycans with truncated and fucosylated structures show no clear response to fucose supplementation.
Transferrin fucosylation remains severely reduced following oral fucose supplementation in FUT8-CDG.
Raw data of ESI-QTOF MS of serum samples in FUT8-CDG.
L-Fucose dosing in patient A and patient B.