Abstract
Background and aim
Adipocytokines, especially leptin is involved in a wide spectrum of proinflammatory functions, in various tissues. This study was carried out to assess the role of serum leptin in autoimmune hepatitis.
Methods
Serum leptin was analyzed in treatment naïve autoimmune hepatitis (AIH, n = 48) patients and compared with the primary biliary cholangitis (PBC, n = 16), chronic hepatitis C (CHC, n = 16) and healthy controls (n = 15). Serum leptin correlation was assessed on liver function tests, disease activity, T regulatory cells (Tregs), and Th17 cells in the liver biopsies and on steroid treatment response in AIH.
Results
Serum leptin was higher in AIH than in PBC, CHC, and HC {AIH: 335 (106.2–580), PBC: 126 (52–381.2), CH: 67 (3.7–133.5) and HC: 66 (40–157.5) ng/ml; P = 0.001}. In AIH cases; serum leptin correlated with hepatic activity index (r = 0.896; P < 0.001); serum transaminases (aspartate aminotransferases (AST) = 0.615, P < 0.001, alanine aminotransferases (ALT) = 0.551, P < 0.001). It had inverse correlation with Treg cells (P = −0.711, P < 0.001) and positively correlated with Th17 cells (r = 0.650, P < 0.001) in the liver biopsy tissue. High serum leptin was found to be associated with steroid partial or nonresponsiveness at 4 weeks (P = 0.002).
Conclusion
Serum leptin is indicative of higher AIH activity and a reduced number of Tregs cells in liver biopsy tissue. Leptin negative cases have more chances of steroid responsiveness and could help in the selection of AIH cases for appropriate therapy.
Keywords: autoimmune hepatitis, leptin, liver biopsy, T helper cells, tregs
Abbreviations: AIH, autoimmune hepatitis; CH, chronic hepatitis; PBC, primary biliary cholangitis; T regs, T regulatory cells; Th17, T helper cells
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease caused by unknown potential factors and tends to progress into cirrhosis or liver failure if not treated on time.1 Various environmental factors such as bacteria, viruses, toxins, and drugs or genetic predisposition are known to trigger AIH. The activity of certain hormones also has been attributed to the pathogenesis of autoimmune disease.1,2 Sex hormones such as estradiol and prolactin could be the reason for the female predisposition of autoimmune diseases.3 In addition to these hormones, relatively less characterized adipocytokines, particularly leptin participates in several processes including inflammation and immunity and can contribute to the progression of autoimmune diseases.4 It can modulate the immune system by stimulating the release of proinflammatory cytokines involved in the induction of pathogenic inflammation and fibrosis of autoimmune diseases.5 Leptin also inhibits T regulatory cells (Tregs) proliferation/functions and increases the Th17 cells proliferation and functions, in in vitro models.6 The present study was aimed to see the status of leptin in AIH and its impact on short-term treatment response.
Patients and methods
Patients and Controls
A prospective study was carried out on treatment-naive AIH-1 patients with fibrosis ≤ F3 (n = 48) diagnosed from January to December 2017 at the Institute of Liver and Biliary Sciences, New Delhi. The specific diagnosis of AIH-1 was established, as described.7 Patients typically had transaminitis, hypergammaglobulinemia, and were positive for the antinuclear antibody (ANA) and/or antismooth muscle antibodies. Liver biopsies were performed in all patients for diagnostic purpose and remaining tissue in paraffin block was used for the study after, rendering the diagnosis. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained to use the data of pathology and clinical profile for this study. The Institutional Ethical Review Board of the Institute of Liver and Biliary Sciences, New Delhi, India, approved the study vide F.25/5/81/ILBS/AC/2015/917 on 22/12/15. Patients with AIH received standard treatment as wysolone starting with 40 mg/d, tapered to a maintenance dose of 20 mg/d for more than 4 wk with azathioprine 1–2 mg/kg per day. These patients were compared with 3 different groups of patients – primary biliary cholangitis (PBC, n = 16); chronic hepatitis C virus (HCV, n = 16), and healthy controls (HCs) (n = 15). Patients with F4 fibrosis, acute on chronic liver failure, acute liver failure, malignancy, pregnancy, overlap syndrome, nonalcoholic steatohepatitis, alcoholics, and the patient already under treatment were excluded.
Enzyme-linked Immunosorbent Assay
A total of 10 ml of blood was collected in EDTA vial from AIH patients and control cases. Plasma was taken out in a separate vial and stored in −80° till the assay procedure. Leptin (KAC2281, Invitrogen, MA, USA) and adiponectin (KHP0041, Invitrogen, MA, USA) were quantified from plasma samples at baseline of various groups using human enzyme-linked immunosorbent assay, in accordance with the manufacturer's recommendations.
Histopathology, Immunohistochemistry, and Immunofluorescence on Liver Biopsies
Liver biopsies were evaluated for histological parameters hepatic activity index (HAI) in accordance with Ishak's modified scoring system,8 and fibrosis in accordance with METAVIR system.9 Immunohistochemistry (IHC) was performed on formalin-fixed and paraffin-embedded sections of liver tissue. CD163 and was assessed in the sinusoidal area in consecutive 20× fields and average was represented as cell count/20×. Double IHC staining was performed with a combination of CD25-FOXP3 to access Tregs. FOXP3 staining was identified with nuclear positivity, and cytoplasmic positivity was showed by CD25 in the similar cells in the portal/periportal septal/periseptal areas. CD25-FOXP3+ cells were enumerated by Image-J software using the IHC profiler. Positive cells were considered and represented as % positive cells. All the portal/periportal/septal and periseptal areas were analyzed for T regs and the average was taken as % cells/portal or septal area. Immunofluorescence was performed on the paraffin-embedded liver section by using an interlukin (IL)-17 monoclonal antibody to assess the T helper cells in the similar areas as for Tregs and represented as average cell count/portal or septal area.
Flow Cytometry
Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll-Hypaque density centrifugation (2500 r/min for 20 min at room temperature). For analysis of Th17 cells, the PBMCs were first washed with Roswell Park Memorial Institute −1640 culture medium and then stimulated with 20 ng/ml phorbol 12-myristate-13-acetate and 1 μg/ml ionomycin in the presence of 2 mmol/ml monensin for 5 h incubation at 37 °C. At that point, cells were stained with CD4-APC, IL23R-PerCP, and RORγ-PE monoclonal antibodies and incubated in the dark for 20 min. After washing with phosphate-buffered saline (PBS) and fixation in accordance with the manufacturer's instruction, the cells were incubated with monoclonal IL-17 antibodies in the dark for 15 min.
For analysis of immune Treg cells, the cell suspension was washed with PBS and stained with CD8-Pecy5, CD25-FITC, FOXP3-PE, and CD3-V450 at 4 °C for 30 min. After washing with PBS and fixation in accordance with the manufacturer's instruction, cells were stained with an enzyme-labeled anti-FOXP3 antibody and incubated for 30 min. For CD4/CD8 analysis, PBMCs were washed with PBS and stained with CD4-FITC, CCR7-PE, CD45RA-V450, CD8-Pecy5, TIM3-APC antibodies followed by incubation, fixation, and then finally washed with PBS.
Clinical Data and Treatment Response
Clinical data and information were collected from the hospital information system. Patients with AIH received steroids as the first-line treatment, and clinical response was assessed as < 2 times of AST/ALT of upper normal limits at week 4 was considered as immune response; decreased transaminases from initial levels but persistent elevation up to 2 times of upper normal limits at 4 weeks was taken as partial response; and no improvement or refractory as nonresponse.10
Statistical Analysis
Statistical analysis was carried out using Statistical Package for Social Sciences (SPSS), version 22.0. Descriptive statistics were presented as proportions, mean ± standard deviation/standard error and median with range. A correlation was made by Pearson/Spearman correlation coefficient. The categorical data analyzed by the chi-square test and the quantitative data by student t-test/Mann-Whitney or one-way ANOVA or Kruskal-Wallis test.
Results
A total of 48 patients with AIH were analyzed in this study. Table 1 displays the general characteristics of the patients about age, gender, and routine laboratory parameters. The average age across the AIH, PBC, CH, and HC groups were comparable (P = 0.227). In the test group patients' distribution amongst the fibrosis, stages (0/1/2/3) were comparable with patients in PBC and CH groups (P = 0.766). As expected, transaminitis and hepatic necroinflammatory activity were higher in AIH as compared with other groups and elevated cholestatic enzymes in PBC. [Table 1].
Table 1.
Basic Attributes in Study Set and Control Groups.
| Autoimmune Hepatitis (AIH) | Primary Biliary Cholangitis (PBC) | Chronic Hepatitis (CH) | Healthy Control | P value | |
|---|---|---|---|---|---|
| n | 48 | 16 | 16 | 25 | |
| Age, yearsa | 46.4 ± 13.1 | 47.5 ± 9.4 | 40.7 ± 12.8 | 40.6 ± 8.6 | 0.227 |
| Gender, M:F | 9:39 | 0:16 | 2:14 | ||
| Hemoglobin, gm/dla | 11.2 ± 2.7 | 10 ± 2.6 | 11.14 ± 2.3 | 0.421 | |
| Total leukocyte count, X10ˆ3/cummb | 6 (5–10) | 6 (4–9) | 4 (4–7) | 0.626 | |
| Platelets, x10ˆ6/cummb | 135 (75–164) | 150 (90–294) | 144 (71–159) | 0.545 | |
| Creatinine, mg/dla | 0.76 ± 0.6 | 0.79 ± 0.6 | 1 ± 0.5 | 0.33 | |
| S. Bil, mg/dlb | 2 (1–10) | 2 (1–4) | 1 (1–1) | 0.611 | |
| Aspartate aminotransaminase, IU/Lb | 105 (77–182) | 96 (55–157) | 44 (29–79) | 0.01 | |
| Alanine aminotransaminase, IU/Lb | 112 (73–149) | 66 (52–147) | 27 (23–67) | 0.005 | |
| S. Alkaline phosphatize, IU/Lb | 102 (79–137) | 180 (115–282) | 103 (97–159) | 0.030 | |
| Gamma glutamyl transpeptidase, IU/Lb | 41 (26–81) | 81 (31–136) | 27 (19–59) | 0.337 | |
| International normalized ratiob | 1 (1–2) | 1 (1–1.7) | 1.5 (1–2) | 0.552 | |
| Thyroid-stimulating hormoneb | 2 (2–4), n = 17 | 3.5 (1.7–8.2), n = 10 | 0.257 AIH vs PBC |
||
| Hepatic activity indexb | 10 (7–14) | 5.5 (4.2–6) | 4 (4–5) | <0.001 | |
| Fibrosis Stage 0/1/2/3 | 2/12/20/12 | 0/4/10/2 | 0/2/9/5 | 0.766 |
AIH, autoimmune hepatitis.
Mean±SD.
Median (interquartile range).
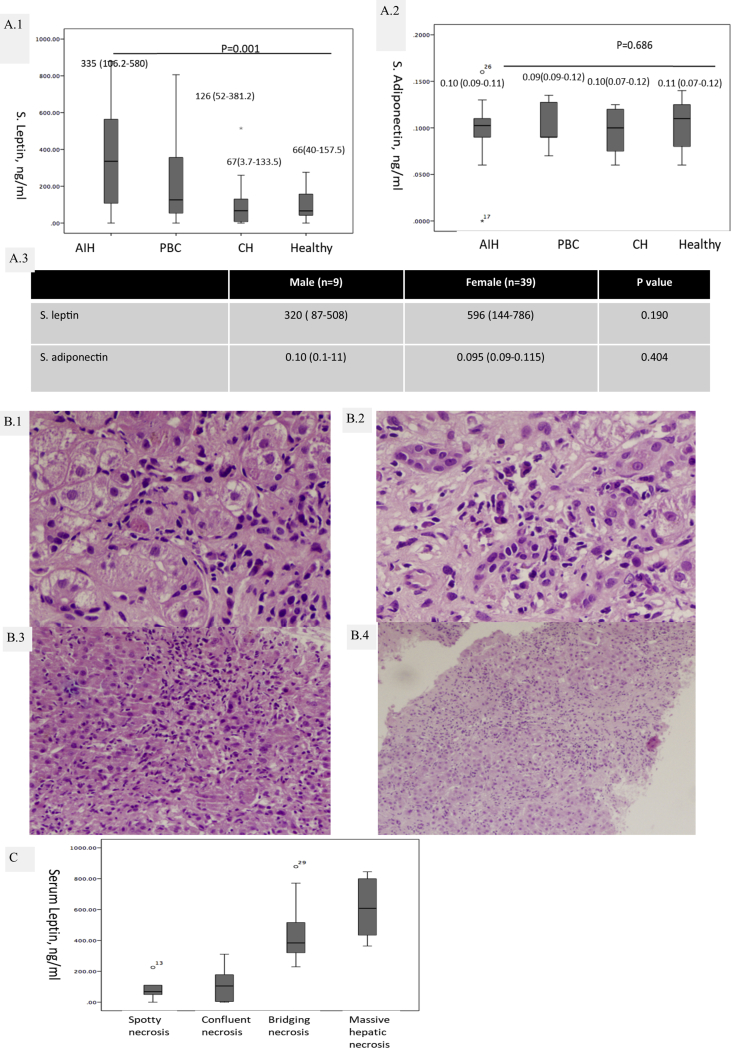
Baseline serum leptin level was higher in AIH [335 {IQR: 106.2–580} ng/ml] as compared with PBC [126 {52–381.2} ng/ml], CH [67 {3.7–133.5} ng/ml] and HCs [66 {40–157.5} ng/ml] (P < 0.001) [Figure 1A.1]. Serum adiponectin levels did not show any difference between the groups [Figure 1A.2]. Elevated levels of serum leptin in patients with AIH were higher in female patients as compared with the male but it did not demonstrate any significant difference {male (n = 9): 320 (87–508) vs the female (n = 39): 596 (144–786); P = 0.190}. In AIH cases, leptin levels correlated with aspartate amino transaminases (r = 0.615, P < 0.001); alanine amino transaminases (r = 0.551, P < 0.001); and HAI (r = 0.896, P < 0.001).
Figure 1.
A.1): levels of serum leptin; (A.2) Serum adiponectin and in autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), chronic hepatitis (CH) and in healthy controls (HCs); (A.3) Comparison of serum leptin and adiponectin in AIH based on gender. Representative photomicrographs of (B.1) Spotty necrosis; (B.2) Confluent necrosis; (B.3) Bridging necrosis; (B.4) Massive hepatic necrosis in AIH [all H&E, 200×] and (C) distribution of serum leptin based on necrosis.
On evaluation of liver biopsies of AIH for necroinflammatory activity spotty necrosis (n = 6) [Figure 1B.1]; confluent necrosis (n = 15) [Figure 1B.2]; bridging necrosis (n = 9) [Figure 1B.3]; and massive hepatic necrosis (n = 18) [Figure 2B.4] was noted. Serum leptin was significantly higher in cases with bridging necrosis and massive hepatic necrosis (P < 0.001) [Figure 1C].
Figure 2.
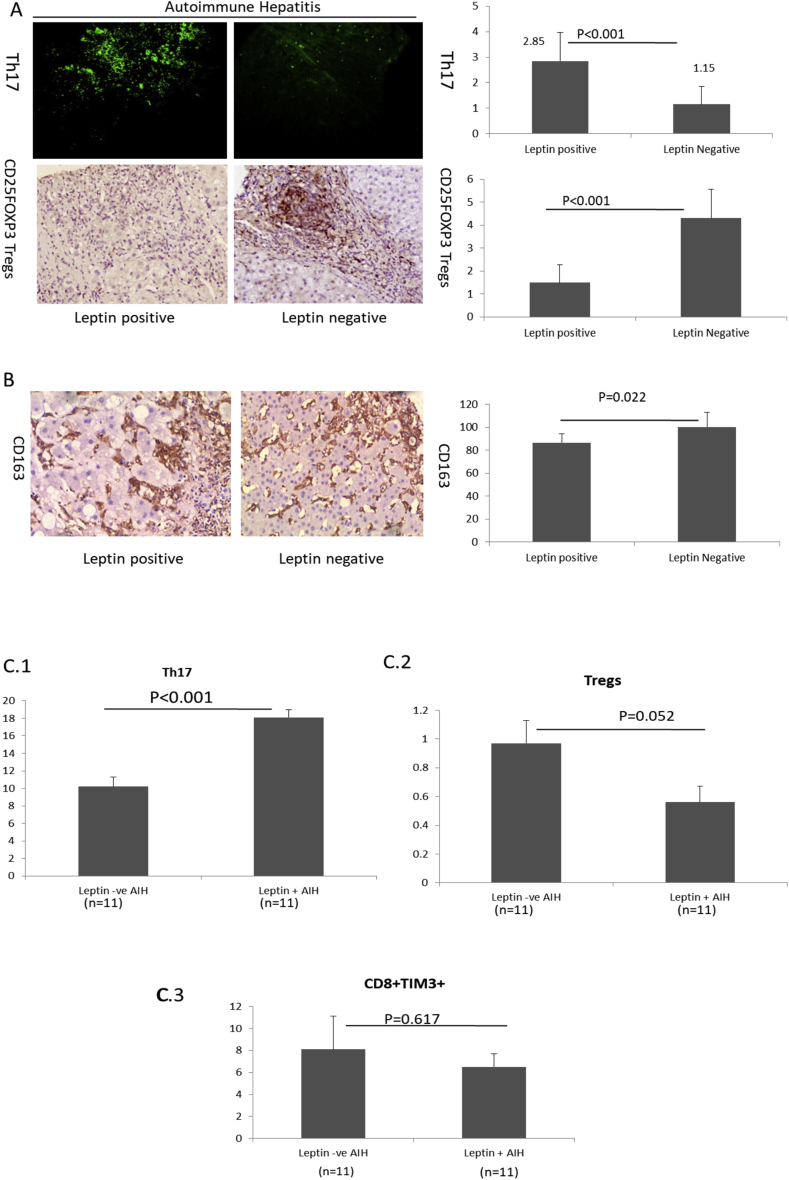
A) Representative images of Th17 cells and Tregs in liver biopsy of leptin positive and negative AIH cases (Left) and respective bar graphs showing the distribution of these cells (Right) (Mean ± SE); ((B) Representative images of CD163 cells in liver biopsy of leptin positive and negative cases (Left) and bar graph showing the distribution of these cells (Right) (Mean ± SE). Distribution of: (C.1) Th17 cells, (C.2) Tregs and (C.3) CD8 cells amongst leptin positive and negative AIH cases (Mean ± SE). AIH, autoimmune hepatitis; Tregs, T regulatory cells.
T helper cells 17 (Th17) sustain substantial proinflammatory activity and Tregs controls the active inflammation.11 In AIH cases, serum leptin positively correlated with Th17 cells (r = 0.650; P < 0.001) and negatively with Tregs (r = −0.711). A typical physiological level of serum leptin is considered as < 200 ng/ml,12 we divided these AIH cases into leptin positive (>200 ng/ml) and leptin negative (≤200 ng/ml). Thirty patients were leptin positive and 18 were leptin negative. Leptin positive patients invariably had a more significant number of Th17 cells (P < 0.001) and a lower percentage of Tregs (P < 0.001) [Figure 2A], and M2 type of macrophages identified by CD163 (P = 0.022) [Figure 2B], in the liver biopsy tissues. Leptin positive cases had higher serum IgG (20.5 ± 6.3 mg/dl) vs 15.5 ± 5.9 mg/dl, P = 0.05) and more ANA seropositivity (17 ANA + Leptin + vs 10 ANA + Leptin-, P = 0.047) than leptin negative cases. There was no difference noted in seropositivity for antismooth muscle antibody (P = 0.634).
Further, we investigated the circulating Th17, Tregs and CD8+Tim3+ cells in PBMCs. PBMCs were available in 22 AIH cases (11 leptin positives and 11 leptin negatives). As noted in liver biopsies; circulating Th17 cells were higher (P < 0.001) [Figure 2C.1] and Tregs were lower (P = 0.052) [Figure 2C.2] in leptin positive than the leptin negative cases. CD8+Tim3+ cells were comparable with both groups (P = 0.617) [Figure 2C.3].
On follow-up after treatment, 23 had a response to steroids, 17 were partially responsive, and eight experienced nonresponses to treatment. Multivariate analysis after adjusting for age, gender, and basal metabolic index; serum leptin was found to be elevated at the baseline, in patients who did not respond or had a partial response to therapy in comparison with cases with the biochemical response at 4 weeks {HR: 5.4, P = 0.001}. However, no difference noted in baseline adiponectin levels in these subgroups of patients with AIH. Baseline serum leptin positivity reasonably predicted the standard treatment response in AIH patients {Leptin positive: response/partial response/no response: 11/11/8 vs leptin negative: 16/2/0; P = 0.002 with a likelihood ratio of 15.4}.
Discussion
The results of this study show that the circulating leptin levels are high in AIH as compared with other autoimmune liver diseases such as primary biliary cholangitis and primary sclerosing cholangitis and HC cases. Serum leptin additionally serves as a surrogate marker of AIH necroinflammatory activity in liver biopsies and correlates with biochemical parameters of disease activity. Leptin positivity in AIH associated with higher Th17 and lower Treg cells both in liver tissue and in circulation, in comparison with those with leptin negative. The data also introduce a further dimension in steroid treatment response in AIH cases. Elevated leptin promotes a proinflammatory status by promoting Th1 and Th17 cells and inhibiting T regs. It modulates both adaptive and innate immunity and leads to the progression of autoimmune diseases.4 Leptin levels were examined in cirrhosis related to chronic viral hepatitis B (CHB) and chronic hepatitis C (CHC), it was suggested that the cirrhosis due to CHB or CHC is associated with higher leptin levels. Increased serum leptin was found to represent a negative prognostic factor for response to lamivudine therapy in CHB.13 On the contrary, another study highlighted the impact of TNF-A and IL-6 on necroinflammatory activity in patients with CHB but not the adipocytokines.14 Leptin levels were also detected to be significantly elevated in CHC with steatosis and associated with liver dysfunction.15 Among the autoimmune liver diseases, adiponectin found to be associated with autoimmune biliary diseases.16
Our study pointed the most excessive levels of leptin detected in AIH as compared with PBC, PSC, and control cases. It correlates with disease activity in the liver parenchyma. It is well perceived that the necroinflammatory activity in AIH is more pronounced than PBC and PSC.17 Leptin acts as an acute phase reactant during in some inflammatory conditions and leads to perpetuation of other proinflammatory cytokines and immune cells that can cause further injury, recruitment of more inflammatory infiltrate, necrosis in the tissues affected18 (e.g. liver injury in AIH). Most of the immune cells express LEPR, which suggests a role for leptin in enhanced immune response.19 This might seem to be the reason for increased Th17 and low Tregs in leptin positive cases as compared with leptin negative AIH cases. CD163 macrophages act as M2 type of macrophages in tissues which controls the anti-inflammatory process.20 Though leptin does not change the phenotype of M2 macrophages under the influence of leptin; M2 macrophages functionally behave as M1 proinflammatory macrophages with the secretion of TNF-α, IL-6, IL-1β, IL-1ra, IL-10, MCP-1, and MIP-1α.21 A recent study in pediatric AIH identified baseline AIH score, and depressed level of IL-2 at baseline can predict the adverse outcome after standard steroid therapy.22 Another study performed on adult patients suggested that the onset of disease < 18 years, increase the chances of relapse and DRB1∗04:01 positivity associated with a favorable clinical outcome.23 Another study highlighted that the incomplete normalization of ALT at 6 months, low serum albumin, and age at presentation of ≤20 years or >60 years were found to comprise poor prognostic factors.24 Considering the variables used in other studies for prognosis, it would be of beneficial use to assess leptin levels. It might also help in triaging the AIH patients in whom, aggressive immunosuppression could be beneficial.
Conflicts of interest
The authors have none to declare.
Funding
This study is part of study funded by Science and Engineering Research Board, Department of Science and Technology, Goverment of India-Grant: Young Scientist Scheme/2014/822.
References
- 1.Manns M.P., Czaja A.J., Gorham J.D. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 2.Kyttaris V.C., Krishnan S., Tsokos G.C. Systems biology in systemic lupus erythematosus: integrating genes, biology and immune function. Autoimmunity. 2006;39:705–709. doi: 10.1080/08916930601061363. [DOI] [PubMed] [Google Scholar]
- 3.Zandman-Goddard G., Peeva E., Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6:366–372. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.La Cava A., Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 5.Matarese G., Leiter E.H., La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70:87–95. doi: 10.1111/j.1399-0039.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E., Carrier Y., Gao W. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Hennes E.M., Zeniya M., Czaja A.J. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 8.Ishak K., Baptista A., Bianchi L. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 9.Bedossa P., Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 10.EASL Clinical Practice Guidelines Autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Lourenço E.V., Liu A., Matarese G., La Cava A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci U S A. 2016;113:10637–10642. doi: 10.1073/pnas.1607101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lord G.M., Matarese G., Howard J.K., Baker R.J., Bloom S.R., Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-inducedimmunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 13.Manolakopoulos S., Bethanis S., Liapi C. An assessment of serum leptin levels in patients with chronic viral hepatitis: a prospective study. BMC Gastroenterol. 2007;7:17. doi: 10.1186/1471-230X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong V.W., Wong G.L., Yu J. Interaction of adipokines and hepatitis B virus on histological liver injury in the Chinese. Am J Gastroenterol. 2010;105:132–138. doi: 10.1038/ajg.2009.560. [DOI] [PubMed] [Google Scholar]
- 15.Ellidokuz E., Cömlekçi A., Ellidokuz H. The role of serum leptin levels in chronic hepatitis C with steatosis. Hepato-Gastroenterology. 2003;50(suppl 2) [cclxix-cclxxii] [PubMed] [Google Scholar]
- 16.Durazzo M., Niro G., Premoli A. Type 1 autoimmune hepatitis and adipokines: new markers for activity and disease progression? J Gastroenterol. 2009;44:476–482. doi: 10.1007/s00535-009-0023-0. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen Canh H., Harada K., Ouchi H. Intractable Liver and Biliary Diseases Study Group of Japan. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017;70:961–969. doi: 10.1136/jclinpath-2016-204271. [DOI] [PubMed] [Google Scholar]
- 18.Bullo M., Garcia-Lorda P., Megias I., Salas-Salvado J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11:525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 19.Procaccini C., Jirillo E., Matarese G. Leptin as an immunomodulator. Mol Aspect Med. 2012;33:35–45. doi: 10.1016/j.mam.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Rőszer Tamás. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat Inflamm. 2015:16. doi: 10.1155/2015/816460. Article ID 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acedo S.C., Gambero S., Cunha F.G., Lorand-Metze I., Gambero A. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In Vitro Cell Dev Biol Anim. 2013;49:473–478. doi: 10.1007/s11626-013-9629-x. [DOI] [PubMed] [Google Scholar]
- 22.Diestelhorst J., Junge N., Jonigk D. Baseline IL-2 and the AIH score can predict the response to standard therapy in paediatric autoimmune hepatitis. Sci Rep. 2018;8:419. doi: 10.1038/s41598-017-18818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirstein M.M., Metzler F., Geiger E. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatology. 2015;62:1524–1535. doi: 10.1002/hep.27983. [DOI] [PubMed] [Google Scholar]
- 24.Ngu J.H., Gearry R.B., Frampton C.M., Stedman C.A. Predictors of poor outcome in patients w ith autoimmune hepatitis: a population-based study. Hepatology. 2013;57:2399–2406. doi: 10.1002/hep.26290. [DOI] [PubMed] [Google Scholar]